Abstract
“Executive functions” (EFs) is an umbrella term for higher cognitive control functions such as working memory, inhibition, and cognitive flexibility. One of the most challenging problems in this field of research has been to explain how the wide range of cognitive processes subsumed as EFs are controlled without an all-powerful but ill-defined central executive in the brain. Efforts to localize control mechanisms in circumscribed brain regions have not led to a breakthrough in understanding how the brain controls and regulates itself. We propose to re-conceptualize EFs as emergent consequences of highly distributed brain processes that communicate with a pool of highly connected hub regions, thus precluding the need for a central executive. We further discuss how graph-theory driven analysis of brain networks offers a unique lens on this problem by providing a reference frame to study brain connectivity in EFs in a holistic way and helps to refine our understanding of the mechanisms underlying EFs by providing new, testable hypotheses and resolves empirical and theoretical inconsistencies in the EF literature.
Keywords: executive functions, working memory, inhibition, flexibility, central executive, brain networks, cognitive control, distributed networks
1. Introduction
The ability to exert control over actions is essential for personal autonomy and one of the most impressive yet poorly understood capacities our brains endow us with. The processes that control actions cover a range of cognitive skills referred to as executive functions (EFs) (Diamond, 2013; Powell and Voeller, 2004). While the precise definition of EFs remains a topic of debate (Duncan and Owen, 2000), there is a common consent in terms of the importance of EFs for adaptive behavior in an ever-changing environment (Jurado and Rosselli, 2007; Norman and Shallice, 1986; Rabbitt, 1997). Numerous theoretical frameworks exist to classify different EFs and to describe how they work together (Burgess et al., 2000; Diamond, 2013; Duncan and Owen, 2000; Luria et al., 1966; Miyake et al., 2000; Norman and Shallice, 1986). According to Miyake (2000) and Diamond (2013), at least three core functions of EF can be identified, which are likely to represent distinct cognitive subsystems that nevertheless share a functional overlap to some degree: updating and monitoring of working memory representations, inhibition and interference control, as well as cognitive flexibility and shifting. Working memory (WM) is a cognitive system that enables to manipulate and maintain restricted chunks of information which are stored in short-term memory (Baddeley et al., 1986; Baddeley and Hitch, 1974; Cowan, 1999; Diamond, 2013; Miller, 1956; Smith and Jonides, 1999). Inhibition has been defined as “being able to control one’s attention, behavior, thoughts, and/or emotions to override a strong internal predisposition or external lure, and instead do what’s more appropriate or needed” (Diamond, 2013). Cognitive flexibility reflects the ability to consider multiple conflicting representations of a single object or event simultaneously (Jacques and Zelazo, 2005) and selectively switch between actions, perspectives, and strategies for appropriate action in a changing environment (Dajani and Uddin, 2015; Diamond, 2013). In addition to flexibly switching between several laboratory tasks, every-day life as well as modern work environments often require performance on two or more tasks at the same time, e.g. driving while having a telephone conversation or simultaneously controlling several displays in air traffic control. In this multitasking context, efficient EFs are essential to select relevant information from the environment and to adjust performance to environmental demands by flexibly shifting between more serial or more parallel processing strategies (Fischer and Plessow, 2015).
2. Centralized Control in Cognition and Cognitive Neuroscience
Substantial theoretical and experimental progress has been made in the past 50 years to describe how humans control their actions by selecting, processing, and prioritizing task-relevant stimuli (often at the expense of simultaneously present yet less relevant signals), and flexibly adapt to changing demands from the environment (Broadbent, 2013; Deutsch and Deutsch, 1963; Kahneman, 1973; Norman and Shallice, 1986; Schneider and Shiffrin, 1977; Shiffrin and Schneider, 1977; Treisman and Gelade, 1980; Wickens, 1991). Early cognitive models have introduced a hypothetical central executive to their cognitive architectures (Baddeley and Hitch, 1974; Norman and Shallice, 1986). One unifying premise underlying these theories is that some sort of central executive system is required that governs exerted control by “dampening” irrelevant and prioritizing relevant salient information or schemes. But while the necessity of higher-order control functions that control lower-order functions is self-evident, it does not explain how this central executive operates, reducing instead to the question of who is in charge of controlling the central executive. This problem of self-recurrency has been widely acknowledged and has led to the criticism that the central executive resembles an all-powerful but ill-defined “homunculus” that directs all processes that are not “automatic” (Monsell and Driver, 2000a). Any theoretical framework of EFs must therefore not only specify how control operates, how it is implemented at the neurophysiological level, and how it integrates other cognitive processes and their interrelatedness, but also formally address the question of what is controlling the controller in order to avoid an infinite hierarchy of central executives or homunculi (Logie, 2016).
One of the most influential theoretical frameworks on the neurophysiological mechanisms of EFs that has pushed beyond the central executive view has been proposed by Miller and Cohen (Miller and Cohen, 2001). This framework emphasizes the importance of the prefrontal cortex (PFC) as a key region to implement a wide range of EFs through active maintenance of goals and the means to achieve them. Rooted in the biased-competition model of selective attention (Desimone and Duncan, 1995), it cast the problem of control as a case of prioritization of competing perceptual or response representations. The role of the PFC is to bias activity in regions responsible for those representation, in accordance with a behavioral goal, that it maintains in memory. Thus, the PFC indirectly acts as a central executive and intervenes in a top-down fashion by selectively prioritizing the relevant process. According to this perspective, exerted control in EF is domain-general and operates on specific subordinated cognitive functions in service of a higher-order task goal. EFs such as inhibition, working memory and cognitive flexibility emerge from the dynamics of the PFC biasing goal-relevant activity patterns in the brain. In the last 20 years, this theoretical model has provided a versatile framework that has generated much empirical evidence supporting the pivotal role of the PFC in many EFs. However, despite progress in understanding the neurophysiological mechanisms of how the PFC represents and implements behavioral goals, the mechanism by which it accounts for EFs remains elusive. Although the PFC may participate to a greater extent than other areas in EFs, increasing evidence suggests that it does not act alone. Damage to the PFC, for instance, may or may not be accompanied by deficits in EFs, and neither site nor size of a prefrontal lesion allow for a clear prediction about the nature of resulting problem in executive functioning (Alvarez and Emory, 2006).
In parallel, the advance of non-invasive neuroimaging techniques such as fMRI, in combination with experimental designs rooted in subtractive logic (Friston et al., 1996; Newman et al., 2001), contributed to the conceptualization of a modular brain architecture (Fodor, 1983; Houk and Wise, 1995; Kanwisher et al., 1997; Minsky, 1988). The results could be construed as a fractionalization of the central executive (Baddeley, 2012; Botvinick et al., 2004, 2001; Braver, 2012; Diamond, 2013; Engle and Kane, 2003; Friston, 1994; Goldman-Rakic et al., 1996; Goschke and Bolte, 2014; Hazy et al., 2007, 2006; Hommel and Wiers, 2017; Koechlin and Summerfield, 2007; Miller and Cohen, 2001; Monsell and Driver, 2000c; Pezzulo et al., 2018; Shenhav et al., 2016, 2013), now a “macroconstruct” in which the central executive consists of discrete subsystems with anatomically segregated and functionally specialized modules. In a typical neuroimaging study, the cognitive process of interest is isolated via correlation to an external manipulation thought to elicit that process, and associated with a brain region of interest. If this association is statistically significant, it is assumed that this process is located within this brain area. This strategy has ascribed different aspects of EFs with specific anatomically circumscribed brain areas. For instance, goal-directed behavior with the ventromedial prefrontal cortex (vmPFC; Hare et al., 2014, 2009; Kahnt et al., 2011; Lim et al., 2011; Sokol-Hessner et al., 2012; Steinbeis et al., 2016; Vassena et al., 2014) and dorsolateral prefrontal cortex (dlPFC; Hare et al., 2014; Kahnt et al., 2011; Milham et al., 2001; Sokol-Hessner et al., 2012; Steinbeis et al., 2016), inhibition with right ventrolateral prefrontal cortex (vlPFC; Aron, 2007; Aron et al., 2014, 2004; Hampshire et al., 2010), conflict monitoring, selective attention, and computation of the expected value of control with anterior cingulate cortex (ACC; Alexander and Brown, 2011; Botvinick et al., 2004; Bush et al., 2002, 1999; Carter and Veen, 2007; Egner and Hirsch, 2005; Kerns, 2004; Kondo et al., 2004; MacDonald et al., 2000; Milham et al., 2001; Ridderinkhof et al., 2004; Shenhav et al., 2016, 2013; van Veen et al., 2001; Vassena et al., 2014; Weissman, 2004; Weissman et al., 2003), and cognitive flexibility and voluntary motor action selection with basal ganglia (BG; Aron et al., 2003; Cameron et al., 2010; Chakravarthy et al., 2010; Pauli et al., 2016; Redgrave et al., 1999; Stocco et al., 2010). The problems with such an approach have been extensively discussed and include subsequent tendencies towards reverse inference and inherent bias in interpretation (Poldrack, 2006; Poldrack and Wagner, 2004). Furthermore, a modular perspective fails to appreciate the activity of this region in the context of the other activities within the brain. The systematic association of a region with an EF such as inhibition could therefore reflect an emergent property of a broader neural network (e.g., involved in detection of salient cues, such as for inhibition; Hampshire et al., 2010), and thus risks to falsely localize higher-level cognitive processes at a lower level of processing (Eisenreich et al., 2017), especially in highly connected regions such as the ACC and basal ganglia.
Although it is intuitive to assume that conceptually distinct EFs must also have distinct anatomical representations (Botvinick and Cohen, 2014), attempts to identify neuroanatomical correlates of the central executive (Baddeley, 2012; Botvinick et al., 2004, 2001; Braver, 2012; Diamond, 2013; Engle and Kane, 2003; Friston, 1994; Goldman-Rakic et al., 1996; Goschke and Bolte, 2014; Hazy et al., 2007, 2006; Hommel and Wiers, 2017; Koechlin and Summerfield, 2007; Miller and Cohen, 2001; Monsell and Driver, 2000c; Pezzulo et al., 2018; Shenhav et al., 2016, 2013) have not yet led to breakthroughs to describe their neurophysiological mechanisms. The idea of subsystems that provide specialized, subsidiary central executive functions is challenged by empirical evidence suggesting that the same putative brain correlates for EFs such as the PFC and ACC have been associated with functionally different EFs (Alexander and Brown, 2011; Botvinick et al., 2004; Braver, 2012; Bush et al., 1999; Carter and Veen, 2007; Hare et al., 2009; Kahnt et al., 2011; Kerns, 2004; Kondo et al., 2004; Lim et al., 2011; MacDonald et al., 2000; Milham et al., 2001; Ridderinkhof et al., 2004; Shenhav et al., 2016, 2013; Steinbeis et al., 2016; van Veen et al., 2001; Vassena et al., 2014; Weissman, 2004; Weissman et al., 2003). This raises the question how a plethora of diverse EFs can arise from a rather static neuroanatomical architecture (Park and Friston, 2013). In complement, different EFs such as inhibition, flexibility, and working memory have been shown to activate large proportions of the cerebrum and converge within PFC, ACC, and posterior parietal cortex (Niendam et al., 2012). Moreover, the putative brain areas underlying EFs also show large functional overlap, such as in goal directed behavior (ACC; Carter and Veen, 2007; dlPFC; Hare et al., 2014; Kahnt et al., 2011; Sokol-Hessner et al., 2012; Steinbeis et al., 2016); vmPFC; Hare et al., 2014, 2009; Kahnt et al., 2011; Sokol-Hessner et al., 2012; Steinbeis et al., 2016), prediction and evaluation of an action (ACC; Alexander and Brown, 2011; Bush et al., 2002; Ridderinkhof et al., 2004; Shenhav et al., 2016, 2013; Vassena et al., 2014); vmPFC; Vassena et al., 2014), and selective attention (ACC; Bush et al., 1999; Milham et al., 2001; Weissman, 2004; Weissman et al., 2003); vmPFC; Lim et al., 2011; dlPFC; Milham et al., 2001), which challenges the functional specificity of these regions. Arguably, the focus on activity of specific regions of interests or single event-related components has therefore substantially limited the possibility to identifying the neural correlates of EFs from a whole brain perspective.
Increasing evidence suggests that these putative brain areas underlying EFs seem to interact. The ACC, for instance, has been found to support PFC in maintaining and updating task-relevant representation in working memory (Banich et al., 2000; D’Esposito and Postle, 2015; Egner and Hirsch, 2005) and signal the need for control (Botvinick et al., 2004; Hazy et al., 2007). Furthermore, studies indicate that cognitive flexibility is orchestrated within BG (Aron et al., 2003), but also supported by PFC (Chakravarthy et al., 2010; Stocco et al., 2010) and ACC (Nicolle and Baxter, 2003). Similarly, close interrelation between PFC and BG were found to support WM processes (Ashby et al., 2005; Baier et al., 2010; Chang et al., 2007; Frank et al., 2001; McNab and Klingberg, 2007; Schroll et al., 2012; Schroll and Hamker, 2013; Voytek and Knight, 2010). Thus, once again, the data suggest that pivotal brain areas that contribute significantly to EFs, such as PFC, ACC, and basal ganglia, act within spatially distributed networks and include other structures that may also be relevant to explain EFs. This point is echoed in studies of neuropsychiatric disorders that exhibit impairments in EFs, and that reveal aberrant development and functional connectivity in spatially distributed brain networks (Assaf et al., 2010; Baker et al., 2014; Bassett et al., 2012; Cerliani et al., 2015; Chai et al., 2011; Cherkassky et al., 2006; dos Santos Siqueira et al., 2014; Du et al., 2016; Fan et al., 2012; Fassbender et al., 2009; Franzen et al., 2013; Garrity et al., 2007; Hull et al., 2017; Itahashi et al., 2014; Jafri et al., 2008; Jang et al., 2011; Liddle et al., 2011; Lin et al., 2015; Manoliu et al., 2014; Meda et al., 2014; Murias et al., 2007; Öngür et al., 2010; Orliac et al., 2013; Paakki et al., 2010; Pomarol-Clotet et al., 2008; Roiser et al., 2013; Rotarska-Jagiela et al., 2010; Salgado-Pineda et al., 2011; Sripada et al., 2014; Sun et al., 2014; Swanson et al., 2011; Tian et al., 2008; Tu et al., 2013; Uddin et al., 2008; van Buuren et al., 2012; Wang et al., 2005; Weng et al., 2010; Whitfield-Gabrieli et al., 2009; Wilson et al., 2011; Woodward et al., 2011).
Thus, mounting evidence suggest that there is no single brain region that sits at the control apex, and, furthermore, efforts to define EF-dedicated neuroanatomical substrates have not been conclusive. The question of how EFs are generated by the brain persists. In the following sections we consider an alternate view to centralized or localized EFs. We argue based on suggestions from the cognitive and computational literature(Barnard and Bowman, 2004; Eisenreich et al., 2017; Vandierendonck, 2016), and in line with the recognized significance of functional connectivity patterns to neural organization and function (Collin et al., 2014; Margulies et al., 2016; van den Heuvel et al., 2012; van den Heuvel and Sporns, 2013), that EFs are neither a strictly top-down generated process nor one that can be localized in specific brain correlates, but rather that EFs are the emerging consequence of communication within a broad network of spatially and functionally dispersed brain areas that integrate different aspects of EFs.
3. Distributed Control and Executive Functions
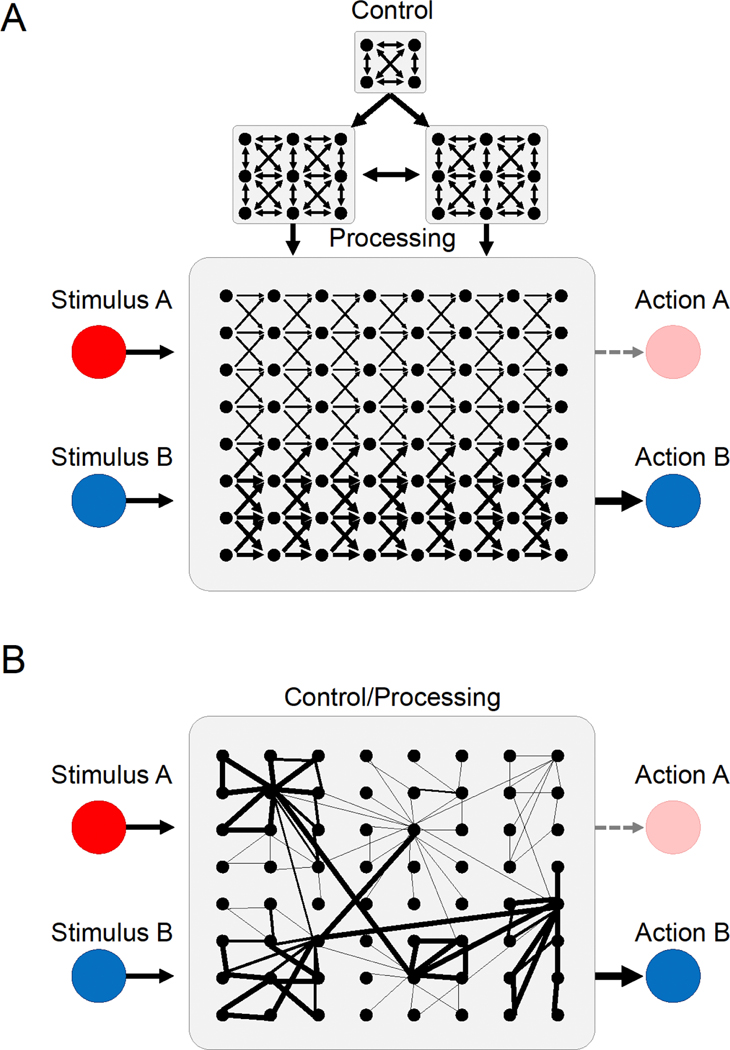
A core assumption underlying many theoretical frameworks on EFs is of hierarchical architecture, namely, that brain areas or circuits underlying EFs regulate, but do not participate in the more basic cognitive processes that they control (Botvinick et al., 2001; Miller and Cohen, 2001; Figure 1A). This top-down view, by design, relies on a centrally operating executive. However, Barnard and Bowman (2004), Vandierendonck (2016) and Eisenreich et al. (2017), have argued that EFs can also be a result of a conceptually and anatomically distributed control system (DCS). One such DCS view is inspired from early connectionist models (Hopfield, 1982; James and Rumelhart, 1986), in which control and controlled processes are co-localized within large numbers of dispersed elements (Figure 1B). In contrast to a hierarchical architecture where core brain regions are assumed to solely exert control on more basic processes, in a DCS, each element can be a controller and an element that is being controlled. In computational models of distributed control, the state of the overall system is often independent from any constituting element (Barnard and Bowman, 2004). In other words, in such systems, behavioral control is analogous to the state of the overall system, and is therefore an emergent function arising from the interaction of all elements (McClelland et al., 2010; Mitchell, 2009). Recent evidence of distributed control in nonbrain biological systems such as in schools of fish, flocks of birds, swarms of insects (Couzin, 2009; Eisenreich et al., 2017; Passino et al., 2008), and herds of baboons (Couzin and Krause, 2003; Strandburg-Peshkin et al., 2015) and in distributed deep learning algorithms (LeCun et al., 2015) highlight the strength and plausibility of a DCS view. Apparent control in these biological distributed systems is often realized via a couple of simple rules that guide the indirect coordination between agents or actions. In schools of fish or flocks of birds, the control over the shape of the swarm and the moving direction is a consequence of the distance kept between adjacent agents and the rule about when to change direction (i.e. follow the group average; Couzin and Krause, 2003). These rules enable fast communication of information (e.g. about the direction of movement) across all agents, without reliance on a top-down controller.
Figure 1.
Organization of a modular executive control network vs. a distributed control network, in which two stimuli (stimulus A and stimulus B) lead to an action outcome (action A vs. action B). A. Top-down controlled, hierarchically organized control-modules govern automatized processing. In this system, control and processing elements are distinct and localized to specific areas. B. Distributed control networks, in which control and processing elements are combined in individual agents. These agents form individual clusters. Adapted and modified from Eisenreich et al. (2017).
Can a DCS framework explain EFs? EFs can be conceptualized as a consequence of the interaction between distributed elements, thus avoiding the problem of any central executive or central executive subsystem. Increasingly evidence suggests that, indeed, properties of EF generation in the brain are consistent with properties of a DCS. One such principle is that all elements can be controlled or be a controller, or, in other words, the emergent property can be observed in any subset of the system. Several studies have highlighted that prefrontal regions, a putative apex of the central executive, as well as posterior regions seem to exhibit both basic and control processing (Awh and Jonides, 2001; Cisek and Kalaska, 2010; Postle, 2006; Sleezer and Hayden, 2016). In line with the DCS view, it has been suggested that working memory (WM), a core EF, is a general property of the cortex, not limited to specific regions (Lee and Baker, 2016; Postle, 2006). This proposal shifts perspective of WM from localized to a highly distributed process wherein information can be maintained in any system engaged in the initial perceptual processing, including any region contributing to the initial percept (Lee and Baker, 2016). The second key principle of a DCS is the existence of local rules capable of generating the emergent phenomenon, such as the distance kept between adjacent agents in a swarm. In the brain, from single neuron firing to neuron populations and between brain area communications, each level is a complex function of its lower level constituents and embedded in a larger-scale organization (Buzsáki, 2006). In this context, EFs such as the initiation vs. the inhibition of an action or switching to another action might be the emergent behavioral outcome from simpler local neurophysiological mechanisms of the participating brain areas, for instance via neuronal interactions resulting in local gain modulation (Abbott, 1997; Chance et al., 2002; Donner and Nieuwenhuis, 2013; Saalmann and Kastner, 2009; Salinas and Thier, 2000) and the collective state of each contributing neuron (Buzsáki, 2006). In particular, evidence supporting biased competition among neuronal populations in generating initiation or inhibition in selective attention (Desimone, 1998; Desimone and Duncan, 1995), suggests that such competition is a general property of neurons across the brain, not limited to any central controller. From this perspective, the emergence of executive control patterns (e.g. initiating or inhibiting an action or switching to another action) observed in putative control structures like the PFC, ACC, and basal ganglia may reflect the overall state of the system, in the same way a single fish in a swarm or a single bird in a flock reflects the state of the overall system (i.e. the trajectory of the movement), without the requirement to be on top of the hierarchy in the control system.
Another key property of a DCS is its robustness to perturbations. In contrast to centralized systems, in which a nonbrain biological systems such as swarm would be vulnerable to the loss of its leading agent, a swarm organized as a DCS has been shown to be robust to degradation (Sumpter, 2006). Similarly, decentralized (i.e. distributed) networks have been shown to be resilient systems which are capable of absorbing large external perturbations without undergoing functional breakdown (Achard, 2006; Bassett and Bullmore, 2006; Bullmore and Sporns, 2009; Buzsáki, 2006). A DCS network organization in the brain may therefore explain how EFs can be preserved to some extent in the face of pathological attack by lesion and substance-related disorders (Ahmadlou et al., 2013; Wang et al., 2015; Yuan et al., 2010), neuropsychological disorders like ADHD (Ahmadlou et al., 2012; Liu et al., 2015; Wang et al., 2009; Xia et al., 2014), schizophrenia (Liu et al., 2008; Micheloyannis et al., 2006; Rubinov et al., 2009; Shim et al., 2014), and restless legs syndrome (Choi et al., 2017), aberrant development such as in autism (Barttfeld et al., 2011; Itahashi et al., 2014), and cognitive decline such as Alzheimer’s (Frantzidis et al., 2014; Stam et al., 2006; Vecchio et al., 2016; Wei et al., 2015; Zeng et al., 2015) and Parkinson’s disease (Berman et al., 2016; Lebedev et al., 2014). As noted previously, damage to the PFC, as well, may or may not be accompanied by deficits in EFs, and neither site nor size of a prefrontal lesion allow for a clear prediction about the nature of resulting problem in executive functioning (Alvarez and Emory, 2006). Thus, EFs do exemplify some robustness in the human brain. One way to investigate the robustness of EFs as a DCS is using ‘lesioned’ networks in computational models, which investigate how an observed profile of anatomical or functional dysconnectivity in a mature network might have been generated by earlier developmental abnormalities (Achard, 2006; Honey and Sporns, 2008; Kaiser et al., 2007; Ravasz and Barabási, 2003; Sporns, 2006). In order to explore the effect of acute damage on overall performance in EFs, nodes or connections are deleted. The vulnerability of a network to damage is then assessed by comparing its efficiency after the lesioning to its intact behavior. In an anatomically informed computational model, in line with the DCS view, deletion of nodes did not impair EFs.
4. A New Era for Executive Functions
Despite the appeal of the DCS view as an elegant theoretical solution for the aforementioned problems with centralized EF frameworks, and despite indirect evidence for the viability of DCS as a putative alternative, direct empirical studies of the neurophysiological mechanisms of a DCS governing EFs are scarce. This may be attributed in part to limited awareness of this framework in the field and in part to inherent difficulties in translating DCS concepts, such as the existence of organizing rules, to testable hypotheses. A key challenge is to derive descriptive measures that could provide testable hypothesis of the organizational rules that drive the network towards emergence of EFs. However, one promising approach to investigate EFs from a DCS perspective is the application of methods from network science, primarily graph theory, to neuroimaging data. A DCS conceptualizes executive functioning as an eminent property of multiple interacting elements of the brain. This is analogous to the network science framework, which aims to summarize, via a family of derived metrics, the organizing principles of a set of connected nodes. Hence, the graph theory framework is naturally aligned with a distributed perspective on EFs, with derived metrics potentially capturing the organizing principles or local rules that guide the behavior of the system. The task then becomes to model the brain as a network in which brain areas or constellations act as nodes (i.e. agents) and functional communication among these nodes are represented as edges, and, critically, to relate the static and dynamic features of the resulting network to EFs. In contrast to putting emphasis on the identification of specialized subsystems of EFs that are assumed to exert control over basic processes, a network analytical approach puts focus on how these brain regions or sub-systems (i.e. network nodes) communicate (i.e. are connected) with each other.
From a network perspective, this means that functionally different EFs such as inhibition, and cognitive flexibility can recruit a set of similar or overlapping brain structures (i.e. PFC, ACC, and other regions), but the connectivity patterns within these networks between perceptual input and motor output are functionally different. The connectivity patterns between the involved agents can, in principle, either result in refraining from an action (i.e. inhibition) or shifting to another action (i.e. cognitive flexibility). In other words, we hypothesize that the emergence of a given EF may lie in a given network state, as determined by organizing rules.
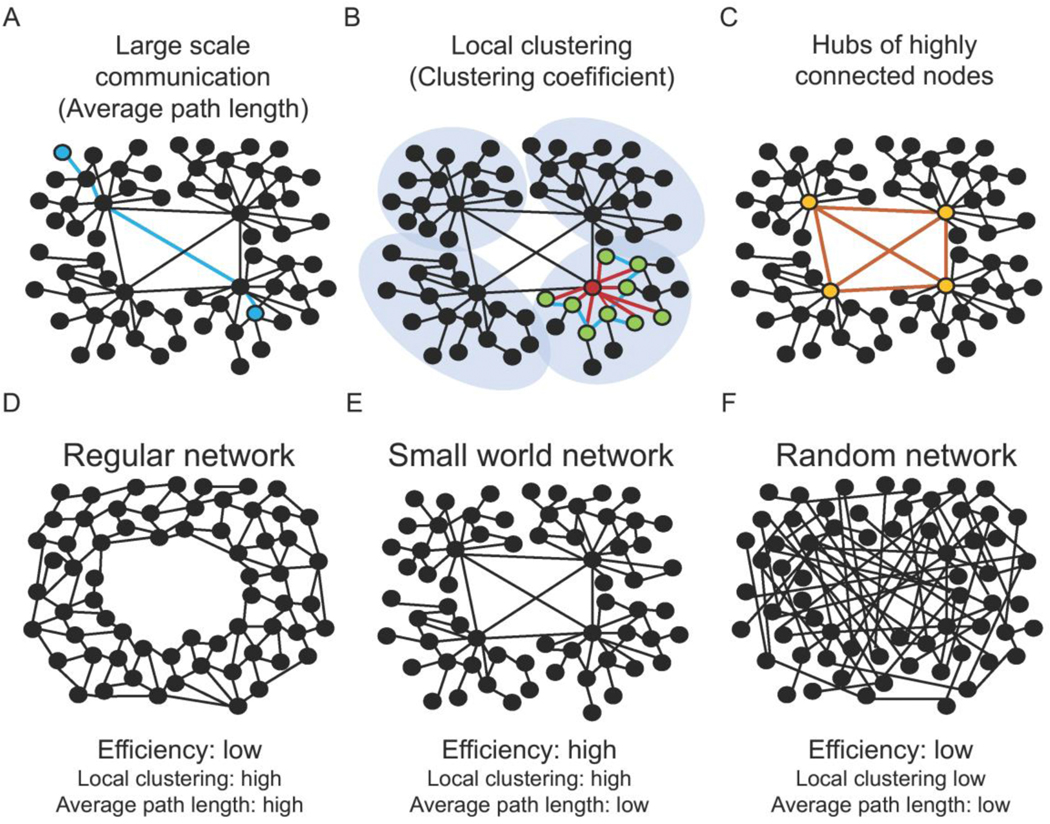
One important characteristic of networks, and putative organizing rule, is the structural and functional efficiency of its architecture. From an evolutionary perspective, brain networks have been evolved to maximize the diversity of possible functional circuits (i.e. enabling functionally different EFs), while minimizing the diversity of structural circuits (i.e. enabling efficient assembly and encoding) (Sporns and Kötter, 2004). Thus, the brain seeks towards minimal complexity of neural architecture with maximal richness of function. This may explain why putative brain correlates of specific EFs (i.e. PFC and ACC) have also been associated with other functionally different EFs (Alexander and Brown, 2011; Botvinick et al., 2004; Braver, 2012; Bush et al., 1999; Carter and Veen, 2007; Hare et al., 2009; Kahnt et al., 2011; Kerns, 2004; Kondo et al., 2004; Lim et al., 2011; MacDonald et al., 2000; Milham et al., 2001; Ridderinkhof et al., 2004; Shenhav et al., 2016, 2013; Steinbeis et al., 2016; van Veen et al., 2001; Vassena et al., 2014; Weissman, 2004; Weissman et al., 2003). From a graph theoretical perspective, the efficiency of large-scale communication between different brain areas is defined by the number of connections necessary to connect all neurons, a measure that is referred to as average synaptic path length (Figure 2A). Additionally, metabolic efficiency in brain networks is realized by predominantly local connectivity (Yoshimura et al., 2005) or local clustering (Figure 2B), which avoids metabolically expensive long-range axonal connections (Kalisman et al., 2005). However, a purely local clustering in the network would dramatically increase the average number of connections necessary to connect all neurons (i.e. the average path length). Thus, two network organization principles—local clustering and average path length —compete with each other to providing network communication that is metabolically efficient (i.e. high local clustering), but also enables effective large-scale communication (i.e. low average path length). Networks, which enable efficient large-scale traffic while maintaining mainly local connectivity are called ‘small-world’ networks (Watts and Strogatz, 1998; Figure 2E). Despite considerable heterogeneity in the methodological approaches, there is an encouraging degree of convergence between small-world properties on the structural (Chen et al., 2008; Hagmann et al., 2007; Y. He et al., 2007; Iturria-Medina et al., 2008, 2007; Sporns and Kötter, 2004) as well as on the functional brain network level (Ferrarini et al., 2009; Liu et al., 2008; Meunier et al., 2009; Salvador, 2004; Schwarz et al., 2008; Wang et al., 2009; Xia et al., 2014). Thus, the small-world properties of neural networks may be a key organizing principle that provides a functional link between brain structure and EFs (Beste et al., 2019a). Namely, we suggest that EFs are the result of the brain’s need to be structurally and functionally efficient. Recent findings highlight that the efficiency of how brain networks communicate (i.e. the small-world network characteristic) is strongly related to the demand on EFs. Differences in small-world properties have been shown for different demands on EFs (Beste et al., 2019; Wolff et al., 2017; Zink et al., 2018). Higher demand on inhibition (Hong et al., 2016), cognitive flexibility and WM capacity, for instance, have been found to lead to decreased small-world properties in the involved functional networks (Wolff et al., 2017), suggesting that EFs emerge when the brain is tipped away from a small-world state. This would predict that training EF capacities should lead to more efficient (i.e. more ‘small-worldish’) functional network communication. Consistent with this prediction, training in EFs such as WM capacity has shown to improve the efficiency of functional networks in the brain (Langer et al., 2013).
Figure 2.
Graph theoretical characteristics of distributed control networks in the brain. Functional integration capacity in neural networks can be quantified by the average path length (A), which describes the average number of edges (marked in blue) to connect each node to any other node in the network (marked in blue). Functional separation capacity in neural networks can be quantified by the clustering coefficient (B), which describes the interconnectivity of neighboring nodes. The clustering coefficient is exemplified in (B), where connections for one node (marked in red) to its immediate neighbors (marked in green) are colored in red and the connections between these neighbors are marked in blue. All real networks lie on a spectrum between very regularly and very randomly connected. Regular networks (D) exhibit a high clustering coefficient and a high average path length. Random networks (F) show a low clustering coefficient and a low average path length. According to Watts and Strogatz(Watts and Strogatz, 1998), a network shows small-world network properties (E) when it demonstrates a high clustering coefficient as in regular networks and a low average path length as in very random networks. In case a network shows small-world properties, highly connected nodes form hubs that integrate high local connections with long range connections (C).
Of particular interest, small-world architecture results in a system that is neither exclusively top-down in information flow, or exclusively distributed. The system necessitates the existence of so-called hub nodes, that are highly connected and serve a key role in facilitating the balance between high-local clustering and low average path length (van den Heuvel and Sporns, 2013; Figure 2C). However, such hub-nodes, due to their high-connectivity, can, in a way, exert control over less-strongly connected nodes along the action cascade and thus may play a key role in driving the functional connectivity state of the system and the emergence of behavior (Figure 2C). Supporting this notion, in a simulated model, deletion of well-connected nodes produced disruptions of functional connectivity (Falcon et al., 2015; Honey and Sporns, 2008; Jirsa et al., 2010) consistent with reported effects of focal human brain lesions on EFs (B. J. He et al., 2007). These facts have also led to the proposal that there exists a connective core network, that is a metabolically costly yet highly integratory collective of brain regions, whose anatomical wiring and topological position within the brain network supports brain-wide communicability and which exerts control over brain dynamics and cortical states (Collin et al., 2014; de Reus and van den Heuvel, 2014; Senden et al., 2017; Shine et al., 2018; van den Heuvel et al., 2012; van den Heuvel and Sporns, 2013). Here we additionally suggest that such a network drives brain states in observance of or striding towards a small-world state, with no single region acting as a core executive or solely responsible solely for the maintenance of behavioral goals (e.g., PFC). It is sensible to suppose that regions previously associated with EFs (i.e. PFC, ACC, basal ganglia) contribute to this network, though the identity of this network, or networks, remains an open question. A large scale meta-analysis of fMRI literature has shown that a superordinate fronto-cingulo-parietal network underlies a range of different EFs such as inhibition, flexibility, and working memory, consistent with expectation, but also integrating the inherent biases in published literature (Niendam et al., 2012). In contrast, in an original research study, Dosenbach et al. (2007), used network analyses on a set of 39 predefined regions of interests (ROIs) to reveal two separate networks of ROIs that both showed small-world characteristics: A frontoparietal network that included dlPFC that was more strongly associated with control adaptation and another network including the ACC, that was more strongly associated with WM processes. These data raise the open question of whether there exists a single superordinate EF network, whether multiple networks exist for different EFs or whether, as we suggest here, EFs are purely emergent phenomena with no underlying network, that arise when the brain responds to disturbances of its small-world state.
5. Conclusion
Despite the experimental and theoretical progress in research on EFs, efforts to identify circumscribed brain areas associated with executive functioning have not satisfactorily described the neurophysiological mechanisms that drive EFs. Increasing evidence from neuroimaging suggests instead that the dynamics of a superordinate, spatially distributed brain network, or networks, underlie EFs. A paradigm change from a centralized view, in which single areas exert top down control over the more basic processes they regulate, towards a distributed control system view, in which each brain network agent controls and is being controlled, provide a new perspective on mechanisms of how EFs emerge, with small-world organization a putative driving principle. Emerging studies that leverage network science, and emphasize emergent network dynamics, pave the wave to a new era of EF research.
Highlights.
Efforts to localize control mechanisms in executive functions have not led to a breakthrough in understanding the central executive
The central executive needs to be reconceptualized as a distributed control system
Executive control is likely the emergent consequence of highly distributed brain processes among brain hub regions
Graph-theory driven analysis of brain networks offers a unique holistic way to study EFs from a distributed control system view
Acknowledgements
This work was supported by a grant from the German Research Foundation (DFG) awarded to Sebastian Markett (MA-6792/3-1) and National Institute of Mental Health awarded to Agatha Lenartowicz (R01MH116268).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, 1997. Synaptic Depression and Cortical Gain Control. Science 275, 221–224. 10.1126/science.275.5297.221 [DOI] [PubMed] [Google Scholar]
- Achard S, 2006. A Resilient, Low-Frequency, Small-World Human Brain Functional Network with Highly Connected Association Cortical Hubs. Journal of Neuroscience 26, 63–72. 10.1523/JNEUROSCI.3874-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadlou M, Adeli H, Adeli A, 2012. Graph theoretical analysis of organization of functional brain networks in ADHD. Clin EEG Neurosci 43, 5–13. [DOI] [PubMed] [Google Scholar]
- Ahmadlou M, Ahmadi K, Rezazade M, Azad-Marzabadi E, 2013. Global organization of functional brain connectivity in methamphetamine abusers. Clinical Neurophysiology 124, 1122–1131. 10.1016/j.clinph.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Alexander WH, Brown JW, 2011. Medial prefrontal cortex as an action-outcome predictor. Nature Neuroscience 14, 1338–1344. 10.1038/nn.2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JA, Emory E, 2006. Executive Function and the Frontal Lobes: A Meta-Analytic Review. Neuropsychol Rev 16, 17–42. 10.1007/s11065-006-9002-x [DOI] [PubMed] [Google Scholar]
- Aron AR, 2007. The neural basis of inhibition in cognitive control. The neuroscientist 13, 214–228. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA, 2014. Inhibition and the right inferior frontal cortex: one decade on. Trends in cognitive sciences 18, 177–185. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA, 2004. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences 8, 170–177. [DOI] [PubMed] [Google Scholar]
- Aron AR, Watkins L, Sahakian BJ, Monsell S, Barker RA, Robbins TW, 2003. Task-Set Switching Deficits in Early-Stage Huntington’s Disease: Implications for Basal Ganglia Function 15, 17. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ell SW, Valentin VV, Casale MB, 2005. FROST: A distributed neurocomputational model of working memory maintenance. Journal of cognitive neuroscience 17, 1728–1743. [DOI] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O’Boyle JG, Schultz RT, Pearlson GD, 2010. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage 53, 247–256. 10.1016/j.neuroimage.2010.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J, 2001. Overlapping mechanisms of attention and spatial working memory. Trends in cognitive sciences 5, 119–126. [DOI] [PubMed] [Google Scholar]
- Baddeley A, 2012. Working memory: theories, models, and controversies. Annual review of psychology 63, 1–29. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Logie R, Bressi S, Sala SD, Spinnler H, 1986. Dementia and working memory. The Quarterly Journal of Experimental Psychology Section A 38, 603–618. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G, 1974. Working memory, in: Psychology of Learning and Motivation. Elsevier, pp. 47–89. [Google Scholar]
- Baier B, Karnath H-O, Dieterich M, Birklein F, Heinze C, Muller NG, 2010. Keeping Memory Clear and Stable--The Contribution of Human Basal Ganglia and Prefrontal Cortex to Working Memory. Journal of Neuroscience 30, 9788–9792. 10.1523/JNEUROSCI.1513-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BTT, Krienen F, Buckner RL, Öngür D, 2014. Disruption of Cortical Association Networks in Schizophrenia and Psychotic Bipolar Disorder. JAMA Psychiatry 71, 109. 10.1001/jamapsychiatry.2013.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z-P, Wright A, Shenker J, 2000. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of cognitive neuroscience 12, 988–1000. [DOI] [PubMed] [Google Scholar]
- Barnard PJ, Bowman H, 2004. Rendering information processing models of cognition and affect compu- tationally explicit: distributed executive control and the deployment of attention 35. [Google Scholar]
- Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Sigman M, 2011. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia 49, 254–263. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, 2006. Small-World Brain Networks. The Neuroscientist 12, 512–523. 10.1177/1073858406293182 [DOI] [PubMed] [Google Scholar]
- Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO, 2012. Altered Resting State Complexity in Schizophrenia. Neuroimage 59, 2196–2207. 10.1016/j.neuroimage.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman BD, Smucny J, Wylie KP, Shelton E, Kronberg E, Leehey M, Tregellas JR, 2016. Levodopa modulates small-world architecture of functional brain networks in Parkinson’s disease: Levodopa Modulates Brain Networks IN PD. Mov Disord. 31, 1676–1684. 10.1002/mds.26713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Stock A-K, Zink N, Ocklenburg S, Akgün K, Ziemssen T, 2019. How minimal variations in neuronal cytoskeletal integrity modulate cognitive control. NeuroImage 185, 129–139. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD, 2001. Conflict monitoring and cognitive control. Psychological review 108, 624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, 2014. The computational and neural basis of cognitive control: charted territory and new frontiers. Cognitive science 38, 1249–1285. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS, 2004. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences 8, 539–546. 10.1016/j.tics.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Braver TS, 2012. The variable nature of cognitive control: A dual-mechanisms framework. Trends Cogn Sci 16, 106–113. 10.1016/j.tics.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent DE, 2013. Perception and communication. Elsevier. [Google Scholar]
- Bullmore E, Sporns O, 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience 10, 186–198. 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T, 2000. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia 38, 848–863. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J, 1999. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biological Psychiatry 45, 1542–1552. 10.1016/S0006-3223(99)00083-9 [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR, 2002. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences 99, 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, 2006. Rhythms of the brain. Oxford Univ. Press, Oxford. [Google Scholar]
- Cameron IGM, Watanabe M, Pari G, Munoz DP, 2010. Executive impairment in Parkinson’s disease: Response automaticity and task switching. Neuropsychologia 48, 1948–1957. 10.1016/j.neuropsychologia.2010.03.015 [DOI] [PubMed] [Google Scholar]
- Carter CS, Veen V van, 2007. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience 7, 367–379. 10.3758/CABN.7.4.367 [DOI] [PubMed] [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C, 2015. Increased Functional Connectivity Between Subcortical and Cortical Resting-State Networks in Autism Spectrum Disorder. JAMA Psychiatry 72, 767. 10.1001/jamapsychiatry.2015.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JDE, Nieto Castañón A, McCarthy JM, Cohen BM, Öngür D, 2011. Abnormal Medial Prefrontal Cortex Resting-State Connectivity in Bipolar Disorder and Schizophrenia. Neuropsychopharmacology 36, 2009–2017. 10.1038/npp.2011.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy VS, Joseph D, Bapi RS, 2010. What do the basal ganglia do? A modeling perspective. Biol Cybern 103, 237–253. 10.1007/s00422-010-0401-y [DOI] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD, 2002. Gain Modulation from Background Synaptic Input. Neuron 35, 773–782. 10.1016/S0896-6273(02)00820-6 [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V, 2007. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage 34, 1253–1269. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC, 2008. Revealing Modular Architecture of Human Brain Structural Networks by Using Cortical Thickness from MRI. Cerebral Cortex 18, 2374–2381. 10.1093/cercor/bhn003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA, 2006. Functional connectivity in a baseline resting-state network in autism: NeuroReport 17, 1687–1690. 10.1097/01.wnr.0000239956.45448.4c [DOI] [PubMed] [Google Scholar]
- Choi JW, Jeong MH, Her SJ, Lee BU, Cha KS, Jung K-Y, Kim KH, 2017. Abnormal Sleep Delta Rhythm and Interregional Phase Synchrony in Patients with Restless Legs Syndrome and Their Reversal by Dopamine Agonist Treatment. J Clin Neurol 13, 340. 10.3988/jcn.2017.13.4.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF, 2010. Neural mechanisms for interacting with a world full of action choices. Annual review of neuroscience 33, 269–298. [DOI] [PubMed] [Google Scholar]
- Collin G, Sporns O, Mandl RC, van den Heuvel MP, 2014. Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cerebral cortex 24, 2258–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin ID, 2009. Collective cognition in animal groups. Trends in cognitive sciences 13, 36–43. [DOI] [PubMed] [Google Scholar]
- Couzin ID, Krause J, 2003. Self-organization and collective behavior in vertebrates. Advances in the Study of Behavior 32, 10–1016. [Google Scholar]
- Cowan N, 1999. An embedded-processes model of working memory. Models of working memory: Mechanisms of active maintenance and executive control 20, 506. [Google Scholar]
- Dajani DR, Uddin LQ, 2015. Demystifying cognitive flexibility: implications for clinical and developmental neuroscience. Trends in neurosciences 38, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reus MA, van den Heuvel MP, 2014. Simulated rich club lesioning in brain networks: a scaffold for communication and integration? Front. Hum. Neurosci. 8. 10.3389/fnhum.2014.00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, 1998. Visual attention mediated by biased competition in extrastriate visual cortex. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 353, 1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J, 1995. Neural mechanisms of selective visual attention. Annual review of neuroscience 18, 193–222. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, 2015. The Cognitive Neuroscience of Working Memory. Annu. Rev. Psychol. 66, 115–142. 10.1146/annurev-psych-010814-015031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch JA, Deutsch D, 1963. Attention: Some theoretical considerations. Psychological review 70, 80. [DOI] [PubMed] [Google Scholar]
- Diamond A, 2013. Executive Functions. Annual Review of Psychology 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Nieuwenhuis S, 2013. Brain-wide gain modulation: the rich get richer. Nat Neurosci 16, 989–990. 10.1038/nn.3471 [DOI] [PubMed] [Google Scholar]
- dos Santos Siqueira A, Biazoli Junior CE, Comfort WE, Rohde LA, Sato JR, 2014. Abnormal Functional Resting-State Networks in ADHD: Graph Theory and Pattern Recognition Analysis of fMRI Data. BioMed Research International 2014, 1–10. 10.1155/2014/380531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE, 2007. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences 104, 11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Pearlson GD, Yu Q, He H, Lin D, Sui J, Wu L, Calhoun VD, 2016. Interaction among subsystems within default mode network diminished in schizophrenia patients: a dynamic connectivity approach. Schizophr Res 170, 55–65. 10.1016/j.schres.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM, 2000. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences 23, 475–483. 10.1016/S0166-2236(00)01633-7 [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J, 2005. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience 8, 1784–1790. 10.1038/nn1594 [DOI] [PubMed] [Google Scholar]
- Eisenreich BR, Akaishi R, Hayden BY, 2017. Control without Controllers: Toward a Distributed Neuroscience of Executive Control. Journal of Cognitive Neuroscience 29, 1684–1698. 10.1162/jocn_a_01139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Kane MJ, 2003. Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychology of learning and motivation 44, 145–199. [Google Scholar]
- Falcon MI, Riley JD, Jirsa V, McIntosh AR, Shereen AD, Chen EE, Solodkin A, 2015. The Virtual Brain: modeling biological correlates of recovery after chronic stroke. Frontiers in neurology 6, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Bernardi S, Van Dam NT, Anagnostou E, Gu X, Martin L, Park Y, Liu X, Kolevzon A, Soorya L, Grodberg D, Hollander E, Hof PR, 2012. Functional deficits of the attentional networks in autism. Brain and Behavior 2, 647–660. 10.1002/brb3.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB, 2009. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res 1273, 114–128. 10.1016/j.brainres.2009.02.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini L, Veer IM, Baerends E, Tol M-J Renken van R.J., Wee NJA Veltman van der D.J., Aleman A, Zitman FG, Penninx BWJH, Buchem MA Reiber van J.H.C., Rombouts SARB, Milles J, 2009. Hierarchical functional modularity in the resting-state human brain. Human Brain Mapping 30, 2220–2231. 10.1002/hbm.20663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Plessow F, 2015. Efficient multitasking: parallel versus serial processing of multiple tasks. Front Psychol 6. 10.3389/fpsyg.2015.01366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor JA, 1983. The modularity of mind. MIT press. [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC, 2001. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cognitive, Affective, & Behavioral Neuroscience 1, 137–160. 10.3758/CABN.1.2.137 [DOI] [PubMed] [Google Scholar]
- Frantzidis CA, Vivas AB, Tsolaki A, Klados MA, Tsolaki M, Bamidis PD, 2014. Functional disorganization of small-world brain networks in mild Alzheimer’s Disease and amnestic Mild Cognitive Impairment: an EEG study using Relative Wavelet Entropy (RWE). Front Aging Neurosci 6. 10.3389/fnagi.2014.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen JD, Heinrichs-Graham E, White ML, Wetzel MW, Knott NL, Wilson TW, 2013. Atypical coupling between posterior regions of the default mode network in attention-deficit/hyperactivity disorder: a pharmaco-magnetoencephalography study. J Psychiatry Neurosci 38, 333–340. 10.1503/jpn.120054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, 1994. Functional and effective connectivity in neuroimaging: a synthesis. Human brain mapping 2, 56–78. [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RSJ, Dolan RJ, 1996. The trouble with cognitive subtraction. Neuroimage 4, 97–104. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD, 2007. Aberrant “Default Mode” Functional Connectivity in Schizophrenia. Am J Psychiatry 8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Cools AR, Srivastava K, 1996. The Prefrontal Landscape: Implications of Functional Architecture for Understanding Human Mentation and the Central Executive [and Discussion]. Philosophical Transactions: Biological Sciences 351, 1445–1453. [DOI] [PubMed] [Google Scholar]
- Goschke T, Bolte A, 2014. Emotional modulation of control dilemmas: The role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia 62, 403–423. 10.1016/j.neuropsychologia.2014.07.015 [DOI] [PubMed] [Google Scholar]
- Hagmann P, Kurant M, Gigandet X, Thiran P, Wedeen VJ, Meuli R, Thiran J-P, 2007. Mapping Human Whole-Brain Structural Networks with Diffusion MRI. PLoS ONE 2, e597. 10.1371/journal.pone.0000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM, 2010. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A, 2009. Self-Control in Decision-Making Involves Modulation of the vmPFC Valuation System 324, 4. [DOI] [PubMed] [Google Scholar]
- Hare TA, Hakimi S, Rangel A, 2014. Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Frontiers in Neuroscience 8. 10.3389/fnins.2014.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC, 2007. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society B: Biological Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC, 2006. Banishing the homunculus: Making working memory work. Neuroscience 139, 105–118. 10.1016/j.neuroscience.2005.04.067 [DOI] [PubMed] [Google Scholar]
- He BJ, Shulman GL, Snyder AZ, Corbetta M, 2007. The role of impaired neuronal communication in neurological disorders: Current Opinion in Neurology 20, 655–660. 10.1097/WCO.0b013e3282f1c720 [DOI] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC, 2007. Small-World Anatomical Networks in the Human Brain Revealed by Cortical Thickness from MRI. Cerebral Cortex 17, 2407–2419. 10.1093/cercor/bhl149 [DOI] [PubMed] [Google Scholar]
- Hommel B, Wiers RW, 2017. Towards a Unitary Approach to Human Action Control. Trends in Cognitive Sciences 21, 940–949. 10.1016/j.tics.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, 2008. Dynamical consequences of lesions in cortical networks. Hum. Brain Mapp. 29, 802–809. 10.1002/hbm.20579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Liu Y, Sun J, Tong S, 2016. Age-Related Differences in the Modulation of Small-World Brain Networks during a Go/NoGo Task. Front. Aging Neurosci. 8. 10.3389/fnagi.2016.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ, 1982. Neural networks and physical systems with emergent collective computational abilities. Proceedings of the national academy of sciences 79, 2554–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC, Wise SP, 1995. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cerebral cortex 5, 95–110. [DOI] [PubMed] [Google Scholar]
- Hull JV, Dokovna LB, Jacokes ZJ, Torgerson CM, Irimia A, Van Horn JD, 2017. Resting-State Functional Connectivity in Autism Spectrum Disorders: A Review. Frontiers in Psychiatry 7, 205. 10.3389/fpsyt.2016.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahashi T, Yamada T, Watanabe H, Nakamura M, Jimbo D, Shioda S, Toriizuka K, Kato N, Hashimoto R, 2014. Altered Network Topologies and Hub Organization in Adults with Autism: A Resting-State fMRI Study. PLoS ONE 9, e94115. 10.1371/journal.pone.0094115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturria-Medina Y, Canales-Rodriguez EJ, Melie-Garcia L, Valdes-Hernandez PA, Martinez-Montes E, Alemán-Gómez Y, Sánchez-Bornot JM, 2007. Characterizing brain anatomical connections using diffusion weighted MRI and graph theory. Neuroimage 36, 645–660. [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y, Sotero RC, Canales-Rodríguez EJ, Alemán-Gómez Y, Melie-García L, 2008. Studying the human brain anatomical network via diffusion-weighted MRI and Graph Theory. Neuroimage 40, 1064–1076. [DOI] [PubMed] [Google Scholar]
- Jacques S, Zelazo PD, 2005. Language and the development of cognitive flexibility: implications for theory of mind., in: Why Language Matters for Theory of Mind, Apr, 2002, University of Toronto, Toronto, ON, Canada; This Chapter Originated from the Aforementioned Conference. Oxford University Press. [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD, 2008. A Method for Functional Network Connectivity Among Spatially Independent Resting-State Components in Schizophrenia. Neuroimage 39, 1666–1681. 10.1016/j.neuroimage.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James L, Rumelhart DE, 1986. PARALLEL DISTRIBUTED PROCESSING, EXPLORATIONS IN THE MICROSTRUCTURE OF COGNITION: VOL 2, PSYCHOLOGICAL AND BIOLOGICAL MODELS. [Google Scholar]
- Jang JH, Jung WH, Choi J-S, Choi C-H, Kang D-H, Shin NY, Hong KS, Kwon JS, 2011. Reduced prefrontal functional connectivity in the default mode network is related to greater psychopathology in subjects with high genetic loading for schizophrenia. Schizophrenia Research 127, 58–65. 10.1016/j.schres.2010.12.022 [DOI] [PubMed] [Google Scholar]
- Jirsa V, Sporns O, Breakspear M, Deco G, McIntosh AR, 2010. Towards the virtual brain: network modeling of the intact and the damaged brain. Archives italiennes de biologie 148, 189–205. [PubMed] [Google Scholar]
- Jurado MB, Rosselli M, 2007. The Elusive Nature of Executive Functions: A Review of our Current Understanding. Neuropsychology Review 17, 213–233. 10.1007/s11065-007-9040-z [DOI] [PubMed] [Google Scholar]
- Kahneman D, 1973. Attention and effort. Citeseer. [Google Scholar]
- Kahnt T, Heinzle J, Park SQ, Haynes J-D, 2011. Decoding different roles for vmPFC and dlPFC in multi-attribute decision making. NeuroImage 56, 709–715. 10.1016/j.neuroimage.2010.05.058 [DOI] [PubMed] [Google Scholar]
- Kaiser M, Martin R, Andras P, Young MP, 2007. Simulation of robustness against lesions of cortical networks. European Journal of Neuroscience 25, 3185–3192. [DOI] [PubMed] [Google Scholar]
- Kalisman N, Silberberg G, Markram H, 2005. The neocortical microcircuit as a tabula rasa. Proceedings of the National Academy of Sciences of the United States of America 102, 880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM, 1997. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of neuroscience 17, 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, 2004. Anterior Cingulate Conflict Monitoring and Adjustments in Control. Science 303, 1023–1026. 10.1126/science.1089910 [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C, 2007. An information theoretical approach to prefrontal executive function. Trends in Cognitive Sciences 11, 229–235. 10.1016/j.tics.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Kondo H, Osaka N, Osaka M, 2004. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. NeuroImage 23, 670–679. 10.1016/j.neuroimage.2004.06.014 [DOI] [PubMed] [Google Scholar]
- Langer N, von Bastian CC, Wirz H, Oberauer K, Jäncke L, 2013. The effects of working memory training on functional brain network efficiency. Cortex 49, 2424–2438. 10.1016/j.cortex.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Lebedev AV, Westman E, Simmons A, Lebedeva A, Siepel FJ, Pereira JB, Aarsland D, 2014. Large-scale resting state network correlates of cognitive impairment in Parkinson’s disease and related dopaminergic deficits. Front. Syst. Neurosci. 8. 10.3389/fnsys.2014.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCun Y, Bengio Y, Hinton G, 2015. Deep learning. nature 521, 436–444. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Baker CI, 2016. Multi-Voxel Decoding and the Topography of Maintained Information During Visual Working Memory. Front. Syst. Neurosci. 10. 10.3389/fnsys.2016.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, Scerif G, Liddle PF, 2011. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. Journal of Child Psychology and Psychiatry 52, 761–771. 10.1111/j.1469-7610.2010.02333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-L, O’Doherty JP, Rangel A, 2011. The Decision Value Computations in the vmPFC and Striatum Use a Relative Value Code That is Guided by Visual Attention. Journal of Neuroscience 31, 13214–13223. 10.1523/JNEUROSCI.1246-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H-Y, Tseng WYI, Lai M-C, Matsuo K, Gau SS-F, 2015. Altered resting-state frontoparietal control network in children with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society : JINS 21, 271–284. 10.1017/S135561771500020X [DOI] [PubMed] [Google Scholar]
- Liu T, Chen Y, Lin P, Wang J, 2015. Small-World Brain Functional Networks in Children With Attention-Deficit/Hyperactivity Disorder Revealed by EEG Synchrony. Clin EEG Neurosci 46, 183–191. 10.1177/1550059414523959 [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T, 2008. Disrupted small-world networks in schizophrenia. Brain 131, 945–961. 10.1093/brain/awn018 [DOI] [PubMed] [Google Scholar]
- Logie RH, 2016. Retiring the central executive. The Quarterly Journal of Experimental Psychology 69, 2093–2109. 10.1080/17470218.2015.1136657 [DOI] [PubMed] [Google Scholar]
- Luria AR, Karpov BA, Yarbuss AL, 1966. Disturbances of active visual perception with lesions of the frontal lobes. Cortex 2, 202–212. [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS, 2000. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288, 1835–1838. [DOI] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, Peters H, Zimmer C, Förstl H, Bäuml J, Wohlschläger AM, Sorg C, 2014. Aberrant Dependence of Default Mode/Central Executive Network Interactions on Anterior Insular Salience Network Activity in Schizophrenia. Schizophrenia Bulletin 40, 428–437. 10.1093/schbul/sbt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, Bezgin G, Eickhoff SB, Castellanos FX, Petrides M, 2016. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences 113, 12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Botvinick MM, Noelle DC, Plaut DC, Rogers TT, Seidenberg MS, Smith LB, 2010. Letting structure emerge: connectionist and dynamical systems approaches to cognition. Trends in cognitive sciences 14, 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Klingberg T, 2007. Prefrontal cortex and basal ganglia control access to working memory. Nature neuroscience 11, nn2024. [DOI] [PubMed] [Google Scholar]
- Meda SA, Ruano G, Windemuth A, O’Neil K, Berwise C, Dunn SM, Boccaccio LE, Narayanan B, Kocherla M, Sprooten E, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, Calhoun VD, Pearlson GD, 2014. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proceedings of the National Academy of Sciences 111, E2066–E2075. 10.1073/pnas.1313093111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D, Achard S, Morcom A, Bullmore E, 2009. Age-related changes in modular organization of human brain functional networks. Neuroimage 44, 715–723. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, Erimaki S, Zervakis M, 2006. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophrenia Research 87, 60–66. 10.1016/j.schres.2006.06.028 [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb AM, Barad V, Cohen NJ, Wszalek TM, Kramer AF, 2001. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain research. Cognitive brain research 12, 467–473. 10.1016/S0926-6410(01)00076-3 [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD, 2001. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience 24, 167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Miller GA, 1956. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological review 63, 81. [PubMed] [Google Scholar]
- Minsky M, 1988. Society of mind. Simon and Schuster. [Google Scholar]
- Mitchell M, 2009. Complexity: A guided tour. Oxford University Press. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD, 2000. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive psychology 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Monsell S, Driver J, 2000a. Banishing the control homunculus. Control of cognitive processes: Attention and performance XVIII 3–32. [Google Scholar]
- Monsell S, Driver J, 2000b. Banishing the control homunculus. Control of cognitive processes: Attention and performance XVIII 3–32. [Google Scholar]
- Monsell S, Driver J, 2000c. Control of cognitive processes: Attention and performance XVIII. MIT Press. [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G, 2007. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry 62, 270–273. 10.1016/j.biopsych.2006.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Twieg DB, Carpenter PA, 2001. Baseline conditions and subtractive logic in neuroimaging. Human Brain Mapping 14, 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle MM, Baxter MG, 2003. Glutamate receptor binding in the frontal cortex and dorsal striatum of aged rats with impaired attentional set-shifting. European Journal of Neuroscience 18, 3335–3342. 10.1111/j.1460-9568.2003.03077.x [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS, 2012. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience 12, 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman DA, Shallice T, 1986. Attention to action, in: Consciousness and Self-Regulation. Springer, pp. 1–18. [Google Scholar]
- Öngür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF, 2010. Default Mode Network Abnormalities in Bipolar Disorder and Schizophrenia. Psychiatry Res 183, 59–68. 10.1016/j.pscychresns.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orliac F, Naveau M, Joliot M, Delcroix N, Razafimandimby A, Brazo P, Dollfus S, Delamillieure P, 2013. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophrenia Research 148, 74–80. 10.1016/j.schres.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Paakki J-J, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, Starck T, Remes J, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Mattila M-L, Zang Y, Kiviniemi V, 2010. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Research 1321, 169–179. 10.1016/j.brainres.2009.12.081 [DOI] [PubMed] [Google Scholar]
- Park H-J, Friston K, 2013. Structural and Functional Brain Networks: From Connections to Cognition. Science 342, 1238411. 10.1126/science.1238411 [DOI] [PubMed] [Google Scholar]
- Passino KM, Seeley TD, Visscher PK, 2008. Swarm cognition in honey bees. Behavioral Ecology and Sociobiology 62, 401–414. [Google Scholar]
- Pauli WM, O’Reilly RC, Yarkoni T, Wager TD, 2016. Regional specialization within the human striatum for diverse psychological functions. PNAS 113, 1907–1912. 10.1073/pnas.1507610113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G, Rigoli F, Friston KJ, 2018. Hierarchical Active Inference: A Theory of Motivated Control. Trends in Cognitive Sciences 22, 294–306. 10.1016/j.tics.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, 2006. Can cognitive processes be inferred from neuroimaging data? Trends in cognitive sciences 10, 59–63. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, 2004. What can neuroimaging tell us about the mind? Insights from prefrontal cortex. Current Directions in Psychological Science 13, 177–181. [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarró S, Gomar J, Vila F, Martínez Á, Guerrero A, Ortiz-Gil J, Sans-Sansa B, Capdevila A, Cebamanos JM, McKenna PJ, 2008. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychological Medicine 38, 1185–1193. 10.1017/S0033291708003565 [DOI] [PubMed] [Google Scholar]
- Postle BR, 2006. Working memory as an emergent property of the mind and brain. Neuroscience 139, 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KB, Voeller KKS, 2004. Prefrontal Executive Function Syndromes in Children. Journal of Child Neurology 19, 785–797. 10.1177/08830738040190100801 [DOI] [PubMed] [Google Scholar]
- Rabbitt P, 1997. Introduction: Methodologies and models in the study of executive function. Methodology of frontal and executive function 1–38. [Google Scholar]
- Ravasz E, Barabási A-L, 2003. Hierarchical organization in complex networks. Physical Review E 67, 026112. 10.1103/PhysRevE.67.026112 [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K, 1999. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 89, 1009–1023. 10.1016/S0306-4522(98)00319-4 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S, 2004. The role of the medial frontal cortex in cognitive control. science 306, 443–447. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Wigton R, Kilner JM, Mendez MA, Hon N, Friston KJ, Joyce EM, 2013. Dysconnectivity in the Frontoparietal Attention Network in Schizophrenia. Frontiers in Psychiatry 4. 10.3389/fpsyt.2013.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DEJ, 2010. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophrenia Research 117, 21–30. 10.1016/j.schres.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AWF, Williams LM, Breakspear M, 2009. Small-world properties of nonlinear brain activity in schizophrenia. Human Brain Mapping 30, 403–416. 10.1002/hbm.20517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S, 2009. Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol 19, 408–414. 10.1016/j.conb.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Pineda P, Fakra E, Delaveau P, McKenna PJ, Pomarol-Clotet E, Blin O, 2011. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophrenia Research 125, 101–109. 10.1016/j.schres.2010.10.027 [DOI] [PubMed] [Google Scholar]
- Salinas E, Thier P, 2000. Gain modulation: a major computational principle of the central nervous system. Neuron 27, 15–21. [DOI] [PubMed] [Google Scholar]
- Salvador R, 2004. Neurophysiological Architecture of Functional Magnetic Resonance Images of Human Brain. Cerebral Cortex 15, 1332–1342. 10.1093/cercor/bhi016 [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM, 1977. Controlled and automatic human information processing: I. Detection, search, and attention. Psychological review 84, 1. [Google Scholar]
- Schroll H, Hamker FH, 2013. Computational models of basal-ganglia pathway functions: focus on functional neuroanatomy. Front. Syst. Neurosci. 7. 10.3389/fnsys.2013.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll H, Vitay J, Hamker FH, 2012. Working memory and response selection: a computational account of interactions among cortico-basalganglio-thalamic loops. Neural Networks 26, 59–74. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Gozzi A, Bifone A, 2008. Community structure and modularity in networks of correlated brain activity. Magnetic resonance imaging 26, 914–920. [DOI] [PubMed] [Google Scholar]
- Senden M, Reuter N, van den Heuvel MP, Goebel R, Deco G, 2017. Cortical rich club regions can organize state-dependent functional network formation by engaging in oscillatory behavior. NeuroImage 146, 561–574. 10.1016/j.neuroimage.2016.10.044 [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD, 2013. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79, 217–240. 10.1016/j.neuron.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM, 2016. Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience 19, 1286. 10.1038/nn.4384 [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W, 1977. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychological review 84, 127. [Google Scholar]
- Shim M, Kim D-W, Lee S-H, Im C-H, 2014. Disruptions in small-world cortical functional connectivity network during an auditory oddball paradigm task in patients with schizophrenia. Schizophrenia Research 156, 197–203. 10.1016/j.schres.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Shine JM, Aburn MJ, Breakspear M, Poldrack RA, 2018. The modulation of neural gain facilitates a transition between functional segregation and integration in the brain. eLife 7, e31130. 10.7554/eLife.31130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleezer BJ, Hayden BY, 2016. Differential contributions of ventral and dorsal striatum to early and late phases of cognitive set reconfiguration. Journal of cognitive neuroscience 28, 1849–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J, 1999. Storage and executive processes in the frontal lobes. Science 283, 1657–1661. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Hutcherson C, Hare T, Rangel A, 2012. Decision value computation in DLPFC and VMPFC adjusts to the available decision time: Value computation adjusts to decision time. European Journal of Neuroscience 35, 1065–1074. 10.1111/j.1460-9568.2012.08076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, 2006. Small-world connectivity, motif composition, and complexity of fractal neuronal connections. Biosystems 85, 55–64. [DOI] [PubMed] [Google Scholar]
- Sporns O, Kötter R, 2004. Motifs in Brain Networks. PLoS Biology 2, e369. 10.1371/journal.pbio.0020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C, Kessler D, Fang Y, Welsh RC, Prem Kumar K, Angstadt M, 2014. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder: Disrupted Network Architecture in ADHD. Human Brain Mapping 35, 4693–4705. 10.1002/hbm.22504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam C, Jones B, Nolte G, Breakspear M, Scheltens P, 2006. Small-World Networks and Functional Connectivity in Alzheimer’s Disease. Cerebral Cortex 17, 92–99. 10.1093/cercor/bhj127 [DOI] [PubMed] [Google Scholar]
- Steinbeis N, Haushofer J, Fehr E, Singer T, 2016. Development of Behavioral Control and Associated vmPFC–DLPFC Connectivity Explains Children’s Increased Resistance to Temptation in Intertemporal Choice. Cerebral Cortex 26, 32–42. 10.1093/cercor/bhu167 [DOI] [PubMed] [Google Scholar]
- Stocco A, Lebiere C, Anderson JR, 2010. Conditional routing of information to the cortex: A model of the basal ganglia’s role in cognitive coordination. Psychological Review 117, 541–574. 10.1037/a0019077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandburg-Peshkin A, Farine DR, Couzin ID, Crofoot MC, 2015. Shared decision-making drives collective movement in wild baboons. Science 348, 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter DJ, 2006. The principles of collective animal behaviour. Philosophical transactions of the royal society B: Biological Sciences 361, 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Lim J, Kwok K, Bezerianos A, 2014. Functional cortical connectivity analysis of mental fatigue unmasks hemispheric asymmetry and changes in small-world networks. Brain and Cognition 85, 220–230. 10.1016/j.bandc.2013.12.011 [DOI] [PubMed] [Google Scholar]
- Swanson N, Eichele T, Pearlson G, Kiehl K, Yu Q, Calhoun VD, 2011. Lateral differences in the default mode network in healthy controls and patients with schizophrenia. Human Brain Mapping 32, 654–664. 10.1002/hbm.21055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Jiang T, Liang M, Zang Y, He Y, Sui M, Wang Y, 2008. Enhanced resting-state brain activities in ADHD patients: A fMRI study. Brain and Development 30, 342–348. 10.1016/j.braindev.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G, 1980. A feature-integration theory of attention. Cognitive psychology 12, 97–136. [DOI] [PubMed] [Google Scholar]
- Tu P-C, Lee Y-C, Chen Y-S, Li C-T, Su T-P, 2013. Schizophrenia and the brain’s control network: Aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophrenia Research 147, 339–347. 10.1016/j.schres.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP, 2008. Network homogeneity reveals decreased integrity of default-mode network in ADHD. Journal of Neuroscience Methods 169, 249–254. 10.1016/j.jneumeth.2007.11.031 [DOI] [PubMed] [Google Scholar]
- van Buuren M, Vink M, Kahn RS, 2012. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophrenia Research 142, 237–243. 10.1016/j.schres.2012.09.017 [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goñi J, Sporns O, 2012. High-cost, high-capacity backbone for global brain communication. Proceedings of the National Academy of Sciences 109, 11372–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, 2013. Network hubs in the human brain. Trends in cognitive sciences 17, 683–696. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS, 2001. Anterior Cingulate Cortex, Conflict Monitoring, and Levels of Processing. NeuroImage 14, 1302–1308. 10.1006/nimg.2001.0923 [DOI] [PubMed] [Google Scholar]
- Vandierendonck A, 2016. A working memory system with distributed executive control. Perspectives on Psychological Science 11, 74–100. [DOI] [PubMed] [Google Scholar]
- Vassena E, Krebs RM, Silvetti M, Fias W, Verguts T, 2014. Dissociating contributions of ACC and vmPFC in reward prediction, outcome, and choice. Neuropsychologia 59, 112–123. 10.1016/j.neuropsychologia.2014.04.019 [DOI] [PubMed] [Google Scholar]
- Vecchio F, Miraglia F, Quaranta D, Granata G, Romanello R, Marra C, Bramanti P, Rossini PM, 2016. Cortical connectivity and memory performance in cognitive decline: A study via graph theory from EEG data. Neuroscience 316, 143–150. 10.1016/j.neuroscience.2015.12.036 [DOI] [PubMed] [Google Scholar]
- Voytek B, Knight RT, 2010. Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences 107, 18167–18172. 10.1073/pnas.1007277107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Fan J, Dong Y, Wang C, Lee TMC, Posner MI, 2005. Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophrenia Research 78, 235–241. 10.1016/j.schres.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, Zhong Q, Wang Y, 2009. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Human Brain Mapping 30, 638–649. 10.1002/hbm.20530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Suh J, Li Z, Li Y, Franklin T, O’Brien C, Childress AR, 2015. A Hyper-connected but Less Efficient Small-world Network in the Substance-Dependent Brain. Drug Alcohol Depend 152, 102–108. 10.1016/j.drugalcdep.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH, 1998. Collective dynamics of ‘small-world’ networks. Nature 393, 440–442. 10.1038/30918 [DOI] [PubMed] [Google Scholar]
- Wei L, Li Y, Yang X, Xue Q, Wang Y, 2015. Altered characteristic of brain networks in mild cognitive impairment during a selective attention task: An EEG study. International Journal of Psychophysiology 98, 8–16. 10.1016/j.ijpsycho.2015.05.015 [DOI] [PubMed] [Google Scholar]
- Weissman DH, 2004. Dorsal Anterior Cingulate Cortex Resolves Conflict from Distracting Stimuli by Boosting Attention toward Relevant Events. Cerebral Cortex 15, 229–237. 10.1093/cercor/bhh125 [DOI] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG, 2003. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. NeuroImage 19, 1361–1368. 10.1016/S1053-8119(03)00167-8 [DOI] [PubMed] [Google Scholar]
- Weng S-J, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS, 2010. Alterations of Resting State Functional Connectivity in the Default Network in Adolescents with Autism Spectrum Disorders. Brain Res 1313, 202. 10.1016/j.brainres.2009.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JDE, Seidman LJ, 2009. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences 106, 1279–1284. 10.1073/pnas.0809141106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens CD, 1991. Processing resources and attention. Multiple-task performance 1991, 3–34. [Google Scholar]
- Wilson TW, Franzen JD, Heinrichs-Graham E, White ML, Knott NL, Wetzel MW, 2011. Broadband neurophysiological abnormalities in the medial prefrontal region of the default-mode network in adults with ADHD. Human Brain Mapping n/a-n/a. 10.1002/hbm.21459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff N, Zink N, Stock A-K, Beste C, 2017. On the relevance of the alpha frequency oscillation’s small-world network architecture for cognitive flexibility. Scientific Reports 7. 10.1038/s41598-017-14490-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Rogers B, Heckers S, 2011. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res 130, 86–93. 10.1016/j.schres.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Foxe JJ, Sroubek AE, Branch C, Li X, 2014. Topological organization of the “small-world” visual attention network in children with attention deficit/hyperactivity disorder (ADHD). Front. Hum. Neurosci. 8. 10.3389/fnhum.2014.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JLM, Callaway EM, 2005. Excitatory cortical neurons form fine-scale functional networks. Nature 433, 868–873. 10.1038/nature03252 [DOI] [PubMed] [Google Scholar]
- Yuan K, Qin W, Liu J, Guo Q, Dong M, Sun J, Zhang Y, Liu P, Wang W, Wang Y, Li Q, Yang W, von Deneen KM, Gold MS, Liu Y, Tian J, 2010. Altered small-world brain functional networks and duration of heroin use in male abstinent heroin-dependent individuals. Neuroscience Letters 477, 37–42. 10.1016/j.neulet.2010.04.032 [DOI] [PubMed] [Google Scholar]
- Zeng K, Wang Y, Ouyang G, Bian Z, Wang L, Li X, 2015. Complex network analysis of resting state EEG in amnestic mild cognitive impairment patients with type 2 diabetes. Front Comput Neurosci 9, 133. 10.3389/fncom.2015.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink N, Stock A-K, Colzato L, Beste C, 2018. Evidence for a neural dual-process account for adverse effects of cognitive control. Brain Struct Funct 1–17. 10.1007/s00429-018-1694-1 [DOI] [PubMed] [Google Scholar]