Abstract
Aims
We aimed to investigate the role of Fasting Plasma Glucose (FPG) and glucose fluctuation in the prognosis of COVID-19 patients stratified by pre-existing diabetes.
Methods
The associations of FPG and glucose fluctuation indexes with prognosis of COVID-19 in 2,642 patients were investigated by multivariate Cox regression analysis. The primary outcome was in-hospital mortality; the secondary outcome was disease progression. The longitudinal changes of FPG over time were analyzed by the latent growth curve model in COVID-19 patients stratified by diabetes and severity of COVID-19.
Results
We found FPG as an independent prognostic factor of overall survival after adjustment for age, sex, diabetes and severity of COVID-19 at admission (HR: 1.15, 95% CI: 1.06–1.25, P = 1.02 × 10−3). Multivariate logistic regression analysis indicated that the standard deviation of blood glucose (SDBG) and largest amplitude of glycemic excursions (LAGE) were also independent risk factors of COVID-19 progression (P = 0.03 and 0.04, respectively). The growth trajectory of FPG over the first 3 days of hospitalization was steeper in patients with critical COVID-19 in comparison to moderate patients.
Conclusions
Hyperglycemia and glucose fluctuation were adverse prognostic factors of COVID-19 regardless of pre-existing diabetes. This stresses the importance of glycemic control in addition to other therapeutic management.
Keywords: Diabetes, Fasting plasma glucose, COVID-19, Coronavirus, Blood glucose fluctuation
Abbreviations: BGM, Blood glucose monitoring; CAD, Coronary artery disease; CRP, C reactive protein; DBP, Diastolic pressure; DM, Diabetes mellitus; %GV, percentage coefficient of variation for glucose; HbA1c, Hemoglobin A1c; LAGE, Largest amplitude of glycemic excursions; PPGE, Postprandial glucose excursions; SBP, Systolic pressure; SDBG, Standard deviation of blood glucose
1. Introduction
Among various comorbidities of COVID-19, diabetes has been reported as the second most common comorbidity after hypertension [1]. The overall proportion of diabetes in COVID-19 patients ranges from 5.3% to 20% in Chinese studies [2], [3], [4], [5], [6]. In a more recent study in Italy, the diabetes prevalence was 17% among COVID-19 patients admitted to Intensive Care Units (ICUs) [7]. In the USA, the COVID-19-Associated Hospitalization Surveillance Network (COVID-NET) reported a diabetes prevalence of 28.3% in hospitalized adult patients [8]. Diabetes was associated with higher mortality in COVID-19: the largest epidemiological investigation in China indicated that the mortality of COVID-19 with diabetes (up to 7.3%) was much higher than that of the patients without any comorbidities (0.9%) [9]. Pre-existing diabetes has been reported as a risk factor to influence the progression and prognosis of COVID-19 [10].
As a metabolic syndrome, the pathogenicity of diabetes involves a chronic, low-grade inflammatory response characterized by compromised innate immune response and elevated level of inflammatory factors, which might exacerbate the inflammatory storm of COVID-19 [11], [12]. On the other hand, viral infections have been known to cause sharp fluctuation of blood glucose level [13], which in turn can induce oxidative stress and inflammation in patients with diabetes [14], [15], [16], and damage the blood vessel endothelium [17], hence creating a vicious circle after a viral infection.
Nevertheless, most studies investigating the independency of diabetes-related phenotypes as risk factors of COVID-19 progression led to conflicting conclusions [10], [18], [19], [20]. The CORONADO study found that HbA1c was not significantly associated with the primary outcomes of COVID-19 (i.e., mechanical ventilation and/or death within 7 days). Their finding suggested that long-term glucose control in diabetes patients has no effect on COVID-19 prognosis. However, they also found that admission plasma glucose was significantly associated with the primary outcome [21]. In-hospital hyperglycemia has been associated with worse COVID-19 prognosis in several previous studies [22], [23], [24], [25]. In addition, treating hyperglycemic patients with insulin infusion had led to a lower risk of severe disease regardless of prior diabetes history. Although the sample size (n = 59) of this study was limited and only COVID-19 patients with moderate disease were included [26].
Hence, we made the hypothesis that the elevated Fasting Plasma Glucose (FPG) levels and glucose fluctuation were risk factors of COVID-19 prognosis, independent of the pre-existing diabetes disease status of COVID-19 patients. In 2,642 patients with a comprehensive spectrum of the severity of COVID-19, we aimed to find clues about the central question: should patients with hyperglycemia be treated with more attention to glycemic control during the COVID-19 infection, regardless of prior diabetes history?
2. Material and Methods
2.1. Study population and inclusion criteria
This was a retrospective study of patients with SARS-Cov-2 infection who were admitted to Huoshenshan hospital from February 1, 2020 to March 24, 2020. Huoshenshan hospital (in Wuhan, China) was an emergency specialty field hospital designated for treating COVID-19. As of 14th April 2020, a total of 2,906 patients were enrolled in this study. The inclusion criteria of COVID-19 patients were: (1) epidemiology history, (2) fever or other respiratory symptoms, (3) typical CT image abnormities of viral pneumonia, and (4) positive result of RT-PCR for SARS-CoV-2 RNA. Exclusion criteria were patients with non-positive results for SARS-CoV-2 RNA and antibodies against SARS-CoV-2 during hospital stay (n = 257) or age younger than 18 years (n = 7). Afterwards, a total of 2,642 patients were included in the analysis. This study protocol was reviewed and approved by the Ethics Review Committee of Huoshenshan Hospital. Informed consent was waived by the ethics committee of the designated hospital for patients with emerging infectious diseases.
According to “Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia (Seventh Edition)” published by the National Health Commission of China, patients with COVID-19 were divided into mild (laboratory confirmed, without pneumonia), moderate (laboratory confirmed and with pneumonia), severe (meeting any of the following criteria: dyspnea, respiratory frequency ≥ 30/minute, blood oxygen saturation < 94%, PaO2/FiO2 ratio < 300, and/or lung infiltrates > 50% of the lung field within 24–48 h) and critical (meeting any of the following criteria: respiratory failure requiring mechanical ventilation, shock or other organ failure that requires intensive care).
The discharge criteria were defined as the following conditions: (1) body temperature returned to normal for at least three days; (2) respiratory symptoms significantly improved; (3) acute exudative lesions were improved considerably on chest radiography; (4) the nucleic acid test of sputum, nasopharyngeal swabs and other respiratory tract samples were negative twice.
2.2. Routine and biochemical examinations
We extracted demographic data, medical history, exposure history, symptoms and signs, laboratory findings, chest CT scans, and the treatment measures from electronic medical records. The date of disease onset was defined as the day when the first symptom showed up. Clinical outcomes were followed up to April 16, 2020. General clinical data such as the gender, age, heart rate, respiration rate, body temperature, systolic pressure (SBP), and diastolic pressure (DBP) were recorded by routine medical examination. We also recorded the results of laboratory tests for peripheral blood parameters of patients within 48 h after admission. Blood samples were obtained under overnight fasting conditions from these patients and measured in routine blood examination. The laboratory examination included blood routine examination (leucocyte count, lymphocyte count, hemoglobin, platelet count), neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP), blood biochemistry (alamine aminotransferase (ALT), albumin, blood urea nitrogen, creatinine, creatine kinase, lactate dehydrogenase (LDH), blood coagulation (prothrombin time, D-dimer) etc. All data were double checked by two physicians (Yu Xu and Bin Wang).
We divided all patients into three groups (i.e., hyperglycemia, hypoglycemia, normoglycemia) based on their fasting plasma glucose level at baseline on admission. We utilized the plasma glucose targets suggested for inpatients by the American Association of Clinical Endocrinologists and American Diabetes Association: hyperglycemia was defined as any blood glucose value > 7.8 mmol/l [27], [28]; hypoglycemia was defined as any blood glucose value ≤ 3.3 mmol/L [28].
Blood Glucose Monitoring (BGM) was conducted by finger-tip testing and recorded at 8-time sessions, which were 03:00, pre-breakfast, post-breakfast, pre-lunch, post-lunch, pre-dinner, post-dinner, and 22:00. The standard deviation of blood glucose (SDBG), the largest amplitude of glycemic excursions (LAGE), postprandial glucose excursions (PPGE) and percentage coefficient of variation for glucose (%GV) were calculated. (PPGE was obtained by calculating the mean absolute value of the post-prandial increment of glucose level above pre-prandial values for each meal. %GV was calculated by dividing the SD of blood glucose by the mean blood glucose and multiplying by 100).
2.3. Outcomes
The primary outcome was survival during hospital stay. The secondary outcome was disease progression, meeting any of the following criteria: (1) severity of COVID-19 changed from mild/moderate into severe/critical during hospital stay; (2) hospital announcement of COVID-19 related death or critical condition; (3) transfer to ICU after>24 h of initial hospital admission.
2.4. Statistical Analysis.
Categorical variables were expressed as frequency rates and percentages (%). Continuous variables were expressed as median and the interquartile range (IQR). If the data were in normal distribution, t-test was used in comparison of two groups. If the data were non-normal distribution data, Mann-Whitney U test was used in comparison between two groups. Categorical variables between groups were compared using the Chi-square test or Fisher exact test.
Multivariate Cox regression analysis was performed to study the associations between clinical characteristics and comorbidities and overall survival (OS) of COVID-19 with adjustment for age, sex, and the severity of COVID-19 at admission to hospital. The possible relationships between progression of COVID-19 and variables were evaluated by logistic linear regression analysis. The multivariable-adjusted model was applied with adjustment of age, sex, diabetes, and the severity of COVID-19 disease at admission to hospital.
The R package lavaan was used to implement the Latent Growth Curve Model to analyze the longitudinal change of FPG over time in COVID-19 patients after admission to hospital. The trajectory over time was modeled as a linear curve with three time points (i.e., FPG measured on three consecutive days from Day 1 of hospital admission) and two latent variables: a random intercept, and a random slope, with fixed coefficients. COVID-19 patients without diabetes were coded as 0. Similarly, COVID-19 patients with moderate disease were coded 0, severe patients coded 1 and critical patients coded 2. The factor loading of the intercept factor were all fixed at 1, whereas the slope factor has fixed values at 0, 1, and 2. In this way of coding, the initial level of FPG was reflected by the intercepts. The growth trajectories of FPG were reflected by the latent slope factor.
To detect any possible non-linear dependency in regression models, the restricted cubic splines was used to flexibly model and visualize the relation of predicted FPG with OS and disease progression of COVID-19. Simpler linear or piecewise-linear models were next fitted to quantify associations: where there was evidence of non-linearity, a two-line piecewise linear model with a single change point was estimated by testing all possible values for the change point and choosing the value with highest likelihood value.
All statistical analyses were performed using STATA program, version 16.0 (STATA Corp., College Station, Tex. US.) and R software, version 4.0.4. P value < 0.05 was considered as statistically significant.
3. Results
3.1. General baseline characteristics
The general baseline characteristics of 2,642 hospitalized patients with COVID-19 were summarized in Table 1 : the median age was 61 years (IQR, 50–68) and 1264 (50.50%) were female. The proportion of patients with smoking (7.03%) or drinking behaviors (4.08%) were both lower than that of the general population (27.7% [29] and 45.8% [30], respectively), probably caused by under-reporting in self-reported questionnaires. Diabetes was the second most common comorbidities (14.94%) after hypertension (32.68%). Overall, 12.15% of COVID-19 patients were taking oral medication for blood glucose control, the proportion was higher for both hypoglycemic (23.53%) and hyperglycemic (51.50%) groups. At admission to hospital, 0.91% of the patients were classified as “mild” in terms of the spectrum of severity of COVID-19, 68.64% as “moderate”, 29.07% as “severe”, 1.38% as critical (Table 1).
Table 1.
Demographics and baseline characteristics of patients with COVID-19.
| Overall (N=2642) |
Normoglycemia (N=2253) |
Hypoglycemia (N=17) |
Hyperglycemia (N=233) |
|||
|---|---|---|---|---|---|---|
| Variables | Count (%) | Count (%) | Count (%) | P-value† | Count (%) | P-value‡ |
| Baseline characteristics | ||||||
| Age (median (Q1-Q3)) | 61 (50-68) | 60 (49-68) | 57 (53-73) | 0.68 | 64 (57-70) | 8.21×10−8 |
| Sex (female) | 1264 (50.50%) | 1135 (50.38%) | 8 (47.06%) | 0.79 | 121 (51.93%) | 0.65 |
| Smoking | 176 (7.03%) | 157 (6.97%) | 0 (0.00%) | 0.63 | 19 (8.15%) | 0.50 |
| Drinking | 102 (4.08%) | 98 (4.35%) | 0 (0.00%) | 1.00 | 4 (1.72%) | 0.06 |
| Oral medication for blood glucose control | 321 (12.15%) | 189 (8.39%) | 4 (23.53%) | 0.05 | 120 (51.50%) | 2.09×10−80 |
| Clinical characteristics at admission | Median (Q1-Q3) | Median (Q1-Q3) | Median (Q1-Q3) | P-value§ | Median (Q1-Q3) | P-value¶ |
| Temperature (°C) | 36.5 (36.3-36.8) | 36.5 (36.3-36.7) | 36.4 (36.1-36.7) | 0.21 | 36.5 (36.3-36.8) | 2.68×10−3 |
| Max temp min temp difference (°C) | 1.1 (0.9-1.5) | 1.1 (0.9-1.5) | 1.4 (0.8-1.7) | 0.39 | 1.3 (0.9-2) | 1.26×10−5 |
| Heart rate, median (IQR), (bpm) | 85 (78-96) | 85 (78-96) | 85 (80-98) | 0.60 | 88 (78.5-98) | 0.01 |
| Respiration rate, median (IQR), (times per min) | 20 (19-21) | 20 (19-21) | 21 (18-21) | 0.97 | 20 (19-22) | 9.30×10−3 |
| SBP, median (IQR), (mmHg) | 130 (120-140) | 129 (120-140) | 127 (116-133) | 0.39 | 132.5 (122-144.5) | 5.39×10−5 |
| DBP, median (IQR), (mmHg) | 80 (74-88) | 80 (74-88) | 82 (73-86) | 0.99 | 80 (74-88) | 0.41 |
| Blood O2 saturation (%) | 97 (93-98) | 97 (93-98) | 98 (93-99) | 0.86 | 95 (90-98) | 2.99×10−5 |
| Length of hospital stay (day) | 13 (8-19) | 13 (8-19) | 15 (10-20) | 0.71 | 17 (10-23) | 2.74×10−6 |
| Symptoms | Count (%) | Count (%) | Count (%) | P-value† | Count (%) | P-value‡ |
| Cough | 1764 (70.87%) | 1585 (70.73%) | 12 (70.59%) | 0.99 | 167 (72.29%) | 0.62 |
| Fever | 1825 (73.26%) | 1642 (73.27%) | 12 (70.59%) | 0.80 | 171 (73.39%) | 0.97 |
| Phlegm | 317 (12.87%) | 294 (13.24%) | 1 (5.88%) | 0.72 | 22 (9.73%) | 0.13 |
| Dyspnea | 1125 (45.36%) | 994 (44.51%) | 8 (47.06%) | 0.83 | 123 (53.48%) | 9.32×10−3 |
| Sore muscle | 744 (30.07%) | 667 (29.92%) | 3 (17.65%) | 0.42 | 74 (32.46%) | 0.43 |
| Fatigue | 1347 (54.27%) | 1208 (54.07%) | 4 (23.53%) | 0.01 | 135 (58.44%) | 0.20 |
| Diarrhea | 139 (5.64%) | 131 (5.90%) | 1 (5.88%) | 1.00 | 7 (3.11%) | 0.08 |
| Vomit | 66 (2.68%) | 58 (2.61%) | 1 (5.88%) | 0.37 | 7 (3.11%) | 0.66 |
| Loss of appetite | 227 (9.22%) | 207 (9.33%) | 0 (0.00%) | 0.39 | 20 (8.85%) | 0.81 |
| Comorbidities | Count (%) | Count (%) | Count (%) | P-value† | Count (%) | P-value‡ |
| Hypertension | 818 (32.68%) | 696 (30.89%) | 8 (47.06%) | 0.15 | 114 (48.93%) | 2.25×10−8 |
| Diabetes | 374 (14.94%) | 224 (9.94%) | 6 (35.29%) | 5.58×10−4 | 144 (61.80%) | 6.17×10−100 |
| CHD | 177 (7.07%) | 151 (6.70%) | 0 (0.00%) | 0.62 | 26 (11.16%) | 0.01 |
| Diabetes (no other comorbidity)* | 140 (5.59%) | 79 (3.51%) | 2 (11.76%) | 0.12 | 59 (25.32%) | 1.37×10−43 |
| Stroke | 39 (1.56%) | 31 (1.38%) | 0 (0.00%) | 1.00 | 8 (3.43%) | 0.02 |
| Coronary stent | 36 (1.44%) | 29 (1.29%) | 0 (0.00%) | 1.00 | 7 (3.00%) | 0.07 |
| Chronic kidney disease | 33 (1.32%) | 25 (1.11%) | 1 (5.88%) | 0.18 | 7 (3.00%) | 0.01 |
| Hyperlipidemia | 30 (1.20%) | 27 (1.20%) | 0 (0.00%) | 1.00 | 3 (1.29%) | 0.76 |
| High uric acid | 30 (1.20%) | 29 (1.29%) | 0 (0.00%) | 1.00 | 1 (0.43%) | 0.36 |
| Atrial fibrillation | 24 (0.96%) | 22 (0.98%) | 0 (0.00%) | 1.00 | 2 (0.86%) | 1.00 |
| Heart failure | 11 (0.44%) | 8 (0.36%) | 1 (5.88%) | 0.07 | 2 (0.86%) | 0.24 |
| Lung cancer | 7 (0.28%) | 5 (0.22%) | 0 (0.00%) | 1.00 | 2 (0.86%) | 0.13 |
| Pulmonary heart disease | 1 (0.04%) | 1 (0.04%) | 0 (0.00%) | 1.00 | 0 (0.00%) | 1.00 |
| No complications | 1284 (51.30%) | 1241 (55.08%) | 5 (29.41%) | 0.03 | 38 (16.31%) | 1.78×10−29 |
| Severity of COVID-19 at admission | Count (%) | Count (%) | Count (%) | P-value† | Count (%) | P-value‡ |
| 0.68 | 1.11×10−9 | |||||
| Mild | 21 (0.91%) | 20 (0.96%) | 0 (0.00%) | 1 (0.47%) | ||
| Moderate | 1589 (68.64%) | 1465 (70.23%) | 12 (80.00%) | 112 (52.34%) | ||
| Severe | 673 (29.07%) | 582 (27.90%) | 3 (20.00%) | 88 (41.12%) | ||
| Critical | 32 (1.38%) | 19 (0.91%) | 0 (0.00%) | 13 (6.07%) | ||
| Outcome | Count (%) | Count (%) | Count (%) | P-value† | Count (%) | P-value‡ |
| Progression | 158 (6.31%) | 107 (4.75%) | 2 (11.76%) | 0.20 | 49 (21.03%) | 1.75×10−22 |
| Death | 64 (2.42%) | 34 (1.51%) | 1 (5.88%) | 0.32 | 25 (10.73%) | 1.42×10−17 |
P-value†: Hypoglycemic group compared to normoglycemic group (the reference) in chi2 test when n > 5 (Fisher's exact when n5).
P-value‡: Hyperglycemic group compared to normoglycemic group (the reference) in chi2 test when n > 5 (Fisher's exact when n5).
P-value§: Hypoglycemic group compared to normoglycemic group (the reference) in Two-sample Wilcoxon rank-sum (Mann-Whitney) test)
P-value¶: Hyperglycemic group compared to normoglycemic group (the reference) in Two-sample Wilcoxon rank-sum (Mann-Whitney) test)
Diabetes (no other comorbidity)*: COVID-19 patients with pre-existing diabetes but without any of the following common comorbidities: hypertension, coronary heart disease (CHD), any type of respiratory disease (i.e., pre-existing chronic obstructive pulmonary disease (COPD), tuberculosis (TB)), or any type of cancer.
3.2. FPG was associated with poorer overall survival and disease progression of COVID-19.
First, we performed multivariate Cox regression analysis on the associations between baseline clinical characteristics and comorbidities and overall survival (OS) of COVID-19 with adjustment for age, sex, and the severity of COVID-19 at admission to hospital (Table 2 ). We found that diabetes was not a significant risk factor for OS (HR diabetes: 1.13, 95% CI 0.57–2.25, P = 0.74) (Table 2). However, FPG was a significant risk factor for OS (HR FPG: 1.12, 95% CI 1.05–1.20, P = 1.33 × 10−3) (Table S1), and remained to be significant after further adjustment for pre-existing diabetes (HR FPG: 1.15, 95% CI 1.06–1.25, P = 1.02 × 10−3) (Table S1). Given that in our data approximately 70% of COVID-19 patients with diabetes were taking oral medication for blood glucose control before and during hospital stay, we additionally adjusted this variable and the results remained to be significant (HR FPG: 1.19, 95% CI 1.09–1.30, P = 7.51 × 10−5) (Table S2).
Table 2.
Multivariate Cox regression analysis on the baseline clinical characteristics, comorbidities and laboratory parameters.
| Variables | N | HR (95%CI) | P-value |
|---|---|---|---|
| Baseline abnormalities | |||
| Temperature (>38.1oC) | 2402 | 5.62 (2.47,12.82) | 3.99×10−5 |
| Respiration rate (>20/min) | 2401 | 2.28 (0.80,6.48) | 0.12 |
| DBP (<80mmHg) | 2394 | 0.67 (0.36,1.23) | 0.19 |
| SBP (<120mmHg) | 2394 | 0.79 (0.40,1.57) | 0.50 |
| Blood O2 saturation (<94%) | 2405 | 0.95 (0.48,1.88) | 0.88 |
| Heart rate (>100bpm) | 2402 | 1.04 (0.51,2.14) | 0.91 |
| Comorbidities | |||
| CHD | 2405 | 3.21 (1.55,6.63) | 1.64×10−3 |
| COPD | 2405 | 4.24 (1.58,11.39) | 4.24×10−3 |
| Chronic kidney disease | 2405 | 2.17 (0.73,6.43) | 0.16 |
| ARDS | 2405 | 0.51 (0.11,2.30) | 0.38 |
| Coronary stent | 2405 | 1.66 (0.5,5.48) | 0.41 |
| Hypertension | 2405 | 1.25 (0.68,2.30) | 0.48 |
| Cancer | 2405 | 0.59 (0.08,4.34) | 0.61 |
| Other lung disease | 2405 | 1.24 (0.47,3.30) | 0.66 |
| Diabetes | 2405 | 1.13 (0.57,2.25) | 0.74 |
| Respiratory failure | 2405 | 1.01 (0.37,2.78) | 0.98 |
| Laboratory parameters abnormalities† | |||
| Creatine kinase isoenzyme [0-24 IU/L] | 2202 | 7.18 (3.45,14.94) | 1.32×10−7 |
| LDH [120-250 IU/L] | 2208 | 6.01 (2.59,13.95) | 2.93×10−5 |
| Eosinophil count [0.02-0.52 *10∼9/L] | 2327 | 4.16 (2.05,8.47) | 8.21×10−5 |
| Myoglobin [0-80 ng/ml] | 1016 | 4.88 (2.10,11.36) | 2.32×10−4 |
| White blood count [3.5-9.5 *10∼9/L] | 2331 | 3.21 (1.72,5.98) | 2.34×10−4 |
| Aspartate aminotransferase [7-45 IU/L] | 2298 | 3.41 (1.74,6.69) | 3.42×10−4 |
| α-hbdh [72-182 IU/L] | 2208 | 4.53 (1.86,11.04) | 8.87×10−4 |
| Lymphocyte count [1.1-3.2 *10∼9/L] | 2330 | 3.63 (1.54,8.56) | 3.21×10−3 |
| BNP [0-100 pg/ml] | 1252 | 2.84 (1.38,5.85) | 4.44×10−3 |
| Urea nitrogen [3.6-9.5 mmol/l] | 2284 | 2.44 (1.28,4.65) | 6.59×10−3 |
| Platelet [125-350 *10∼9/L] | 2328 | 2.46 (1.26,4.8) | 8.13×10−3 |
| CRP [0-4 mg/L] | 2207 | 12.37 (1.63,94.09) | 0.02 |
| GGT [10-60 IU/L] | 2285 | 2.23 (1.14,4.33) | 0.02 |
| Alkaline phosphatase [45-125 IU/L] | 2284 | 2.32 (1.09,4.96) | 0.03 |
| Fasting plasma glucose [3.3-7.8 mmol/l] | 2286 | 2.10 (1.05,4.20) | 0.04 |
| DD [0-0.55 mg/L] | 1964 | 4.45 (1.01,19.59) | 0.05 |
| Albumin [34-54 g/L] | 2297 | 1.95 (0.94,4.04) | 0.07 |
| Alanine aminotransferase [9-50 IU/L] | 2294 | 1.76 (0.92,3.39) | 0.09 |
| Creatinine [57-111 umol/L] | 2277 | 1.54 (0.79,3.01) | 0.20 |
| Hemoglobin [115-150 g/L] | 2329 | 1.45 (0.74,2.82) | 0.28 |
| Creatine kinase [24-190 IU/L] | 2201 | 1.36 (0.67,2.78) | 0.40 |
| Uric acid [202-416 umol/L] | 2270 | 1.21 (0.63,2.34) | 0.57 |
| Total protein [65-85 g/L] | 2298 | 1.04 (0.49,2.19) | 0.92 |
The multivariate Cox regression analysis was adjusted for age, sex, and severity of COVID-19 at admission to hospital.
†To make Hazard Ratios (HR) comparable for all the risk factors, the continuous variables in the laboratory tests were converted into binary variables (i.e. abnormal or normal) using their normal ranges as the cut-offs. The values of cut-offs were retrieved from the reference ranges for medical tests used by Huoshenshan hospital (shown in the brackets).
Abbreviations: HR, hazard ratio; DBP, diastolic blood pressure; SBP, systolic blood pressure; CHD, coronary heart disease; CORD, chronic obstructive pulmonary disease; ARDS: acute respiratory distress syndrome; LDH, lactate dehydrogenase; α-hbdh, α-hydroxybutyrate dehydrogenase; BNP, B-type natriuretic peptide; CRP, C-reactive protein; GGT, gamma-glutamyl transferase; DD, D-dimer; IL6, interleukin 6.
Next, we investigated the influence of diabetes and FPG on disease progression of COVID-19 in multivariate logistic regression analysis, with adjustment of age, sex, and severity of COVID-19. The results were in line with those of OS analysis: diabetes was not a significant risk factor for progression (OR diabetes: 0.76, 95% CI 0.46–1.27, P = 0.30) (Table S1), whereas FPG showed significant associations (OR FPG: 1.25, 95% CI 1.16–1.35, P = 9.13 × 10−9) (Table S1) and remained to be significant after further adjustment for oral medication of blood glucose control (OR FPG: 1.27, 95% CI 1.17–1.37, P = 3.33 × 10−9) (Table S2).
3.3. FPG showed non-linear associations with overall survival and disease progression.
As shown in Table 1, we divided patients into three groups according to their FPG levels at admission: hypoglycemic (FPG < 3.3 mmol/L), normoglycemic (3.3 mmol/L ≤ FPG < 7.8 mmol/L) and hyperglycemia (FPG ≥ 7.8 mmol/L). We observed that both hypoglycemic and hyperglycemic COVID-19 patients had higher death rate and progression rate in comparison to that of the overall group. Going by this observation, we investigated whether there was evidence of non-linearity in the associations of FPG with COVID-19 prognosis. We applied restricted cubic splines to flexibly fit regression models of FPG with OS and disease progression with adjustment for age, sex, the severity of COVID-19 at admission and stratification by diabetes.
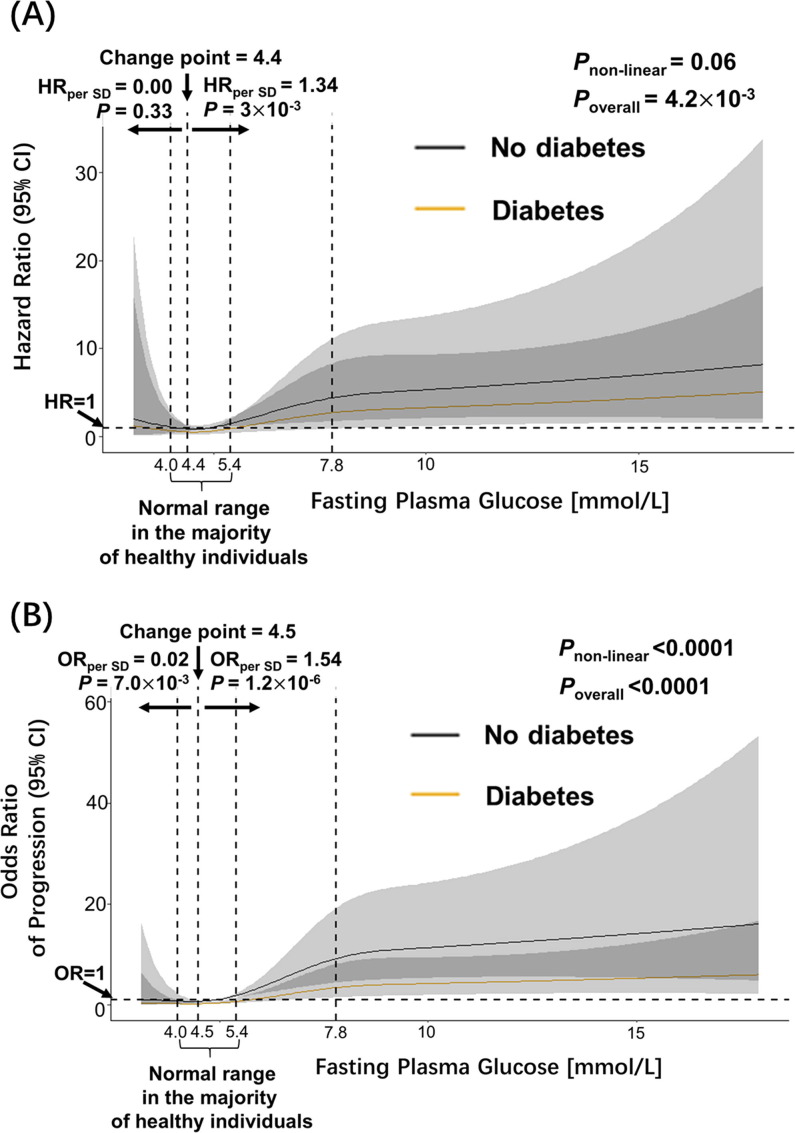
FPG showed J-shaped associations with OS of COVID-19 (Fig. 1 A): when FPG level was above 4.4 mmol/L, the estimated hazard ratio per one SD increase of FPG was 1.34 (95% CI 1.11–1.62). However, there was only marginal significance of non-linearity for OS analysis (Pnon-linear = 0.06), because FPG was not significantly associated with OS of COVID-19 below the point of FPG of 4.4 mmol/L. Moreover, we observed significant non-linearity (Pnon-linear < 0.0001) of the FPG association with disease progression (Fig. 1 B): when FPG was below 4.5 mmol/L, higher FPG was protective (OR = 0.02 per SD increase of fasting glucose; (95% CI 0.001–0.341)); whereas when above 4.5 mmol/L, FPG became a risk factor of COVID-19 disease progression: the estimated odds ratio of progression per one SD increase of FPG was 1.54 (95% CI 1.30–1.84).
Fig. 1.
The J-shaped association between FPG and overall survival and progression of COVID-19. Dashed vertical lines represent the range of FPG levels between 4.0 and 5.4 mmol/L (the normal range in majority of healthy individuals), and 7.8 mmol/L (the cut-off point of FPG level considered hyperglycemia). (A) For overall survival analysis, the estimates were adjusted for age, sex, the severity of COVID-19 at admission to hospital, stratified by pre-existing diabetes. (B) The logistic regression models predicting disease progression were adjusted for age, sex, the severity of COVID-19 at admission to hospital, stratified by pre-existing diabetes. The P-values for overall association (Poverall) and P-values for non-linearity (Pnon-linear) are displayed for each outcome in the figures. HR: hazard ratio. OR: odds ratio.
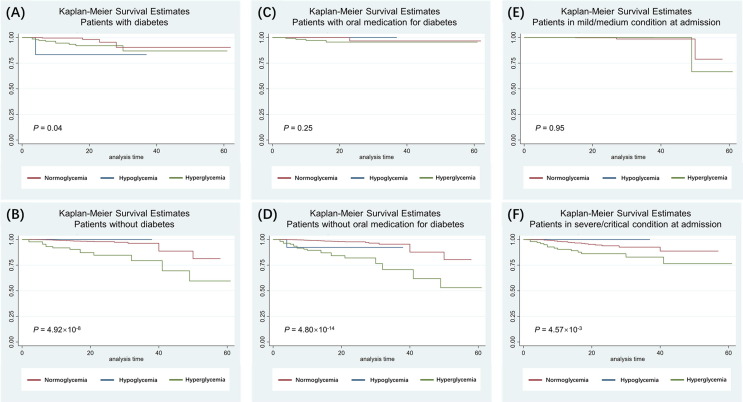
We plotted Kaplan-Meier curves to show the effect of glycemic status on the OS of patients with COVID-19, stratified by various clinical features (Fig. 2 & Figure S1 ). Overall, patients in normoglycemic group (red line) tended to survive longer in contrast to patients with hyperglycemic status (green line). It's worth noting that the adverse effect of hyperglycemia on OS was much stronger in patients without pre-existing diabetes in comparison to patients with diabetes (Fig. 2 A & B). In addition, glycemic status had no significant influence on OS in COVID-19 patients who was taking oral medication for blood glucose control (Fig. 2 C), or in patients in mild/moderate condition at admission to hospital (Fig. 2 E).
Fig. 2.
Kaplan-Meier curves for COVID-19 patients stratified into various clinical feature groups.The glycemic status of COVID-19 patients was determined by the FPG level measured at admission to hospital. Red line: normoglycemic group. Blue line: hypoglycemic group. Green line: hyperglycemic groups. The difference in survival between the three groups were compared using the log-rank test, and the P-values were displayed at the bottom left corner in each graph. (A & B) Kaplan-Meier curves for COVID-19 patients stratified by pre-existing diabetes status. (C & D) Kaplan-Meier curves for COVID-19 patients stratified by oral medication for blood glucose control. (E & F) Kaplan-Meier curves for COVID-19 patients stratified by severity of COVID-19 at admission. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
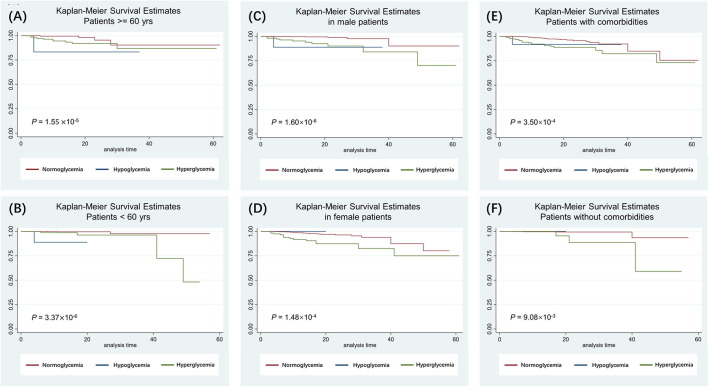
The differences of Kaplan-Meier survival curves were also statistically significant for patients stratified by age groups (using 60 years as the cut-off) (Figure S1 E & F), sex (Figure S1 C & D), or any type of comorbidities (Figure S1 E & F) which included hypertension, coronary heart disease (CHD), any type of respiratory disease (i.e., pre-existing chronic obstructive pulmonary disease (COPD), tuberculosis (TB)), or any type of cancer. The survival probabilities for the patients with hyperglycemia were always lower than that of the normoglycemic patients, suggesting a survival benefit of normoglycemia regardless of age group, sex, or comorbidities. However, the effect of hypoglycemic status was less conclusive due to the small number of patients in this group (n = 17): it showed protective effect in patients without diabetes and in patients in severe or critical condition at admission to hospital, but adverse effect in other sub-groups (Fig. 2 & Figure S1 ).
3.4. Blood glucose fluctuation was associated with disease progression of COVID-19
In addition to baseline FPG, we investigated the influence of blood glucose fluctuation on COVID-19 prognosis. We calculated the blood glucose fluctuation indexes SDBG, LAGE, PPGE, and %GV for 101 COVID-19 patients whose Blood Glucose Monitoring (BGM) data was available.
The range of the blood glucose fluctuation indexes are: SDBG (3.08 [IQR, 2.02–4.39]), LAGE (8.70 [IQR,5.20–11.40]), PPGE (6.07 [IQR, 3.30–8.60]), %GV (26.45 [IQR,18.88–34.22]). According to the “diabetes blood sugar fluctuation management expert consensus” published by Chinese Medical Association Endocrinology Branch in 2017 [31], SDBG > 2.0, PPGE > 2.2, and LAGE > 4.4 were considered as abnormal blood glucose fluctuations cut-offs. Hence, the blood glucose fluctuation indexes of the 101 COVID-19 patients were all above the normal ranges. This could be explained by the fact that BGM was only conducted on COVID-19 patients who have manifested symptoms of dysfunctional glucose metabolism during hospital stay.
In these 101 patients, 67.33% were with previously diagnosed diabetes, among which 34.67% were with diabetes but without any other comorbidities. None of the patients with BGM data was in the mild spectrum of COVID-19, 65% was classified in moderate type, 33% in severe type, and 2% in critical type. However, the disease severity spectrum was not significantly different between patients with or without BGM data (Fisher’s exact P-value = 0.55). No death event occurred in these 101 patients. Eight out of the 101 patients (7.92%) had disease progression during hospital stay (Fisher’s exact P-value = 0.49).
We investigated whether the 4 blood glucose fluctuation indexes were associated with disease progression of COVID-19 using multivariate logistic regression analysis with adjustment of age, sex, diabetes and severity of COVID-19. Two of the blood fluctuation indexes were significantly associated with disease progression of COVID-19: SDBG (OR: 1.54, 95% CI (1.05, 2.27), P = 0.03), and LAGE (OR: 1.18, 95% CI (1.01,1.39), P = 0.04) (Table S1). The associations remained significant after additional adjustment for oral medication for blood glucose control: SDBG (OR: 1.84, 95% CI (1.14, 2.95), P = 0.01), LAGE (OR: 1.29, 95% CI (1.05,1.57), P = 0.01) (Table S2).
3.5. FPG and blood glucose fluctuation indexes were associated with elevated inflammatory biomarkers regardless of diabetes and COVID-19 severity
Inflammation played an important role in the pathogenesis of both diabetes and COVID-19. To delineate the relationship between dysfunctional glucose metabolism and inflammation after coronavirus infection, we analyzed the association between FPG levels and inflammatory biomarkers with adjustment for age, sex, diabetes, and the severity of COVID-19 at admission.
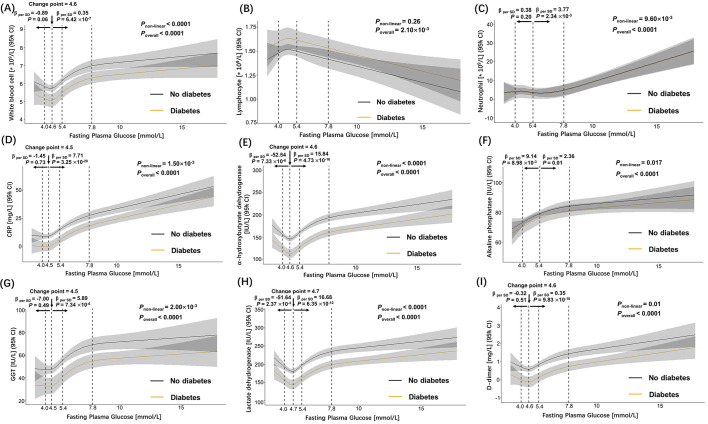
Overall, admission FPG showed significant positive associations with admission inflammatory biomarkers such as white blood cell absolute count, neutrophil count, C-reactive protein (CRP), alkaline phosphatase, α-hydroxybutyrate dehydrogenase (α-hbdh), GGT, lactate dehydrogenase, D-dimer; and negative association with lymphocyte count (Figure S2). The associations were generally in non-linear J-shape: for example, the estimated increase of white blood cell count per one SD increase of FPG was 0.35 (95% CI 0.21–0.48)×10- 9/L when the level of FPG was above 4.6 mmol/L. The slope was most steep between the range from FPG of 4.6–7.8 mmol/L, slowly tapering off above FPG of 7.8 mmol/L. When FPG was below 4.6 mmol/L, its association with white blood cell count was insignificant (Figure S2 A). Similar non-linear association patterns were observed for neutrophil, CRP, GGT, and D-dimer: their increase associated with the elevated level of FPG remained flat until the level of FPG raised above the change points as shown accordingly in Figure S2, respectively. For α-hydroxybutyrate dehydrogenase and lactate dehydrogenase, the associations were significantly negative below the change points and became significantly positive when FPG raised above the change point (Figure S2 E & H). One exception was alkaline phosphatase (Figure S2 F), which showed strong positive association with FPG (7.36 (95% CI 2.28–16.00) IU/L per one SD increase of FPG) when FPG was below 5.4 mmol/L, but the association weakened when FPG increased above 5.4 mmol/L. Notably, when FPG was in the range of 4.0 to 10 mmol/L, COVID-19 patients without pre-existing diabetes (Figure S2 black lines) had significantly higher levels of white blood cell count, CRP, α-hydroxybutyrate dehydrogenase, GGT, lactate dehydrogenase, and D-dimer in comparison to COVID-19 patients with diabetes (Figure S2 orange lines).
In addition, we investigated the linear associations between FPG and blood fluctuation indexes with the baseline levels of inflammation-related laboratory parameters with adjustment for age, sex, diabetes, and the severity of COVID-19 at admission (Table 3 ). In consistent with the non-linear regression models (Figure S2), FPG was significantly associated with most baseline inflammatory biomarkers (Table 3). Although only 4 inflammatory biomarkers: CRP, creatine kinase, D-dimer and interleukin-6 (IL-6), showed significant associations with glucose fluctuation indexes. There were no significant associations between FPG and blood fluctuation indexes, but this could be caused by the limited number of patients (n = 97) with BGM data.
Table 3.
Linear correlation analysis between blood glucose fluctuation indexes and laboratory parameters of inflammation.
| FPG |
SDBG |
LAGE |
PPGE |
%GV |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | N | Coeff | P | N | Coeff | P | N | Coeff | P | N | Coeff | P | N | Coeff | P |
| FPG | na | na | na | 97 | 0.255 | 0.166 | 97 | 0.124 | 0.100 | 97 | 0.06 | 0.487 | 97 | −0.004 | 0.878 |
| White blood cell count | 2294 | 0.102 | 1.17×10−10 | 95 | 0.083 | 0.405 | 95 | 0.198 | 0.414 | 95 | 0.303 | 0.150 | 95 | −0.339 | 0.602 |
| Lymphocyte count | 2293 | −0.181 | 5.46×10−4 | 95 | −0.212 | 0.524 | 95 | −0.285 | 0.727 | 95 | −0.302 | 0.670 | 95 | −1.428 | 0.511 |
| Neutrophil count | 2295 | 0.009 | 2.38×10−7 | 95 | 0.002 | 0.298 | 95 | 0.005 | 0.224 | 95 | 0 | 0.952 | 95 | −0.007 | 0.577 |
| Monocyte count | 2293 | 0.094 | 0.179 | 95 | −0.49 | 0.672 | 95 | −0.576 | 0.839 | 95 | −0.079 | 0.974 | 95 | −6.357 | 0.399 |
| Eosinophil count | 2290 | −0.092 | 0.477 | 95 | −0.351 | 0.815 | 95 | 0.432 | 0.906 | 95 | −0.998 | 0.754 | 95 | −0.503 | 0.959 |
| CRP | 2185 | 0.015 | 3.45×10−29 | 93 | 0.014 | 0.05 | 93 | 0.03 | 0.089 | 93 | 0.025 | 0.111 | 93 | 0.012 | 0.798 |
| ALP | 2288 | 0.006 | 3.93×10−6 | 93 | −0.004 | 0.389 | 93 | −0.011 | 0.300 | 93 | −0.007 | 0.493 | 93 | −0.016 | 0.598 |
| Creatine kinase | 2206 | 0.000 | 0.509 | 94 | 0.007 | 0.021 | 94 | 0.017 | 0.013 | 94 | 0.004 | 0.517 | 94 | 0.009 | 0.620 |
| α−hbdh | 2212 | 0.006 | 3.07×10−21 | 94 | 0.004 | 0.143 | 94 | 0.005 | 0.508 | 94 | 0.003 | 0.642 | 94 | −0.013 | 0.508 |
| GGT | 2289 | 0.005 | 2.81×10−8 | 95 | −0.001 | 0.76 | 95 | −0.002 | 0.737 | 95 | −0.004 | 0.466 | 95 | 0.002 | 0.915 |
| LDH | 2211 | 0.004 | 3.10×10−15 | 94 | 0.004 | 0.086 | 94 | 0.007 | 0.299 | 94 | 0.004 | 0.503 | 94 | −0.010 | 0.558 |
| DD | 1950 | 0.149 | 1.24×10−11 | 81 | 0.436 | 7.95×10−3 | 81 | 0.808 | 0.047 | 81 | 0.805 | 0.024 | 81 | 2.386 | 0.030 |
| IL6 | 1168 | 0.000 | 0.375 | 62 | −0.038 | 0.136 | 62 | −0.084 | 0.172 | 62 | −0.053 | 0.335 | 62 | −0.366 | 0.036 |
Abbreviations: FPG, fasting plasma glucose; SDBG, standard deviation of blood glucose; %GV, percentage coefficient of variation for glucose; LAGE: largest amplitude of glycemic excursions; PPGE, postprandial glucose excursions; CRP, C-reactive protein; ALP, Alkaline; phosphatase; α-hbdh,α-hydroxybutyrate dehydrogenase GGT, gamma-glutamyl transferase; LDH, lactate dehydrogenase; DD, D-dimer; IL6, interleukin 6.
Linear regression analysis adjusted for age, sex, diabetes, and the severity of COVID-19 at admission to hospital.
3.6. The growth trajectory of FPG over time was steeper in patients with severe COVID-19 regardless of pre-existing diabetes
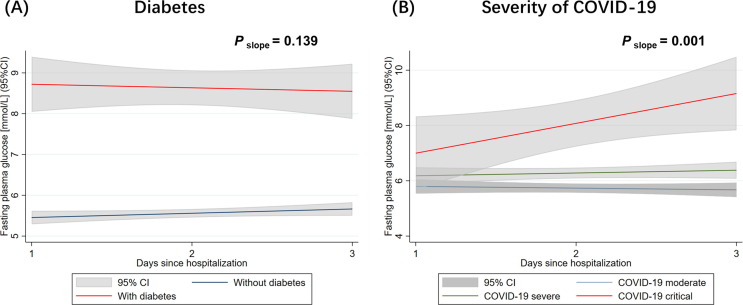
We used the latent growth curve model to analyze the longitudinal change of FPG over time in COVID-19 patients after admission to hospital. We included 625 patients whose FPG levels have been measured on three consecutive days from Day 1 of hospital admission.
First, we used diabetes as a predictor for the variations in intercept and slope of the change of FPG ( Fig. 3 A). The regression intercept predicted by diabetes was 3.263 (Table S3), which indicated that the initial status of FPG in COVID-19 patients with diabetes was 3.263 mmol/L higher in comparison to patients without diabetes on Day1 of hospital stay. The slope predicted by diabetes is negative (-0.189) but the P-value of the variations in slopes was insignificant (P slope = 0.139) (Table S3), which suggested there was no significant difference in terms of FPG changes over time for patients with diabetes and without diabetes ( Fig. 3 A).
Fig. 3.
Change of FPG within the first 3 days of hospitalization in COVID-19 patients. The lines represent the prediction for FPG on time points from a linear regression analysis, stratified by pre-existing diabetes status (A); or stratified by the severity of COVID-19 at admission to hospital (B). The P-values for the variations of slopes in the latent growth curve model were displayed in each group.
Then we used the severity of COVID-19 at admission as a predictor for the variations in intercept and slope of the change of FPG (Fig. 3 B). The slope predicted by the severity of COVID-19 was positive (0.296) with a significant P-value (P slope = 0.001) (Table S3), which suggested that the growth trajectory of FPG for severe patients was steeper than that of moderate patients. In other words, the positive gains of FPG over time for severe patients was significantly more than the positive gains over time for moderate patients.
We then applied a combined model with two regressors (i.e., diabetes and severity of COVID-19) to predict the latent growth factors. The intercepts and slopes in the combined model were similar with those predicted by diabetes and severity of COVID-19 individually (Table S3).
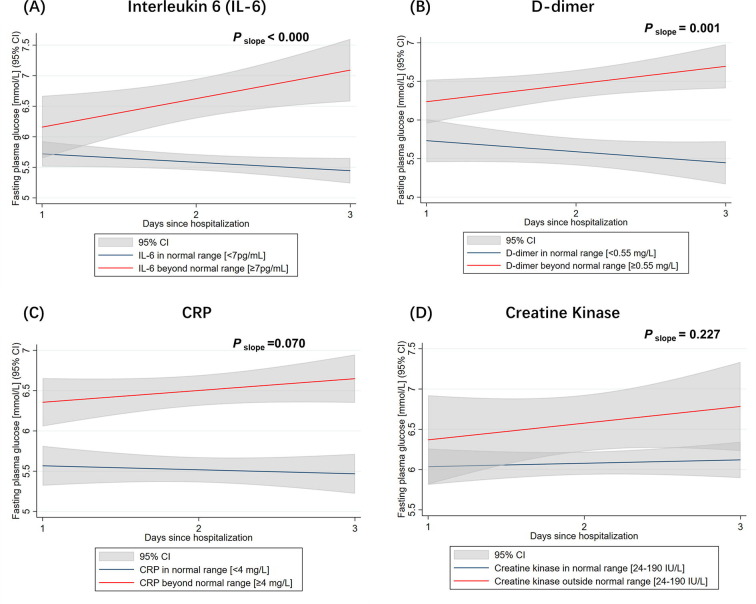
Because CRP, creatine kinase, D-dimer and interleukin-6 (IL-6) were the 4 inflammatory biomarkers significantly associated with glucose fluctuation indexes (Table 3), we also applied these 4 inflammatory biomarkers as predictors for the variations in intercept and slope of the change of FPG (Figure S3) (Table S4). For patients whose baseline levels of IL-6 or D-dimer were beyond the normal range at admission, the positive gain of FPG over time was significantly more than that in patients whose baseline levels of IL-6 or D-dimer were within normal range (Figure S3 A & B). However, no significant difference in FPG slopes was observed for CRP and creatine kinase (Figure S3 C & D). Cox regression analysis showed that 3-day change of FPG was associated with worse prognosis after adjustment of the baseline IL-6 and D-dimer (Table S5).
4. Discussion
Our findings agreed with a couple of previous studies that, in multivariate analysis after adjustment for age and sex, diabetes was not an independent risk factor for in-hospital death of COVID-19 patients [18], [32], [33]. Moreover, our findings extended the previous work by showing that abnormal glycemic profile was an independent risk factors of COVID-19 survival regardless of the pre-existing diabetes status. In addition, the growth trajectory of FPG over the first 3 days of hospital stay was steeper in patients with more severe COVID-19, whereas there was no significant difference in the growth trajectories of FPG in patients with and without pre-existing diabetes. This is generally in keeping with the findings of Montefusco et. al. that COVID-19-associated new-onset hyperglycemia was associated with worse clinical outcomes (i.e., higher demand for oxygen support or positive-pressure ventilation), and higher glycemic variability was observed in patients with acute COVID-19 as compared to healthy controls. Notably, they reported that glycemic abnormality persisted in some patients who recovered from COVID-19 [23].
We observed J-shaped associations between FPG and COVID-19 mortality and disease progression, with lowest mortality within the range of normal blood sugar levels (4.0 to 5.4 mmol/L) (Fig. 1). The influence of hyperglycemia on adverse outcomes of COVID-19 were generally stronger in patients without diabetes than in patients with diabetes (Fig. 2 A & B), indicating importance of blood glucose monitoring in COVID-19 patients without pre-existing diabetes. We also observed that the risk conferred by hyperglycemia became insignificant in the group of COVID-19 patients taking glucose-lowering medication before and during hospital stay (Fig. 2 C), which agreed with the findings in a previous study where multivariable-adjusted hazard ratios of hyperglycemia decreased when glucose-lowering medication was adjusted in the model [34].
Chronic low-grade inflammation is a character feature of diabetes [35]. On the other hand, infection of SARS-CoV-2 may increase the secretion of hyperglycemic hormones, such as glucocorticoid and catecholamines, which results in elevated blood glucose and glucose variability. Indeed, our findings indicated that dysfunctional blood glucose metabolism was associated with over-active immune response, independent of pre-existing diabetes and severity of COVID-19 (Table 3 & Figure S2).
It was also worth pointing out that our study suffered from the intrinsic limitation of the observational study design in the way that it cannot elucidate cause-and-effect relationship between hyperglycemia and COVID-19 outcomes. Although, previous studies provided valuable clues supporting both sides: the CORONADO study observed an age- and sex-independent association between hyperglycemia at admission and the severity of COVID-19 and they postulated that this observation was rather the consequence of the severity of the infection than a causal primary factor [21]. Several other studies were also in favor of this “hyperglycemia as consequence” theory, that cytokine storm initiated by COVID-19 infection disrupted beta cell function, which can result in hyperglycemia in both acute and post-COVID-19 phase [15], [16], [23]. On the other hand, the studies of Sardu et. al. suggested that elevated blood glucose may itself cause an inflammatory response, leading to severe COVID-19 disease. Not only they found that a decrease in glucose levels between baseline and 24 h was associated with a lower rate of progression to severe disease [22], but also that insulin infusion therapy reduced the IL-6 and D-dimer levels and the risk of progression to severe disease in patients with hyperglycemia [26]. Our study also showed that elevated FPG levels in the first 3 days after admission was associated with a higher risk of death and disease progression even after adjustment of baseline IL-6 and D-dimer levels (Table S5). Unfortunately, IL-6 and D-dimer measurements were not taken as frequently as FPG in our data, so we cannot examine how the inflammatory biomarkers changed along with blood glucose levels.
Taking together, these observations were suggestive of a mutual causality between hyperglycemia, blood glucose fluctuation and COVID-19 prognosis: the acute hyperglycemia upregulates ACE2 expression in beta cells, facilitating viral cell entry[15], [36]. The virus’s entry into beta cells through ACE2 in turn may cause an acute beta-cell dysfunction that could lead to further uncontrolled glucose homeostasis [37]. This is also consistent with previous animal studies that showed acute glucose fluctuation caused significant increase of inflammatory cytokines in mice [38]. Further studies with a larger cohort or randomized controlled trials are needed to fully elucidate the therapeutic effect of glucose-lowering medications on COVID-19 prognosis.
Some limitations of our study should be acknowledged. First, in terms of predictive biomarkers of COVID-19 prognosis, the prognostic values of FPG and glycemic variability were admittedly not as strong as many clinical and laboratory parameters (Table 2). However, our findings provided suggestive evidence about the influences of glucose metabolism abnormalities in the inflammatory state after COVID-19 infection. Second, only a limited number of COVID-19 patient had BGM data available (n = 97). In addition, this sub-group was not a representative sample of the general population of COVID-19 patients. Rather, they were a group of individuals who had already shown signs of dysfunctional glucose metabolism. Third, in this study we used FPG instead of glycated hemoglobin (A1C) or glycated albumin, which have been suggested as better indicators for long-term glucose monitoring [39], but not available due to our data set limitations. Fourth, the diabetes disease status was collected from the medical records, which in most cases did not differentiate diabetes types. According to a recent study about the prevalence of diabetes in Chinese adults, approximately 5.8% of newly diagnosed diabetes are type 1 diabetes [40]. Whilst both type 1 and type 2 diabetes are characterized by having higher than normal blood glucose levels, their pathophysiology is fundamentally different. Reassuringly, a preliminary study suggested that there was no increase of risk for people with type 1 diabetes hospitalized for COVID-19 [21]. In future studies, it is important to distinguish COVID-19 patients with type1 and type 2 diabetes and investigate whether they have different complications and prognosis of COVID-19.
Abnormal glycemic status is an independent risk factor for poor prognosis of COVID-19. Patients with high FPG and larger glucose fluctuations could have higher risk of disease progression of COVID-19. We suggest that blood glucose management should be better optimized for COVID-19 patients during hospitalization and in follow-up period after discharge from hospital to avoid aggravation of glucose metabolism.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all members of the Department of Epidemiology of the Army Medical University for their professional suggestions, and all members of the Department of Respiratory Disease of the Xinqiao Hospital and Huoshenshan Hospital for sample collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2021.109041.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
References
- 1.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song F., Shi N., Shan F., et al. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J.J., Dong X., Cao Y.Y., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Zangrillo A., Zanella A., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg S., Kim L., Whitaker M., et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang A., Zhao W., Xu Z., et al. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo W., Li M., Dong Y., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman E.L., Savelieff M.G., Hayek S.S., et al. COVID-19 and Diabetes: A Collision and Collusion of Two Diseases. Diabetes. 2020;69(12):2549–2565. doi: 10.2337/dbi20-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceriello A., De Nigris V., Prattichizzo F. Why is hyperglycaemia worsening COVID-19 and its prognosis? Diabetes Obes Metab. 2020;22(10):1951–1952. doi: 10.1111/dom.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall R.J., Armart P., Hulme K.D., et al. Glycemic Variability in Diabetes Increases the Severity of Influenza. mBio. 2020;11(2) doi: 10.1128/mBio.02841-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang C.M., Hsieh C.J., Huang J.C., et al. Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus. Acta Diabetol. 2012;49(Suppl 1):S171–S177. doi: 10.1007/s00592-012-0398-x. [DOI] [PubMed] [Google Scholar]
- 15.Mahrooz A., Muscogiuri G., Buzzetti R., et al. The complex combination of COVID-19 and diabetes: pleiotropic changes in glucose metabolism. Endocrine. 2021;72(2):317–325. doi: 10.1007/s12020-021-02729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Accili D. Can COVID-19 cause diabetes? Nat Metab. 2021;3(2):123–125. doi: 10.1038/s42255-020-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quagliaro L., Piconi L., Assaloni R., et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 18.Shi Q., Zhang X., Jiang F., et al. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center. Retrospective Study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 19.Riddle M.C., Buse J.B., Franks P.W., et al. COVID-19 in People With Diabetes: Urgently Needed Lessons From Early Reports. Diabetes Care. 2020;43(7):1378–1381. doi: 10.2337/dci20-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L., She Z.G., Cheng X., et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31(6):1068–1077 e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cariou B., Hadjadj S., Wargny M., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sardu C., D'Onofrio N., Balestrieri M.L., et al. Hyperglycaemia on admission to hospital and COVID-19. Diabetologia. 2020;63(11):2486–2487. doi: 10.1007/s00125-020-05216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montefusco L., Ben N.M., D'Addio F., et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021;3(6):774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bode B., Garrett V., Messler J., et al. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L., Wei W., Yang K., et al. Glycemic control before admission is an important determinant of prognosis in patients with coronavirus disease 2019. J Diabetes Investig. 2021;12(6):1064–1073. doi: 10.1111/jdi.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sardu C., D'Onofrio N., Balestrieri M.L., et al. Outcomes in Patients With Hyperglycemia Affected by COVID-19: Can We Do More on Glycemic Control? Diabetes Care. 2020;43(7):1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umpierrez G.E., Hellman R., Korytkowski M.T., et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 28.Moghissi E.S., Korytkowski M.T., DiNardo M., et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parascandola M., Xiao L. Tobacco and the lung cancer epidemic in China. Transl Lung Cancer Res. 2019;8(Suppl 1):S21–S30. doi: 10.21037/tlcr.2019.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu W., Xu J., Taylor A.W., et al. Analysis of the alcohol drinking behavior and influencing factors among emerging adults and young adults: a cross-sectional study in Wuhan, China. BMC Public Health. 2019;19(1):458. doi: 10.1186/s12889-019-6831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mu Y. Experts Consensus on Management of Glycemic Variability of Diabetes Mellitus. Chinese Med Front J. Electronic Ed. 2017;14(17):7. [Google Scholar]
- 32.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maddaloni E., D'Onofrio L., Alessandri F., et al. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II) Cardiovasc Diabetol. 2020;19(1):164. doi: 10.1186/s12933-020-01140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H., Tian S., Chen T., et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020;22(10):1897–1906. doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 36.Bornstein S.R., Rubino F., Khunti K., et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lisco G., De Tullio A., Giagulli V.A., et al. Hypothesized mechanisms explaining poor prognosis in type 2 diabetes patients with COVID-19: a review. Endocrine. 2020;70(3):441–453. doi: 10.1007/s12020-020-02444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu N., Shen H., Liu H., et al. Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro-inflammatory cytokine expression in vivo. Cardiovasc Diabetol. 2016;15(1):109. doi: 10.1186/s12933-016-0427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freitas P.A.C., Ehlert L.R., Camargo J.L. Glycated albumin: a potential biomarker in diabetes. Arch Endocrinol Metab. 2017;61(3):296–304. doi: 10.1590/2359-3997000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang X., Yan X., Zhou H., et al. Prevalence and identification of type 1 diabetes in Chinese adults with newly diagnosed diabetes. Diabetes Metab Syndr Obes. 2019;12:1527–1541. doi: 10.2147/DMSO.S202193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.