Abstract
To identify single immunodominant marker proteins which can replace complex virion antigen in serodiagnostic assays, we investigated in detail the molecular fine specificity of antibody responses in different individuals with latent or active human cytomegalovirus (HCMV) infection. An overview of the HCMV proteins recognized by human antibodies was obtained by immunoblotting. For selected immunodominant proteins the epitope fine specificity of the antibody response was determined by a peptide-scanning enzyme-linked immunosorbent assay (ELISA). Epitope clusters were synthesized as combination peptides and were used for further serologic analysis of immunoglobulin M (IgM) and IgG reactivities with panels of sera from different groups of patients in comparison to those with cytomegalovirus (CMV) virion antigen. Several serum samples had significantly higher reactivities with peptides than with the CMV virion antigen. However, individual serum samples occasionally recognized diverse peptide epitopes, stressing the importance of using combinations of peptides in serologic assays. From these studies we were able to define a specific combination of peptides derived from pp52 (UL44) and pp150 (UL32) for the specific and highly sensitive early detection of HCMV IgM, whereas a combination of peptides from pp150 (UL32), gB (UL55), and pp28 (UL99) was selected to give optimal and specific reactivity with HCMV IgG. On the basis of the results obtained with these peptide combinations, new, highly specific serodiagnostic assays were constructed. These assays had sensitivities of 98.9 and 96.4% for IgG and IgM, respectively, in comparison with the results obtained with the “gold standard,” the virion antigen-based ELISA. From the results of this study we conclude that specific combinations of highly defined synthetic peptides can replace complex HCMV virion extracts used in current serodiagnostics and may add to further standardization of HCMV serology.
Human cytomegalovirus (HCMV), a beta herpesvirus, is widespread in human populations. HCMV is naturally transmitted via saliva, urine, or breast milk but can also be transmitted sexually. Alternatively, HCMV may be transmitted by blood donation and organ transplantation (8). Infection of immunocompetent hosts with HCMV rarely causes clinical symptoms, whereas in patients with suppressed cellular immune functions or after intrauterine infection, HCMV may cause a variety of clinical syndromes. Transplant recipients can develop a broad range of clinical symptoms during infection, and these may mimic symptoms related to rejection of the transplanted organ. On the basis of clinical manifestations alone, HCMV infection may be difficult to discriminate from transplant rejection and other infections, therefore requiring laboratory confirmation. Specific diagnosis of HCMV infection is based on different approaches. The most direct methods include culture of the virus (5) or detection of viral components, like viral DNA (28), RNA (1, 6), and antigens (29), in body fluids or tissue biopsy specimens. Serologic assays are widely used for donor selection and to support the diagnosis of HCMV infection in the host and to determine whether it is an active or latent infection (12). Although it indirectly reflects viral activity, serology provides a cheap alternative method that can readily be automated for routine use. In current serologic assays complex viral lysates are commonly used (12). However, the use of these viral lysates has disadvantages because they consist of many viral antigens whose exact compositions are difficult to standardize. Preparation of lysates requires culture of HCMV in fibroblasts, resulting in potential contamination with cellular proteins. Since many transplant recipients may temporarily develop autoantibody responses, a false-positive reactivity may result (25). Another problem can arise, since herpesviruses share multiple protein homologues, which can give rise to cross-reactivity in assays based on complex viral lysates (26). In order to overcome these problems the viral lysate should be replaced by a defined antigen preparation, preferably consisting of a combination of HCMV-specific and immunodominant antigens, in order to achieve the highest sensitivity and specificity. Besides recombinant proteins, synthetic peptides corresponding to immunodominant antigenic determinants of HCMV proteins can be used to detect antibodies to the parent protein (12). Combinations of such defined immunodominant proteins or peptides may ideally be suited as replacements for complex protein-antigen mixtures.
Although the 235-kb HCMV genome of strain AD169 has been sequenced and more than 200 open reading frames have been identified, only a limited number of HCMV polypeptides have been designed as targets for human antibody responses. The combination of proteins which should be included in the antigen mixture for HCMV serodiagnostic assays is not yet fully defined. However, a number of HCMV proteins may be good candidates (10). The tegument protein pp150 (UL32) is recognized by most HCMV-positive individuals during both latent infection and an activated or a reactivated state of the viral infection (11, 23, 33). During primary infection the antibody response to pp150 may be delayed (9). On the other hand, the major tegument protein (pp65 [UL83]) is recognized early during infection, but antibodies seem to disappear during later stages (22, 32). Proteins recognized in healthy individuals, furthermore, include the nonstructural protein pp52 (UL44) (18), the tegument protein pp28 (UL99) (13), and the viral glycoproteins gB (UL55) (15, 30) and gH (UL75) (24).
In the present study we analyzed in detail the fine specificities of antibody responses to HCMV during acute and latent infections with the aim of defining the immunodominant HCMV proteins and the dominant epitope domains on these proteins. First, immunoglobulin G (IgG) and IgM responses to HCMV proteins were characterized by immunoblotting. Subsequently, the antibody interaction with several immunodominant proteins was analyzed in more detail by a peptide-scanning enzyme-linked immunosorbent assay (ELISA) (PEPSCAN). After defining the most reactive epitope clusters for each protein, soluble peptides and combination peptides were synthesized. The ELISA reactivities of these peptides were compared with results of a standard ELISA based upon an HCMV (strain AD169) lysate. Some peptides showed clearly enhanced reactivity with sera compared to that with the lysate. The most-reactive peptides were subsequently combined to create fully peptide-based diagnostic assays for the detection of IgG and IgM in human sera with high sensitivities and specificities.
MATERIALS AND METHODS
Sera.
Serum samples were randomly collected from 122 healthy American blood donors (courtesy of C. A. Horwitz, Mount Sinai Medical Center, Minneapolis, Minn.) and 151 healthy Belgian blood donors (Blood Transfusion Centre, Antwerp, Belgium); 147 commercially available serum samples from blood donors (Trina, Greissensee, Switzerland) were also used. Nine IgM-positive serum samples from 9 transplant recipients (a large volume of sample taken shortly after seroconversion) and 83 follow-up serum samples (2 ml of each sample) from 19 patients with active primary or recurrent HCMV infection were obtained from renal transplant recipients from The Netherlands (H. Weiland, Universital Hospital, Leiden, The Netherlands). Active HCMV infection in these patients was diagnosed by isolation of virus from blood leukocytes by shell vial culture (5) and was confirmed by routine serology (see below). Sera were obtained from all patients at weekly intervals following transplantation, and the sera were analyzed for this study from 2 weeks before until 4 weeks following seroconversion. The time of seroconversion was determined by routine IgM and IgG serology with the IMX CMV-M (Abbott, North Chicago, Ill.) and the Vironostika CMV IgM II (Organon Teknika, Boxtel, The Netherlands) assay kits. The results of routine serologic analysis were confirmed for all serum samples by a noncommercial ELISA (16) with purified virion as the antigen, as indicated below. This assay was used as the “gold standard” in all experiments.
Cell culture and antigen preparation.
Human fetal lung fibroblasts (HLFs) were grown in a 1:1 mixture of Ham’s F12 medium and Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah). For preparation of the antigen, HLF cells were infected with HCMV AD169 at 0.01 PFU per cell for 10 days until a 100% cytopathic effect was achieved for the cells (16). The cells were harvested, and the cytoplasmic fraction of the infected cells was recovered by hypotonic detergent-mediated lysis of the HCMV-infected cells and by taking the supernatant after removal of the nuclei by Ficoll centrifugation as described by Van Loon et al. (31). The supernatant was centrifuged at 13,000 × g for 5 min, and the pellet containing soluble virions, capsids, and dense body particles (virion material) was resuspended by sonication in 50 mM phosphate buffered-saline (PBS) at pH 7.2.
SDS-PAGE and Western immunoblot analysis.
All protein samples were boiled for 5 min in sample buffer containing 0.2 M Tris-HCl (pH 6.8), 4% sodium dodecyl sulfate (SDS), 0.18% glycerol, 0.02% β-mercaptoethanol, bromophenol blue, and 3 M urea and were then centrifuged at 13,000 × g for 1 min. The polypeptides were separated by standard SDS-polyacrylamide gel electrophoresis (PAGE). After electrophoresis in a 10% separation gel, the proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), which were subsequently cut into 3-mm strips. Nonspecific binding of serum to the strips was prevented by incubation for 1 h at room temperature with blocking buffer (4% dried milk powder with 5% horse serum in PBS). Human sera used for IgM detection were first treated with GullSorb (Gull Laboratories, Salt Lake City, Utah) to remove the IgG by the protocol provided by the manufacturer. Sera were diluted 1:100 in blocking buffer and were incubated with the blot strips for 3 h at room temperature. The blot strips were washed three times in PBS containing 0.05% Tween 20 (PBST), horseradish peroxidase (HRP)-conjugated anti-human IgG or IgM antibody (DAKO, Glostrup, Denmark) was added, and the mixture was incubated for 1 h at room temperature. After washing, the blot strips were developed by using 0.3% 4-chloro-1-naphthol in PBS as the substrate. To determine the position of known HCMV immunogenic proteins, a mixture of monoclonal antibodies to defined HCMV marker proteins was used. The mixture contained monoclonal antibodies from Goodwin Institute (Plantation, Fla.) and Organon Teknika reactive with pp150 (UL32), pp72 (UL122), pp65 (UL83), pp52 (UL44), and pp28 (UL99).
PEPSCAN.
For fine mapping of the pp150, pp65, pp52, and pp28 epitopes, a PEPSCAN was performed (3, 4). Peptide synthesis and immunological screening were performed at ID-DLO, Lelystad, The Netherlands. For PEPSCAN, peptides of 12 amino acids in length with an overlap of 11 amino acids were synthesized by automated solid-phase peptide synthesis on chemically activated polyethylene pins. The immune reactivities of the peptides were analyzed with HCMV-positive sera as described by Middeldorp and Meloen (17).
Peptide synthesis.
Peptides derived from the most reactive epitope domains of pp150, gB, pp28, and p52 were synthesized by 9-fluorenylmethoxycarbonyl chemistry (Applied Biosystems, Inc., Foster City, Calif.) with a 433A peptide synthesizer from Applied Biosystems, Inc. (2). Peptides were purified by high-pressure liquid chromatography with an RP-C2/C18 column (Pharmacia). Table 1 presents the amino acid sequences of the peptides used.
TABLE 1.
Systematic one-letter representation of amino acid sequences of peptides used in this study
| Protein (gene) | Amino acid positions | Amino acid sequence |
|---|---|---|
| pp150 (UL32) | 595–614 | TPTPVNPSTAPAPAPTPTFAC |
| 615–636 | CQTPVNGNSPWAPTAPLPGDM | |
| 595–636 | TPTPVNPSTAPAPAPTPTFACCQTPVNGNSPWAPTAPLPGDM | |
| 1011–1048 | KSGTGPQPGSAGMGGAKTPSDAVQNILQKIEKIKNTEE | |
| pp52 (UL44) | 266–293 | FLTEEPFQRGDPFDKNYVGNSGKSRGGG |
| 295–312 | GGSLSSLANAGGLHDDG | |
| gB (UL55) | 792–809 | SADGTTVTSGSTKDTSLQ |
| 60–81 | SEAVSHRANETIYNTTLKYGDV | |
| pp28 (UL99) | 15–45 | TTPGEPLKDALGRQVSLRSYDNIPPTSSSDEGEDDDC |
| 130–160 | CETDDLDEEDTSIYLSPPPVPPVQVVAKRLPRPDTPRT |
ELISA.
Micro-ELISA plates were coated with the selected peptides in 50 mM sodium bicarbonate buffer (pH 9.6) for 16 h at 4°C. The peptides were used either in a free form or after coupling to bovine serum albumin (BSA) (19). Free binding sites were saturated by incubation with 3% BSA in PBS. After 1 h of incubation at 37°C the wells were washed four times with PBST. Sera were diluted 1:100 in PBST with 20% normal goat serum containing 0.5% Triton X-100, and the mixture was incubated for 1 h at 37°C. For IgM detection, the sera were first treated with Gullsorb as described above. After washing, HRP-conjugated swine antibody to human IgG or rabbit antibody to human IgM (DAKO) diluted 1,000 times in PBST containing 1% BSA was added for 1 h. The wells were washed four times with PBST and the bound HRP label was detected with 3,3′,5,5′-tetramethylbenzidine as substrate for 30 min in the dark, after which the coloring reaction was stopped by the addition of 1 M H2SO4. The absorbance was measured at 450 nm. The virion ELISA with plates coated with sonified virion material at pH 9.6 was performed as described above, except that human serum was diluted in PBST containing 40% normal goat serum containing 0.5% Triton X-100.
RESULTS
Immunoblot analysis.
Molecular characterization of the IgM and IgG immune responses against HCMV was performed by analyzing serum samples from various HCMV-infected individuals on immunoblot strips containing total lysates of HCMV-infected HLF cells. The samples included sera from HCMV-seropositive healthy individuals and sequential serum samples from renal allograft recipients who developed active primary HCMV infection.
Sera from healthy HCMV-seropositive individuals.
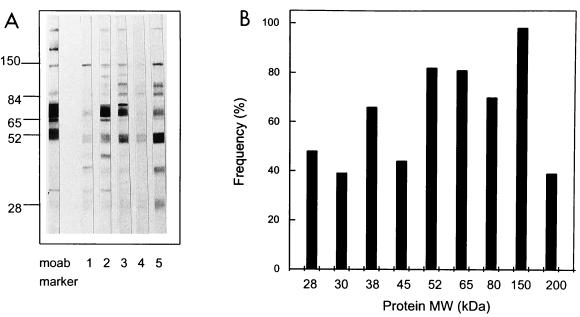
First, the blot strips were randomly probed with 72 serum samples from healthy seropositive blood donors selected by virion ELISA (16) at a 1:100 dilution and analyzed for IgG reactivity. A representative example of individual IgG responses on immunoblots of sera from five donors in this group is shown in Fig. 1A. The HCMV IgG recognition patterns for all sera are summarized in a frequency plot (Fig. 1B). Sera from HCMV-seronegative individuals had no detectable bands in any of the experiments. As shown in the frequency plot, a protein at 150 kDa is recognized by sera from 100% of the HCMV-positive individuals, followed by proteins of 52 and 65 kDa by sera from approximately 80% of the individuals, a protein of 38 kDa by sera from 74% of the individuals, and a protein of 28 kDa by sera from 55% of the individuals. Proteins with molecular masses of 150, 65, 52, 38, and 28 kDa most likely correspond to viral phosphoproteins pp150 (UL32), pp65 (UL83), pp52 (UL44), pp38 (UL80a), and pp28 (UL99), respectively, because they migrated to the positions identified by the reference monoclonal antibodies used. Next to these most reactive HCMV proteins, which correspond to the proteins found in previous studies by other investigators (27, 33), some additional undefined proteins with molecular masses of 200, 80, 45, and 30 kDa were also identified in 40 to 80% of the serum samples (Fig. 1B).
FIG. 1.
Overview of HCMV-specific IgG responses of sera from HCMV-seropositive healthy individuals. (A) An example of the of IgG responses of sera from five healthy donors against HCMV-encoded proteins by immunoblot analysis. The antigen applied to the blot was a total lysate of HCMV-infected fibroblasts. Lane moab, monoclonal antibody mixture to HCMV proteins pp150, pp65, pp52, and pp28; lanes 1 to 5, HCMV-positive sera. (B) A frequency table from the immunoblot of the IgG response of 72 healthy donors whose sera were reactive with HCMV-encoded proteins. The frequency of occurrence of sera reactive to the immunodominant proteins visible on the blot is plotted. MW, molecular mass.
Radioimmunoprecipitation analysis (RIPA) largely confirmed the results described above and revealed one additional band at 11 kDa in some sera. This additional band may be related to gB (UL55), as described by others (7). These data indicate that the immunodominant proteins are mainly recognized by epitopes resistant to denaturation (data not shown).
HCMV recognition pattern in primary infected renal allograft recipients.
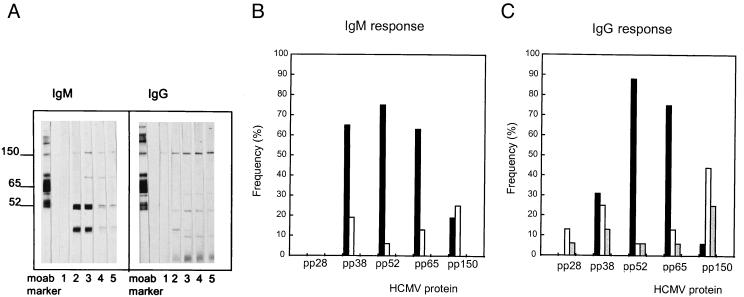
The development of IgM and IgG antibody responses against HCMV proteins during active HCMV infection following renal transplantation was analyzed by immunoblotting as described above. Mock-infected fibroblast antigen was used as a control for autoreactive IgM, which frequently develops during transplant rejection periods. Confirming previous data (12), the proteins most strongly recognized by human IgM include species with molecular masses of 150, 65, 52, and 38 kDa, corresponding to the proteins pp150 (UL32), pp65 (UL83), pp52 (UL44), and pp38 (UL80a), as shown in Fig. 2B.
FIG. 2.
Summary of the chronological appearance of IgM and IgG antibodies against HCMV proteins in renal transplant recipients with primary infections. (A) An example of immunoblot analysis of the IgM and IgG responses of a patient with primary HCMV infection monitored weekly. Lanes moab, monoclonal antibody mixture; lanes 1 to 5, weekly follow-up serum samples. (B and C) Frequency distribution of HCMV proteins recognized by the IgM (B) and IgG (C) responses of samples from allograft recipients (n = 16) with primary infections taken at and shortly after seroconversion, as defined by routine HCMV IgM serology. Symbols: ■, at seroconversion; □, secondarily recognized infection; ░⃞, infection recognized later.
To characterize the antigens first recognized by these patients, the development of IgM and IgG antibody responses over time (weekly after transplantation) was monitored by immunoblotting. The results shown in Fig. 2B and C indicate delayed IgM and IgG responses to pp150 compared to the time to the development of antibody responses to pp65, pp52, and pp38. The important immunodominant proteins for early diagnosis of HCMV infection are therefore pp38 (UL80a), pp52 (UL44), and pp65 (UL83). Surprisingly, responses to pp28 of the IgM class were not detected, whereas an IgG response to pp28 was readily detectable.
PEPSCAN analysis of immunodominant proteins of HCMV.
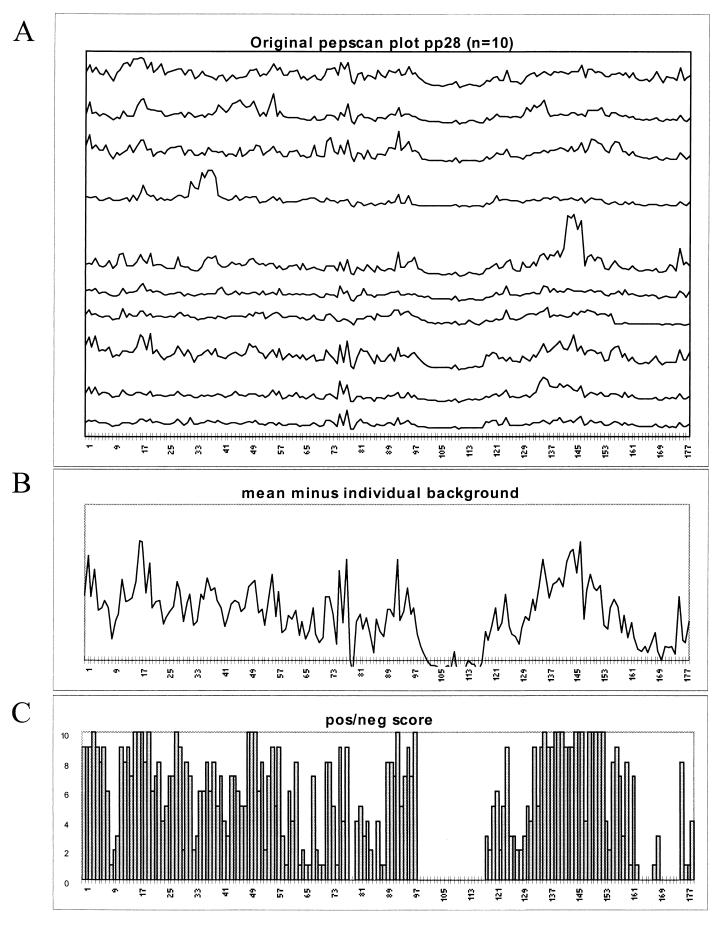
From the most immunoreactive polypeptides identified by the immunoblot analysis, amino acid sequence information was used for systematic synthesis of all possible overlapping 12-mer peptides on polypropylene pins. For the identification of antigenic peptide sequences (epitopes), the reactivities of all overlapping 12-mer peptides were analyzed by ELISA (PEPSCAN) with HCMV-seropositive human sera (n = 14 for pp150, n = 9 for pp65, n = 10 for pp52, and n = 10 for pp28) that were reactive with the tested protein on immunoblotting. An example of the results of the PEPSCAN analysis of pp28 (UL99) is shown in Fig. 3. Figure 3A shows that individual sera recognize multiple regions within the protein sequence and that the numbers and positions of epitopes vary between the sera. Figure 3B and C, however, indicates that the overall reactivities of the sera show a preference for domains in the N- and C-terminal regions of pp28. With the results of the PEPSCAN analysis of pp28, two 30-amino-acid peptide regions could be identified, and these were overlapping with epitopes recognized by most (>60%) sera (amino acids 15 to 45 and 130 to 160). Similarly, the PEPSCAN analysis of pp52 revealed two commonly recognized epitopes, which were selected for peptide synthesis (i.e., amino acids 266 to 293 and 295 to 312). The PEPSCAN analysis of pp65 did not reveal dominant epitopes consistently recognized by all sera. Therefore, it was not possible to define a specific peptide which was able to replace the whole pp65 protein. The PEPSCAN analysis of pp150 resulted in the identification of three epitope cluster regions (i.e., amino acids 595 to 614, 615 to 636, and 1011 to 1048) which are positionally equivalent to epitopes described previously (9, 20).
FIG. 3.
(A) Plot of PEPSCAN reactivities (OD450) of all overlapping dodecapeptides of pp28 with 10 individual serum samples from HCMV-infected healthy donors. (B) Mean OD450 value for all sera, individually corrected for the background reactivity. (C) Overview of peptides with reactivities of greater than three times the standard deviation above the negative background established for each serum, as defined previously (17).
Analysis of peptide recognition by individual sera.
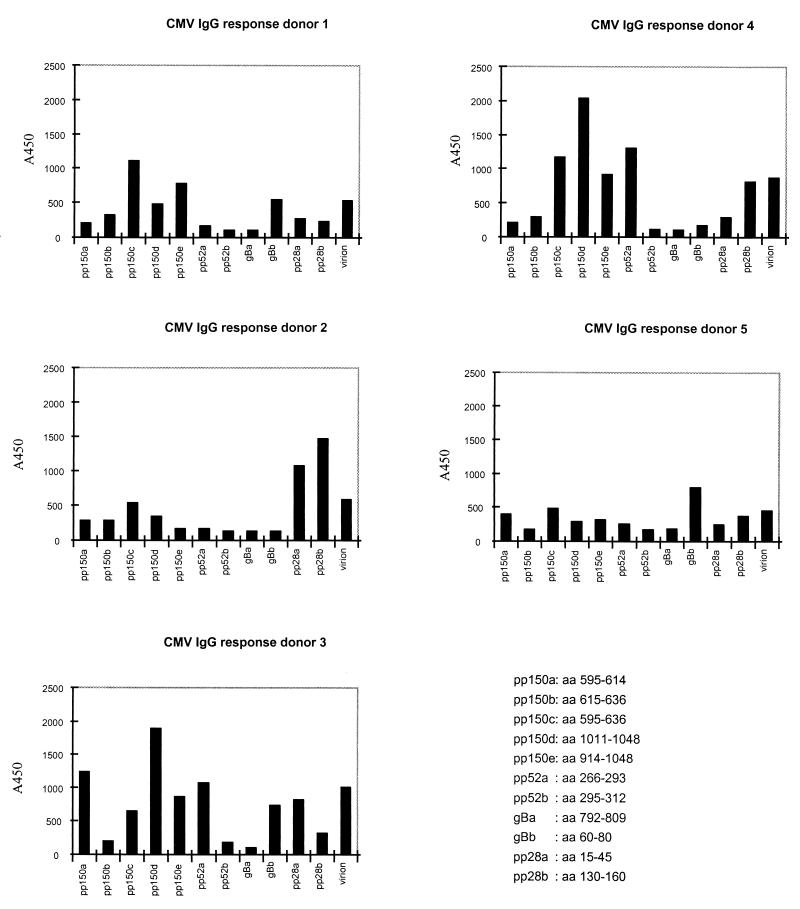
The reactivities of sera with distinct peptides, selected on the basis of PEPSCAN analysis, were compared with the responses against virion antigens by ELISA in order to define the diagnostic potential of peptides relative to those of intact proteins. The IgG antibody responses were monitored with sera from 49 healthy blood donors by ELISA with HCMV virion antigens and the selected HCMV epitopes described above. All serum samples were also tested by a reference HCMV ELISA method (16). The IgG reactivities of individual sera revealed a rather diverse recognition of the peptides, as shown in Fig. 4 for five serum samples. Serum from donor 4 has a high level of reactivity to peptides of pp150, whereas for sera from donor 2, a high level of reactivity to pp28 and a low level of recognition of pp150 peptides were seen. The differences in epitope specificities of the antibodies in each serum sample stress the importance of using a mixture of peptides in further studies. The reactivity with a single peptide compared to that with the virion antigen was higher for some sera, reflecting the dominance of the epitope in the overall immune response. This was found for both IgG and IgM responses.
FIG. 4.
Overview of the IgG reactivities of five serum samples from HCMV-seropositive healthy blood donors with different peptides. aa, amino acids.
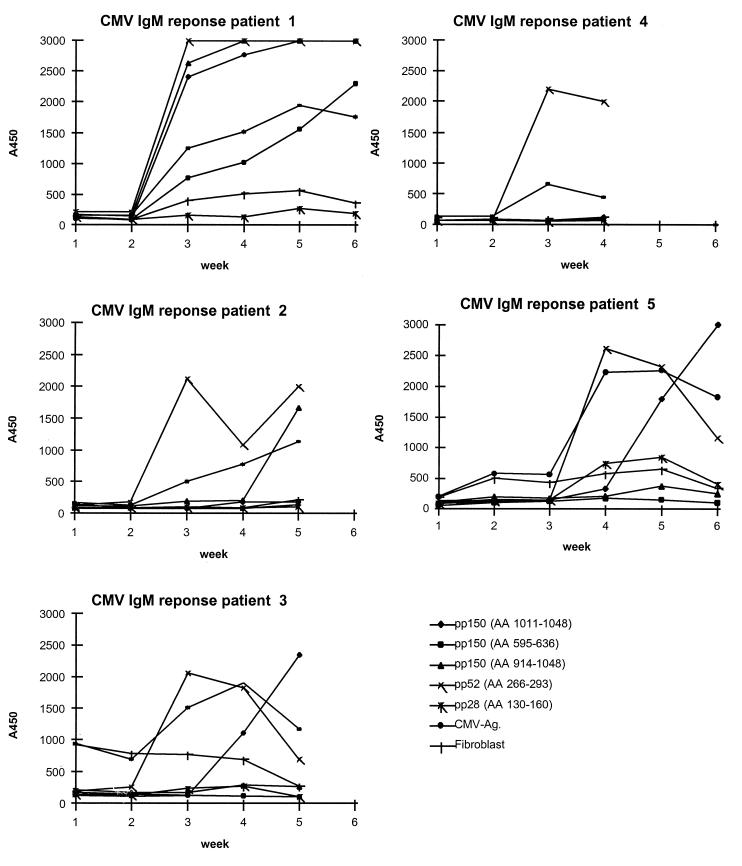
The development of IgM antibody reactivity over time (weekly after transplantation) was monitored in renal transplant patients by ELISA for the different HCMV antigens and the selected HCMV epitopes described above by using mock-infected fibroblasts as a control for the autoreactive IgM response that frequently develops during transplant rejection periods. The results of five follow-up series are shown in Fig. 5. The IgM reactivity with both virion and peptide antigens was positive at seroconversion and declined during convalescence for all subjects. The point of seroconversion was identical whether virion or peptide antigen was used, but with peptides, significantly higher responses (optical densities at 450 nm [OD450]) were obtained at seroconversion, allowing a more accurate diagnosis. Importantly, no false-positive reactivity (non-HCMV-specific binding) was observed by the peptide ELISA compared to the reactivity observed with the cell culture-derived virion antigen, as illustrated for patients 3 and 5 (Fig. 5). Responses to a peptide derived from pp52 were detected early, whereas antibody responses to peptides derived from pp150 were delayed. pp28 was recognized less frequently than the pp150 and pp52 peptides were. In this way we could define a number of specific peptides, listed in Table 1, with optimal reactivity for human IgM or IgG. These peptides were used for further studies.
FIG. 5.
Overview of the development of epitope-specific IgM responses of sera from five patients with a primary HCMV infection following renal transplantation. All sera were tested by a reference virion ELISA and a control ELISA with mock-infected fibroblast extracts as control for the autoreactive IgM that frequently develops during transplant rejection periods. Ag, antigen; AA, amino acids.
Selection of optimal combinations of soluble peptides for detection of IgM or IgG.
Synthetic peptides that spanned single or multiple immunoreactive peptide regions as determined by PEPSCAN analysis were synthesized separately by automated solid-phase synthesis, purified by high-pressure liquid chromatography, and tested with panels of sera from healthy donors and allograft recipients. The most reactive peptides or combinations of peptides which could replace the reactivity of the whole protein were evaluated in various combinations to achieve maximal reactivity for particular patient populations or antibody classes. A summary of the results is presented in Table 2. For the optimal detection of HCMV-reactive IgG, the combination of epitopes from pp150 (amino acids 1011 to 1048), pp28 (amino acids 130 to 160), and BSA-coupled gB (amino acids 60 to 81) was selected on the basis of their individual overall reactivities with the different panels tested (HCMV combination IgG ELISA). With these peptide combinations, HCMV IgG reactivity was analyzed with a panel of sera (n = 420) randomly selected from healthy blood donors. Of these sera, 190 were HCMV positive (45% of the entire panel), and the HCMV combination IgG ELISA detected HCMV in 188 of the 190 samples. None of the HCMV-negative samples showed any reactivity, despite the presence of antibodies to other human herpesviruses (i.e., herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus [data not shown]). For the optimal detection of HCMV-reactive IgM, peptides derived from pp150 (amino acids 1011 to 1048) and pp52 (amino acids 266 to 293) were selected as described above (HCMV combination IgM ELISA). HCMV IgM reactivity was analyzed with a panel (n = 83) of sera from renal transplant patients with active HCMV infections that were confirmed by isolation of virus from the blood. Of these, 63 serum samples (76% of the entire panel) were from patients with active HCMV primary infection, and all 63 samples had a positive IgM reaction in the combination IgM ELISA. Of the remaining 20 serum samples from patients with recurrent HCMV infection, 11 (55%) had low HCMV IgM titers by both the virion and combination IgM ELISAs (data not shown).
TABLE 2.
Overview of antibody reactivities of individual epitopes and combinations of HCMV peptide epitopes by ELISAa
| Protein (gene) | Amino acid positions | No.
of serum samples positive/total no. tested (%)
|
|
|---|---|---|---|
| IgG | IgM | ||
| pp150 (UL32) | 595–614 | 16/49 (33) | NT |
| 615–636 | 19/49 (39) | NT | |
| 595–636 | 26/49 (53) | 5/9 (55) | |
| 1011–1048 | 50/57 (88) | 6/9 (67) | |
| pp52 (UL44) | 266–293 | 15/49 (31) | 8/9 (89) |
| 295–312 | 0/49 (0) | 9/9 (100) | |
| gB (UL55) | 792–809 | 0/49 (0) | 4/9 (45) |
| 60–81 | 30/49 (61) | 3/9 (34) | |
| pp28 (UL99) | 15–45 | 24/49 (84) | 4/9 (45) |
| 130–160 | 41/49 (84) | 0/9 (0) | |
| Combination IgG | 188/190 (98.9) | NT | |
| Combination IgM | NT | 80/83 (96.4) | |
Sera from HCMV-seropositive blood donors were tested for peptides with IgG reactivity. Nine serum samples from nine transplant recipients obtained after seroconversion were tested for IgM reactivity. Positivity was an OD450 value greater than three times the standard deviation of the mean for a panel of five HCMV-negative serum samples. NT, not tested.
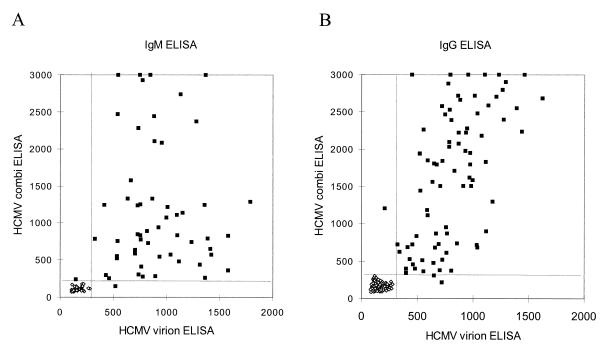
The performance of the HCMV combination IgG ELISA, in which the most reactive peptides (pp150, pp28, and gB) were used as indicated above, in defining the HCMV serologic status of healthy individuals was evaluated with 190 serum samples from HCMV-positive blood donors. The same set of sera was tested by the virion ELISA. Figure 6 shows the results of a comparison of both ELISAs. Good agreement (98.9%) regarding IgG positivity was observed. Two serum samples had discrepant results, and both of these were positive by immunoblot analysis. One of the serum samples was positive by the virion ELISA but negative by the combination ELISA, and the other serum sample was negative by the virion ELISA and positive by the combination ELISA. There was little relation between the reactivities (OD450) of the peptide-based and virion-antigen ELISAs, as indicated by the scattered distribution of the OD450 for both IgM and IgG comparisons.
FIG. 6.
Comparison of OD450 values of HCMV-peptide combination ELISAs (y axis) and HCMV virion ELISA (x axis) for the detection of anti-HCMV antibodies in the sera of renal transplant patients (IgM) (A) and healthy individuals (IgG) (B).
Similarly, 83 follow-up serum samples from 29 allograft recipients with active HCMV infection were tested for IgM reactivity by the HCMV combination IgM ELISA and the virion ELISA (Fig. 6A). The IgM combination ELISA containing only two peptides, derived from pp52 and pp150, showed a good agreement (96.4%) with the virion IgM ELISA. Follow-up serum samples from one patient were consistently negative by the combination ELISA but positive by the virion ELISA and immunoblot analysis. The reactivity (OD450) of the HCMV combination IgG ELISA generally was higher than that of the virion ELISA for the same serum sample, most importantly resulting in an improved signal-to-background ratio, which allowed a better discrimination of weakly seropositive specimens.
DISCUSSION
HCMV encodes a broad range of proteins, which are exposed to the host immune system during active viral infection. Depending on the immunogenicity of the individual HCMV proteins that are expressed and the strength and specific reactivity of the host response, detectable antibody levels will appear in the serum, allowing serologic detection. Serology is still widely used to define HCMV immune status and to support the diagnosis of active infection determined by more direct methods such as virus isolation, PCR, nucleic acid sequence-based amplification, or antigenemia assay. Progression to the use of more defined (standardized) serologic tools requires the precise definition of the HCMV proteins and their epitopes that are recognized by human IgM and IgG at different stages of HCMV infection. Previous studies have used RIPA and immunoblotting to characterize immunodominant polypeptides of purified HCMV virions (10, 21, 32). In this study we initially used this approach to confirm the nature of the most immunoreactive HCMV-encoded proteins recognized by sera from both healthy individuals and renal allograft recipients. In contrast to previous studies, we used whole-cell extracts for immunoblot analysis in order to allow identification of all possible HCMV antigenic polypeptides, not just structural virion proteins.
Our results indicate that the IgG response in healthy HCMV carriers is characterized by the recognition of proteins pp150, pp65, pp52, pp38, and pp28 (Fig. 1), corresponding to the HCMV genes UL32, UL83, UL44, UL80a, and UL99, respectively, confirming the results of other investigators (10, 13, 33). Furthermore, we identified some additional immunodominant proteins of 200, 80, 45, and 30 kDa that were reactive with 40 to 80% of the sera but for which we could not define the potential reading frame on the HCMV genome.
The immune responses of transplant recipients show IgM and IgG reactivities to the same set of HCMV proteins, pp150, pp65, pp52, and pp38 (Fig. 2), whereas only an IgG response to pp28 could be detected. The IgM and IgG antibodies to these proteins in follow-up sera from these patients had different kinetics, reflecting the diversity of immune responses at the molecular level. Recognition of immunodominant protein pp150 is delayed in the primary response to HCMV, which is in agreement with the data of Landini (12). On the other hand, pp52 is the earliest recognized HCMV protein in terms of both the IgM and the IgG responses of most patients analyzed. This immunoblot analysis allowed us to define a number of immunoreactive polypeptides which contain nonconformational epitopes that strongly interact with human IgM and IgG antibodies. Such polypeptides are obvious candidates for more detailed epitope mapping by the PEPSCAN approach. Our PEPSCAN analysis of pp150 revealed epitopes similar to those described by Landini (11). Data from PEPSCAN analysis of both pp28 and pp52 showed two clear epitope regions for each protein that have not been described before. PEPSCAN analysis of pp65 did not reveal a common dominant epitope, confirming previous data (22) that suggested that pp65 is mostly recognized via conformational epitopes.
Comparison of the immune response to individual peptides derived from PEPSCAN analysis shows a recognition frequency similar to that for the whole protein in the various serum samples studied, confirming that immunoblotting detects antibody binding predominantly mediated by nonconformational epitopes. This was also suggested by the highly similar results of immunoblotting and RIPA (data not shown). Comprehensive evaluation of the immunoreactivities of individual peptides alone and in various combinations with human IgM and IgG allowed the definition of combinations of peptides with optimal diagnostic performance in detecting acute-phase seroconversion (IgM) and immune status (IgG) (Table 2).
The synthetic peptides that provided highly dominant immunoreactive epitopes were not necessarily the same for IgM and IgG antibodies, not even within the same patient. In many instances synthetic peptides gave significantly better responses than intact proteins or complex antigen extracts, resulting in improved serologic performance. This may be due to the improved accessibility of defined peptide epitopes in comparison to those of native or even recombinant (fragments of) HCMV proteins.
Due to the highly defined immunochemical character of the selected HCMV peptides, it was not necessary to use control assays for analysis of autoreactive antibodies in sera from patients with transplant rejection or autoimmune diseases (Fig. 5). In sera from HCMV-negative donors who were seropositive for other human herpesviruses (herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus), no false-positive cross-reactivity was detected with the selected peptide combinations. These findings illustrate the high degrees of specificity of defined peptide reagents for serologic analysis.
Individual patients may develop antibody responses to different epitopes with different kinetics and strengths after the onset of infection, as indicated by the data presented in Fig. 4 and 5, stressing the importance of using a mixture of different epitopes for specific antibody detection. Therefore, combinations of peptides had to be selected to cover all antibody reactivities, as was previously found for recombinant antigen fragments (14, 33).
Specific combinations of peptides for use in the serodiagnosis of IgM and IgG reactivities were compared in an ELISA format with the total viral lysate. The best combination of peptides for use in the detection of HCMV IgG (HCMV combination IgG ELISA) consisted of peptides derived from three HCMV proteins, pp150 (UL32), gB (UL55), and pp28 (UL99), and had a sensitivity of 98.9% (Fig. 6). The combination IgM ELISA contained only peptides from pp150 (UL32) and pp52 (UL44) and had a sensitivity of 96.4% relative to the results of the ELISA with the virion lysate. The sensitivity of the current peptide ELISA for the detection of HCMV antibodies, however, is still not 100%. Therefore, the possibility of using additional markers must be considered. These markers may be derived from known immunoreactive proteins, like pp38 encoded by UL80a, or from the immunodominant proteins at 200, 80, 45, and 30 kDa, for which no reading frame has yet been defined.
In conclusion, we have described in detail the interaction of human IgM and IgG antibodies with defined immunodominant polypeptides of HCMV. This allowed the selection and synthesis of highly defined, individual peptide reagents which proved to be well suited as replacements for complex viral lysates as antigens in ELISAs. The availability of highly defined HCMV reagents, which are relatively cheap compared to the cost of cell culture-derived antigens, is important for further standardization of serology and will contribute to the more accurate serodiagnosis of HCMV infection and determination of HCMV immune status.
ACKNOWLEDGMENTS
We thank Wouter Puijk and Evert van Dijk for the PEPSCAN analysis.
REFERENCES
- 1.Blok M J, Goossens V J, Vanherle S J, Top B, Tacken N, Middeldorp J M, Christiaans M H, van Hooff J P, Bruggeman C A. Diagnostic value of monitoring human cytomegalovirus late pp67 mRNA expression in renal-allograft recipients by nucleic acid sequence-based amplification. J Clin Microbiol. 1998;36:1341–1346. doi: 10.1128/jcm.36.5.1341-1346.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fields G B, Noble R L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 3.Geysen H M, Meloen R H, Barteling S J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci USA. 1984;81:3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geysen H M, Rodda S J, Mason T J, Tribbick G, Schoofs P G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987;102:259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- 5.Gleaves C A, Smits T F, Shuster E A, Pearson G R. Comparison of standard tube and shell vial culture techniques for the detection of cytomegalovirus in clinical specimens. J Clin Microbiol. 1985;21:217–221. doi: 10.1128/jcm.21.2.217-221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gozlan J, Laporte J P, Lesage S, Labopin M, Majman A, Gorin N C, Petit J C. Monitoring of cytomegalovirus infection and disease in bone marrow recipients by reverse transcription-PCR and comparison with PCR and blood and urine cultures. J Clin Microbiol. 1996;34:2085–2088. doi: 10.1128/jcm.34.9.2085-2088.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes K, Alford C, Britt W. Antibody response to virus-encoded proteins after cytomegalovirus mononucleosis. J Infect Dis. 1987;156:615–621. doi: 10.1093/infdis/156.4.615. [DOI] [PubMed] [Google Scholar]
- 8.Ho M. Cytomegalovirus. New York, N.Y: Plenum Medical Book Company; 1990. [Google Scholar]
- 9.Landini M P, Rossier E, Schmitz H. Antibodies to human cytomegalovirus structural polypeptides during primary infection. J Virol Methods. 1988;22:309–317. doi: 10.1016/0166-0934(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 10.Landini M P, Guan M X, Jahn G, Lindenmaier W, Mach M, Ripalti A, Necker A, Lazzarotto T, Plachter B. Large-scale screening of human sera with cytomegalovirus recombinant antigens. J Clin Microbiol. 1990;28:1375–1379. doi: 10.1128/jcm.28.6.1375-1379.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landini M P, Ripalti A, Sra K, Pouletty P. Human cytomegalovirus structural proteins: immune reaction against pp150 peptides. J Clin Microbiol. 1991;29:1868–1872. doi: 10.1128/jcm.29.9.1868-1872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landini M P. New approaches and perspectives in cytomegalovirus diagnosis. Prog Med Virol. 1993;40:157–177. [PubMed] [Google Scholar]
- 13.Landini M P, Lazzarotto T, Maine G T, Ripalti A, Flanders R. Recombinant mono- and polyantigens to detect cytomegalovirus-specific immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol. 1995;33:2535–2542. doi: 10.1128/jcm.33.10.2535-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer H, Bankier A T, Landini M P, Brown C M, Barrell B G, Ruger B, Mach M. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988;62:2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer H, Masuho Y, Mach M. The gp58/116 complex of the human cytomegalovirus represents the aminoterminal part of the precursor molecule and contains a neutralizing epitope. J Gen Virol. 1990;71:2443–2450. doi: 10.1099/0022-1317-71-10-2443. [DOI] [PubMed] [Google Scholar]
- 16.Middeldorp J M, Jongsma J, Ter Haar A, Schirm J, The T H. Detection of immunoglobulin M and G antibodies against cytomegalovirus early and late antigens by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984;20:763–771. doi: 10.1128/jcm.20.4.763-771.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middeldorp J M, Meloen R H. Epitope-mapping on the Epstein-Barr virus major capsid protein using systematic synthesis of overlapping oligopeptides. J Virol Methods. 1988;21:147–159. doi: 10.1016/0166-0934(88)90061-4. [DOI] [PubMed] [Google Scholar]
- 18.Mocarski E S, Pereira I, Michael N. Precise localization of genes on large animal virus genomes: use of λgt11 and monoclonal antibodies to map the gene for a cytomegalovirus protein family. Proc Natl Acad Sci USA. 1985;82:1266–1270. doi: 10.1073/pnas.82.4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris R E, Saelinger C B. A simple reliable method for producing electron dense markers of uniform size for use in immunoelectron microscopy. J Immunol Methods. 1982;49:237–246. doi: 10.1016/0022-1759(82)90124-7. [DOI] [PubMed] [Google Scholar]
- 20.Novak J, Sova P, Krchnak V, Hamsikova E, Zavadova H, Roubal J. Mapping of serologically relevant regions of human cytomegalovirus phosphoprotein pp150 using synthetic peptides. J Gen Virol. 1991;72:1409–1413. doi: 10.1099/0022-1317-72-6-1409. [DOI] [PubMed] [Google Scholar]
- 21.Nowak B, Sullivan C, Sarnow P, Thomas R, Bricout F, Nicolas J C, Fleckenstein B, Levine A J. Characterization of monoclonal antibodies and polyclonal immune sera directed against human cytomegalovirus virion proteins. Virology. 1984;132:325–338. doi: 10.1016/0042-6822(84)90039-4. [DOI] [PubMed] [Google Scholar]
- 22.Ohlin M, Plachter B, Sundqvist V A, Steenbakkers P G, Middeldorp J M, Borreback C A. Human antibody reactivity against the lower matrix protein (pp65) produced by cytomegalovirus. Clin Diagn Lab Immunol. 1995;2:325–329. doi: 10.1128/cdli.2.3.325-329.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plachter B, Wiecdzorek L, Scholl B C, Ziegelmaier R, Jahn G. Detection of cytomegalovirus antibodies by an enzyme-linked immunosorbent assay using recombinant polypeptides of the large phosphorylated tegument protein pp150. J Clin Microbiol. 1992;30:201–206. doi: 10.1128/jcm.30.1.201-206.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen L, Matkin C, Spaete R, Pachl C, Merigan T C. Antibody response to human cytomegalovirus glycoprotein gB and gH after natural infection in humans. J Infect Dis. 1991;164:835–842. doi: 10.1093/infdis/164.5.835. [DOI] [PubMed] [Google Scholar]
- 25.Revello M G, Percivalle E, Gerna G. Immunoglobulin M to the membrane of uninfected fibroblasts in primary human cytomegalovirus infections. Microbiology. 1986;9:127–138. [PubMed] [Google Scholar]
- 26.Severi B, Landini M P, Govoni E. Human cytomegalovirus morphogenesis: an ultrastructural study of the late cytoplasmic phases. Arch Virol. 1988;98:51–64. doi: 10.1007/BF01321005. [DOI] [PubMed] [Google Scholar]
- 27.Spaete R R, Gehrz R, Landini M P. Human cytomegalovirus structural proteins. J Gen Virol. 1994;75:3287–3308. doi: 10.1099/0022-1317-75-12-3287. [DOI] [PubMed] [Google Scholar]
- 28.Spector S A, Wong R, Hsia K, Pilcher M, Stempien M J. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest. 1998;101:497–502. doi: 10.1172/JCI1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The T H, Van den Berg A P, Harmsen M C, Van der Beij W, Van Son W J. The cytomegalovirus antigenemia assay: a plea for standardisation. Scand J Infect Dis Suppl. 1995;99:25–29. [PubMed] [Google Scholar]
- 30.Urban M, Winkler T, Landini M P, Britt W, Mach M. Epitope-specific distribution of IgG subclasses against antigenic domains on glycoproteins of human cytomegalovirus. J Infect Dis. 1994;169:83–90. doi: 10.1093/infdis/169.1.83. [DOI] [PubMed] [Google Scholar]
- 31.Van Loon A M, Heessen F W A, Van der Loght J T M, Van der Veen J. Direct enzyme-linked immunosorbent assay that uses peroxidase-labeled antigen for determination of immunoglobulin M antibody to cytomegalovirus. J Clin Microbiol. 1981;13:416–422. doi: 10.1128/jcm.13.3.416-422.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Zanten J, Van der Giessen M, Van Son W J, The T H. Antibody responses to human cytomegalovirus-specific polypeptides studied by immunoblotting in relation to viral load during cytomegalovirus infection. J Med Virol. 1993;39:80–87. doi: 10.1002/jmv.1890390115. [DOI] [PubMed] [Google Scholar]
- 33.Vornhagen R, Plachter B, Hinderer W, The T H, Van Zanten J, Matter L, Schmidt C A, Sonneborn H H, Jahn G. Early serodiagnosis of acute human cytomegalovirus infection by enzyme-linked immunosorbent assay using recombinant antigens. J Clin Microbiol. 1994;32:981–986. doi: 10.1128/jcm.32.4.981-986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]