Summary
The rapid propagation of novel human coronavirus 2019 and its emergence as a pandemic raising morbidity calls for taking more appropriate measures for rapid improvement of present diagnostic techniques which are time‐consuming, labour‐intensive and non‐portable. In this scenario, biosensors can be considered as a means to outmatch customary techniques and deliver point‐of‐care diagnostics for many diseases in a much better way owing to their speed, cost‐effectiveness, accuracy, sensitivity and selectivity. Besides this, these biosensors have been aptly used to detect a wide spectrum of viruses thus facilitating timely delivery of correct therapy. The present review is an attempt to analyse such different kinds of biosensors that have been implemented for virus detection. Recently, the field of nanotechnology has given a great push to diagnostic techniques by the development of smart and miniaturised nanobiosensors which have enhanced the diagnostic procedure and taken it to a new level. The portability, hardiness and affordability of nanobiosensor make them an apt diagnostic agent for different kinds of viruses including SARS‐CoV‐2. The role of such novel nanobiosensors in the diagnosis of SARS‐CoV‐2 has also been addressed comprehensively in the present review. Along with this, the challenges and future position of developing such ultrasensitive nanobiosensors which should be taken into consideration before declaring these nano‐weapons as the ideal futuristic gold standard of diagnosis has also been accounted for here.
Keywords: biosensors, Covid‐19, nanobiosensors, nanotechnology, virus
Abbreviations
- 2D
two dimensional
- 4,4′‐DAAB 4,4′
diaminoazobenzene
- ACE 2
angiotensin‐converting enzyme 2
- AgNCs
silver nanoclusters
- AgNPs
silver nanoparticles
- AIDS
acquired immune deficiency syndrome
- AuNIs
gold nanoislands
- AuNPs
gold nanoparticles
- CDC
Centers for Disease Control and Prevention
- CNTs
carbon nanotubes
- Covid‐19
coronavirus disease of 2019
- CRISPR
clustered regularly interspaced short palindromic repeats
- CT
computed tomography
- DETECTR
DNA endonuclease‐targeted CRISPR trans reporter (DETECTR) technology
- DNA
deoxyribonucleic acid
- E
envelope protein
- EC
electrochemical
- EIS
electrochemical impedance spectroscopy
- ELISA
enzyme‐linked immunosorbent assays
- ER
endoplasmic reticulum
- EU
European Union
- EUA
emergency use authorisation
- F1ab
opening reading frame 1a/b
- FDA
Food and Drug Administration
- FEB
field effect biosensing
- FET
field‐effect transistor
- FRET
fluorescence resonance energy transfer
- FTO
fluorine‐doped tin oxide electrode
- GCE
glassy carbon electrode
- GO
graphene oxide
- H1N1
hemagglutinin type 1 and neuraminidase type 1 – ‘Influenza’ A (H1N1) virus
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV DNAs
human immunodeficiency virus oligonucleotides
- HIV
human immunodeficiency virus
- HIV‐1
human immunodeficiency virus‐1
- HPV
human papilloma virus
- HSV‐2
herpes simplex virus type 2
- HTLV‐1
human T‐cell lymphotropic virus‐1
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- Kb
kilobyte
- LFIA
lateral‐flow immunochromatographic assay
- LFICS
lateral flow immunochromatographic strips
- LSPR
localised surface plasmon resonance
- M
membrane glycoprotein
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- MWNT
multiwalled carbon nanotube
- N
nucleocapsid protein
- NAATs
nucleic acid amplification tests
- ORFs
open reading frames
- PCR
polymerase chain reaction
- PEP‐PDA
peptide‐functionalised polydiacetylene
- pfu/mL
plague forming units/mL
- pH
potential of hydrogen
- pM
picomolar
- POC
point of care
- PPT
plasmonic photothermal
- QDs
quantum dots
- QDs‐DNA
quantum dots‐DNA
- RNA
ribonucleic acid
- rRT‐PCR
real‐time reverse‐transcription polymerase chain reaction
- RSV
respiratory syncytial virus
- RT‐LAMP
reverse transcription‐loop‐mediated isothermal amplification
- RT‐PCR
reverse transcription polymerase chain reaction
- S
spike glycoprotein
- SARS‐CoV
severe acute respiratory syndrome‐coronavirus
- SARS‐CoV‐2 VOC 202012/01
variant of concern, the year 2020, month 12, variant 01
- SARS‐CoV‐2
severe acute respiratory syndrome‐coronavirus‐2
- SEF
surface‐enhanced fluorescence
- SEIRA
surface‐enhanced infrared absorption spectroscopy
- SERS
surface‐enhanced Raman spectroscopy
- SPR
surface plasmon resonance
- ssDNA
single‐stranded DNA
- SWCNTs
single‐walled carbon nanotube
- TiO2
titanium dioxide
- US
United States
- WHO
World Health Organization
- WNV
West Nile virus
- ZKV
Zika virus
- ZnO
zinc oxide
1. INTRODUCTION
It is becoming evident day by day that while some viruses like human immunodeficiency virus (HIV) and hepatitis are killing millions of people some emergent viruses like severe acute respiratory syndrome‐coronavirus (SARS‐CoV) in 2002–2003, swine influenza A (H1N1) in 2009, Ebola haemorrhagic fever eruption in 2014 and the most recent SARS‐CoV‐2 (Covid‐19) are becoming a global menace resulting in pandemics. 1 From the wide palate of viruses posing a threat to mankind, respiratory viruses are the deadliest affecting infants, children, elderly people, immunocompromised or patients with co‐morbidity. 2 Despite significant progress in the prevention, diagnosis and treatment over the past 100 years, the recovery rate for these viral diseases is far from satisfactory where over 95% of these deaths are due to the deficiency of correct diagnosis and treatment. Concerning treatment, major hindrances include imprudent use of antimicrobials, the emergence of multi‐drug resistance in pathogens, the appearance of novel variants of pathogenic viruses and so forth. 3 Similarly, traditional diagnostic methods such as microscopy, immunology and the polymerase chain reaction (PCR) approaches although effective are plagued by issues like inaccuracy and ineptness. 2 Although both diagnosis and treatment go hand in hand, early prognosis gives the treatment of these infections an upper edge, so the development of rapid and sensitive identification methods is paramount for better dissemination of therapeutics in these challenging times. 4 , 5 In the current scenario, the main strategy to control the Covid‐19 pandemic depends on testing and diagnosis of the disease at early stages. Reverse transcription PCR (RT‐PCR) testing remains the primary method for diagnosing SARS‐CoV‐2 although some clinicians may utilise chest computed tomography (CT) scans as a more reliable way to assess the stage of the disease. 6 However, while the former is quite a time taking process the latter cannot detect the early onset of the disease. Besides, CT scans cannot distinguish between Covid‐19 and viral pneumonia patients. It can be said that the present diagnostic modalities are the rate‐limiting factors in terms of the number of persons that can be tested. Thus, for better management of Covid‐19, more reliable, sensitive and quick diagnostic methods are paramount.
Biosensors are deductive devices where biological identification entities such as enzymes, antibodies or nucleic acids are bound with another component called transducer and detector which identify the analyte and give a digitised output. 7 Viral biosensors offer an elating secondary means to conventional diagnostic analyses owing to their pocket‐friendly, delicate, speedy, miniaturised and portable nature, which are critical parameters of efficient diagnostic agents. 8 In this regard, electrochemical (EC)‐based DNA‐sensing biosensors were successfully employed for diagnosing the Ebola virus while a paper‐based biosensor was used for the identification of the chikungunya virus. 9 , 10 Recently, the field of nanotechnology has given a great push to imaging and diagnosis technologies. In this context, comprehending the biosensing concept is the basic groundwork required for developing nanobiosensors for uninterrupted monitoring of human wellness. Several nanomaterials like nanowires, nanorods, nanoparticles and thin films made up of nanocrystalline matter have been preferably used in nanobiosensors owing to their superlative electronic and mechanical properties which help in reinforcing better biological signalling and transduction mechanisms. 11 High sensitivity and better amplification of signals owing to the intrinsic properties of nanomaterials like tunnelling and quantum effects, the high surface‐to‐volume ratio for the better surface area makes these nanobiosensors unique for point‐of‐care tests. 12 The portability, hardiness and affordability of nanobiosensor make them an apt diagnostic agent for the prognosis of a wide array of diseases like cancer, diabetes, malaria, HIV and bilharzia. Recently, a membrane‐based EC sensitive nanobiosensor was designed for the detection of West Nile viral particles. 13
It is already known that nanobiosensor‐based approaches provide a more meticulous and targeted method for the detection of the virus. In the current scenario of Covid‐19 infection, EC‐based biosensors, surface‐enhanced Raman spectroscopy (SERS)‐based biosensors, field‐effect transistor (FET)‐based biosensors and surface plasmon resonance (SPR)‐based biosensors are already being implemented for the diagnosis of Covid‐19 14 although the results are not that satisfactory. Presently, nanobiosensors for Covid‐19 infections are being considered for early diagnosis of the disease. Nanomaterials such as gold and carbon have garnered a lot of attention in this context for detecting the virus and its biomolecule. 15 These nanomaterials when attached with analyte‐like complementary single‐stranded nucleic acid aptamer could be a novel approach for sensing SARS‐CoV‐2 by identifying spike protein S1 in clinical samples. 16 The present review is an elaborate compilation of such kind of nanobiosensors which are futuristic devices which can be tailor‐made and modulated to become indispensable diagnostics tools for correct authentication of Covid‐19 or any such viral antigens with ease.
2. INCIDENCE OF VIRAL DISEASES
Infectious diseases caused by viruses have been discovered at a speedy pace lately and have been the root cause of many epidemics, endemics and pandemics for every demographic region. These obligate parasites upon entry inside a host cell utilising receptor‐ligand interaction chemistry are responsible for causing many diseases. Illustrious representation of human viruses that cause evidential health problems comprise HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV). Similarly, other viruses like rotavirus, astroviruses, Zika virus (ZKV), West Nile virus, influenza, respiratory syncytial virus cause significant damage to the organs they affect posing as great threats to human health, consequently leading to morbidity and mortality. 1
The year 2014–2016 witnessed the outbreak of Ebola in West Africa which took the world by surprise with its unexplainable magnitude and aggressiveness and raised questions about the readiness of public health systems. 17 In February 2016, the public failed to comprehend the implications of the ZKV after the World Health Organization (WHO) stated it as an international civic health emergency owing to its relationship in cases of a rare congenital condition called microcephaly in Brazil. 18 The first report of human virus infection such as yellow fever was first reported in the US army at the starting of the 20th century and found its way into Brazil as an outbreak in 2017. Another outbreak in 2018 of an unusual virus‐like the Lassa virus in Nigeria resulted in 100 deaths due to haemorrhagic fever. The transition of the ZKV, from an unknown pathogen to a deadly one was swift and quick. The western hemisphere which was devoid of this virus till 2015 reported about 800,000 cases in pen and paper. Moreover, the Asian descent of ZKV, accountable for the 2015–2016 epidemic, caused severe congenital defects in children known as congenital Zika syndrome if the women contracted it during pregnancy. 19 The Nipah virus made its first registered appearance in Malaysia in 1998 where the virus is believed to be transferred from fruit bats to domestic pigs. It is believed to be a major reason for two epidemic outbreaks that occurred in 2001 and 2007 in Bangladesh. The outbreak of this exotic virus was first reported in Kerala, India, which caused severe inflammation of the brain claiming 14 lives. 20
In the contemporary world, three extremely pathogenic influenza viruses, CoVs, SARS‐CoV‐1 the Middle East respiratory syndrome CoV (MERS‐CoV), and the recently emerged SARS‐CoV‐2 are the major viruses that have resulted in different respiratory diseases throughout the world. 21 The first influenza viruses which are believed to have come from birds and then to horses before spreading to humans was the root cause of the first pandemic in 1918 which killed around 50 million people in 2 years around the globe. Similarly, the Asian flu and the Hong Kong flu of the 1950s and 1970s killed roughly 2 million people. 22 The delayed spectre of the 1918 flu, along with more recent rounds of avian flu, had led a galore of experts to fear the emergence of another deadly strain of influenza which became true recently with the outbreak of the Covid‐19 pandemic. As the present SARS‐CoV‐2 has emerged as the deadliest virus in the past 100 years affecting millions, worldwide research presently is going on to decipher this mysterious pathogen. The focus of the entire scientific fraternity is now to find a definite therapeutic intervention and diagnostic tool which will help to rein this devastating virus. Before designing an implementing diagnostics technique for the identification of the pathogen which is the need of the hour, we need first to know about Covid‐19 which is summarised below.
3. COVID‐19 DISEASE
Unusual and unprecedented pneumonia cases emerged with an unknown cause in Wuhan city in China during December 2019. The WHO had identified it as coronavirus disease‐19 (Covid‐19) and on 13 March 2020, WHO declared the disease as a pandemic. Covid‐19 is caused by a new pathogen that is 80% similar to the genome of an already existing virus, that is, SARS‐CoV, identified in China in 2002, and 50% similar to MERS‐CoV, identified in the Middle East region in 2012. Since the new pathogen is more similar to SARS‐CoV, it is named SARS‐CoV‐2 by the International Viral Classification Commission. 23 SARS‐CoV‐2 caused serious health issues across the globe and as a consequence, it had led to a socio‐economic burden. This is, because, the virus can spread among people either with or without any symptoms. 24
3.1. Clinical presentations
WHO published that all age groups are susceptible to Covid‐19. It generally spreads through direct or indirect contact with the infected person. The droplets containing viral particles can spread while coughing or sneezing and it can be transmitted from the contaminated objects as well. RNA trace was detected in rectal swabs suggesting that the transmission is possible through fecal‐oral. 25 The clinical presentation of Covid‐19 includes fever, sore throat, cough, headache, myalgia, shortness of breath, pneumonia, conjunctivitis, decreased leukocyte count, dysfunction of some organs such as liver and respiratory failure in some cases. Besides, the symptoms observed in patients keep on changing from time to time, and with each new wave of Covid‐19, some new symptoms are detected. However, some of these symptoms are very common for flu, cold and respiratory problems and so, the diagnosis of the disease in the early stage without clinical symptoms and in the second stage with clinical symptoms is not possible without proper testing done in laboratories. Around 80%–85% of people are either with no clinical presentation or with a subclinical presentation. Mostly, the disease can work for 3–14 days without any clinical symptoms. 26
3.2. Virology
Coronaviruses are large, spherical, enveloped viruses with a positive‐sense, single‐stranded RNA genome of 27–32 kb. 27 Coronaviridae family consists of two subfamilies, that is, Coronavirinae and Torovirinae. So far, four genera of Coronavirinae have been identified which are Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus. Human coronaviruses HCoV‐229E and NL63 belong to the Alphacoronavirus genera and MERS‐CoV, SARS‐CoV, SARS‐CoV‐2, HCoV‐OC43 and HCoV‐HKU1 belong to Betacoronavirus genera.
The genome of SARS‐CoV‐2 mainly consists of 10 open reading frames (ORFs). The first ORF (ORF1a/b) covers two thirds of RNA and it is translated into two large polyproteins. The other ORFs covering one third of the RNA genome code the main structural proteins that are spike glycoprotein (S), an envelope protein (E), nucleocapsid protein (N) and membrane glycoprotein (M). Apart from these main four structural proteins, several accessory proteins are also present whose functions are not known. They do not take part in the viral replication process. Numerous scientists discovered that SARS‐CoV‐2 needs angiotensin‐converting enzyme 2 (ACE 2) as the receptor for entering the host cells, just like SARS‐CoV. For the pathogenesis of contamination, the binding of the virus to the cell receptors is significant. 28 The virus binds to the ACE 2 receptor through the spike protein, following the entry of cells through endosomes. Once inside the cell, the viral genome is released and translated into viral polyproteins. 29 The RNA and capsid protein of the virus are getting replicated and transcribed in the cytoplasm. All the other structural proteins are getting transcribed and translated in the endoplasmic reticulum and are transported to the golgi complex. They are further assembled and released from the host cells (Figure 1). 30 The natural host for SARS‐CoV‐2 are bats, however not much is known about their intermediate host and this knowledge is pressing to prevent further spread of this pandemic. In this regard, a recent study was done to anticipate the potential intermediate hosts of SARS‐CoV‐2 by assessing the composition and variations of coronavirus spike proteins and host ACE2 receptors. 31 Thus, owing to its unique features and aggressive nature, designing treatment and detection strategies of the Covid‐19 pathogen has been the dilemma of researchers worldwide.
FIGURE 1.
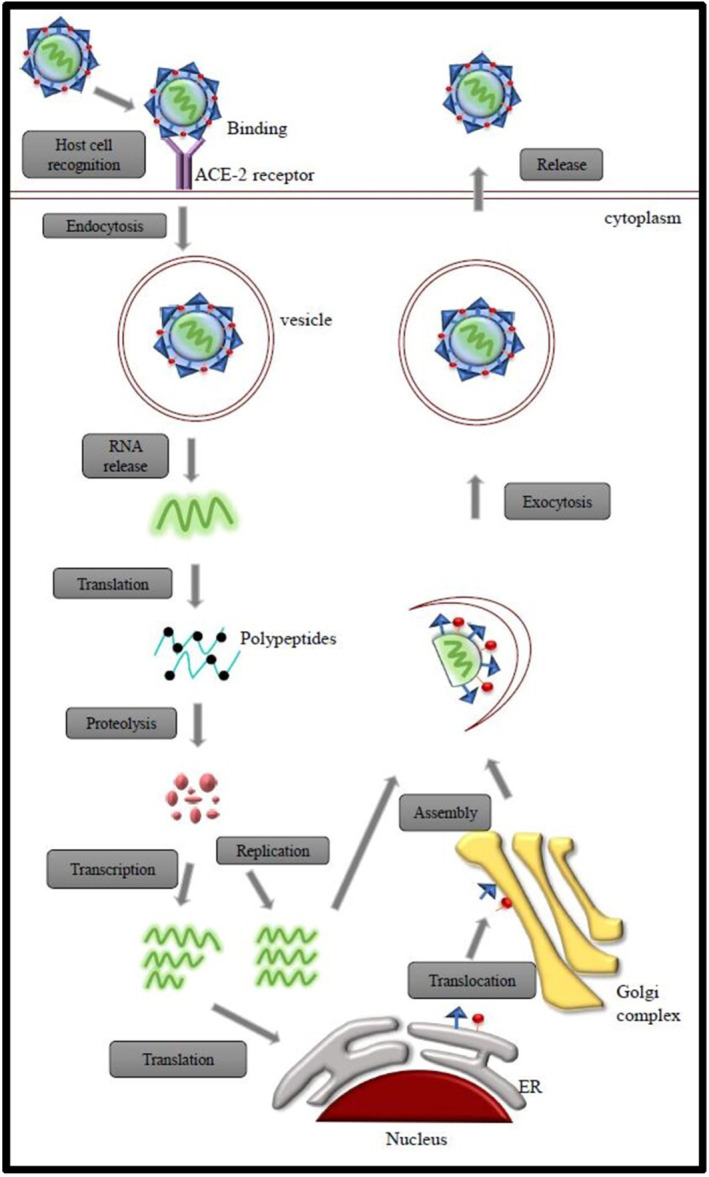
Mode of entry and life cycle of SARS‐CoV‐2 in host cells
3.3. Fight against COVID‐19: Need of the hour
Even after the identification of drugs and the development of vaccines, we cannot ensure full protection against the pathogen because of the news of the emergence of new strains of the virus. Recently, United Kingdom authorities reported to WHO on 14 December 2020, a new variant of SARS‐CoV‐2 which is referred to by them as SARS‐CoV‐2 VOC 202012/01 (Variant of concern, the year 2020, month 12, variant 01). It is not phylogenetically related to the SARS‐CoV‐2 and contains 23 nucleotide substitutions. It first appeared in South East England and within few weeks spread across many countries. The preliminary analyses have shown that the disease severity is the same as the old strain. Also, another mutant strain, that is, the VOC 202012/01 variant due to deletion in the 69/70 del position was found which has been reported in 31 different countries/areas. Another variant 501Y.V2 has been reported in three areas of South Africa and four other countries which is due to N501Y mutation. The severity of the disease is not known clearly which needs further investigation (https://www.ncbi.nlm.nih.gov/sars‐cov‐2/).
The success of any therapy relies on a fast diagnosis of the disease. Owing to its unique features and aggressive nature, before designing treatment strategies, early detection of Covid‐19 pathogen and identifying the strain of the virus which is causing the disease is paramount. Conventional detection methods like RT‐PCR and CT scans are considered as standard measures for SARS‐CoV‐2 detection; however, both have their shortcomings. Researchers are coming up with more advanced RT‐PCR techniques which can be helpful for pathogen detection. Recently, Park et al. and Yu et al. have devised a reverse transcription loop‐mediated isothermal amplification (RT‐LAMP) assay for detecting genomic RNA of SARS‐CoV‐2. The authors were capable of devising a modified RT‐PCR technique that was very sensitive and was immune to cross‐reactivity. 32 , 33 Similarly, Lin et al. upgraded the above technique by coupling RT‐LAMP with a pH indicator to allow easy readout of the amplification reaction demonstrated by a change in colour. 34 Moreover, RT‐LAMP/Cas12 DETECTR fluorescent assay was developed which was able to detect SARS‐CoV‐2 from respiratory swab RNA extracts within 40 min. This system was as efficient and sensitive and can be considered equivalent to the US FDA EUA‐approved CDC assay for the detection of SARS‐CoV‐2. 35
Furthermore, the diagnosis of SARS‐CoV‐2 is carried by using many other techniques. It can either be direct identification by detecting viral RNA or antigens or indirect identification by detecting antibody responses. The former is reasoned as the gold standard technique for detecting active infection while the latter is the cornerstone for the detection of the previous contact with the virus. 36 Most of the testing methods used for the diagnosis of SARS‐CoV‐2 are listed out in Table 1.
TABLE 1.
List of available diagnostic tools for detection of Covid‐19 disease
| Molecular testing | Antigen testing | Antibody testing | |
|---|---|---|---|
| Testing methods | Nucleic acid amplification tests – real‐time reverse‐transcription polymerase chain reaction, isothermal nucleic acid amplification – CRISPR‐based assay | Rapid diagnostic immunoassays | POC (disposable immunochromatographic lateral flow assays), to enzyme‐linked immunosorbent assays or chemiluminescent immunoassays |
| Mechanism of testing | Detection of specific target viral genes and regions | Detection of the presence of viral particles | Detection of antibodies, especially immunoglobulin G, immunoglobulin M and immunoglobulin A, that are specific for SARS‐CoV‐2 antigens |
| Specimen | Nasal or throat swabs | Nasal or throat swabs | Plasma, serum or whole blood |
| Diagnosis period | First 5 days after symptoms onset | Less than 5–7 days after symptoms | First 1 week after symptoms onset |
| Time required for getting results | Within 1–4 h or up to a week | Within an hour | Within 1–3 days of testing |
| Diagnosis of stage of infection | Active infection | Past infection | Active infection |
| Approval status | FDA approved | EU approved | FDA approved |
| Accuracy | High | The only accuracy of positive results is high | Needs another antibody testing for improved accuracy |
| Sensitivity | High | Moderate | High |
| Specificity | High | High | High |
| Point of care testing | Only a few are available | Available | Available |
| Cost | Moderate | Low | Low |
| Advantages | Most accurate for active infection | Time and cost‐effective | Identification of people with immunity whose antibodies can be used to treat Covid‐19 patients |
| Limitations | Cannot determine the past infection | Might require a molecular test to confirm negative antigen results | Cannot determine the present infection and possibility of false‐positive results |
Despite all the above‐mentioned techniques, what the need of the hour is to devise diagnostic methods which are portable, sensitive, pocket friendly and do not require skilled technicians or extensive laboratory facilities. In this area, innovative methods like biosensors can be enforced to improve prognosis and can decide the outcome of the treatment.
4. BIOSENSORS IN VIRAL DISEASE DETECTION
The first biosensor was created in 1956 although the biosensors concept came in 1903. 37 Biosensors are devices that involve in transforming biological responses into electrical signals. The purpose of a biosensor is to detect or sense a specific biological material which may be proteins, antibodies, immunological molecules, enzymes ans so forth. 38 Biosensors are user‐friendly to operate and provide exceptional performance, rapid response, high specificity and sensitivity, compact size, portability and real‐time analysis. Since the 1980s, the number of publications regarding biosensors has come into the limelight which has increased exponentially and today, for example, over 55,000 related items on the PubMed database can be found on this topic. 39
Biosensors are used in detecting various biological materials like nucleic acids, proteins, cancer biomarkers, explosives, microorganisms like bacteria, viruses. It is also used in food processing for the detection of toxins, environmental screening, diagnosis in the clinical field and bioterrorism. Researchers are focusing on fabrication quality and biosensor development, nowadays, to enhance the sensitivity and specificity of various techniques. Also, to enhance them, researchers are expanding affinity between creative surface chemistries and using nanomaterials like nanoparticles, nanofilm or quantum dot (QD) for the amplification of signal. 40 , 41 , 42 , 43
The biosensor consists of three components, namely bioreceptor, transducer and detector (Figure 2). Bioreceptor acts as a template to detect the specific target which is usually a biologically sensitive material. Various materials are used as bioreceptors and these are selected based on the target like antibody which is used for detecting antigen and enzymes are used for screening substrates. Bioreceptors are immobilised onto the transducer so that the interaction of receptor and target is more efficient. The transducer is the second component and the word trans means change and the word ducer means energy. As the name suggests, it converts the interaction of the target or bioanalyte with the bioreceptor into an electrical signal. The third component is the detector system which receives the electrical signal from the second component and amplifies it to read and study the response. 11
FIGURE 2.
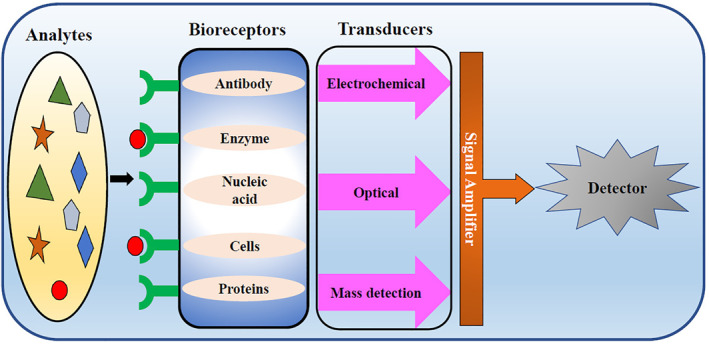
Schematic representation of a biosensor
Many types of biosensors are used for various purposes. According to the mechanism of transduction, biosensors are classified as EC, optical and piezoelectric biosensors. EC biosensors are the best choice in many clinical applications. They are mainly employed for the detection of nucleic acids, proteins and cancer biomarkers. They are classified into potentiometric, amperometric, cyclic voltammetry and impedimetric transducers. Optical biosensors are involved in measuring the change in optical characteristics after interaction of receptor and target. They may be direct or indirect optical biosensors. The former focuses on the measurement of change on the transducer surface while the latter is designed with labels to screen the binding events. Piezoelectric biosensors are used in detecting hormones, cells, bacteria and so on. It transforms either mass or thickness of materials into an electrical output. 43 Similarly, plasmonic‐based biosensors owing to their rapid sampling, broad linear range, high sensitivity and high selectivity are promising tools for virus detection. Sensing based on plasmonic platforms such as SPR, localised SPR (LSPR), SEF, SERS and SEIRA has broadened the application of such biosensors for disease detection. 44
Based on the interaction of bioreceptor and analyte, it is classified as affinity sensor which involves the detection event, metabolism sensor involving chemical change after the interaction and catalytic sensor that employs the conversion of an auxiliary substrate and then, the signal is produced. 45 According to the type of biosensors used, biosensors are classified as immunosensors, enzymatic biosensors, DNA aptamer biosensors, peptide‐based biosensors and whole‐cell biosensors. 46
A wide array of viruses can be easily detected using these biosensors. According to statistics, there are many articles under the topic ‘biosensor and virus’ published on the Web of Science. In 2000, there were 57 publications related to this topic while in 2005 their number increased to 117. Similarly in 2010 around 200 publications focused on this area of detection of viruses. 47 Castillo‐Henriquez et al. surveyed to analyse the success of biosensors for virus detection and compiled data that highlighted the use of biosensors in terms of bacterial and viral detection from 223 papers published between 2010 and 2020. 48 Some of the works of researchers regarding virus detection by biosensors lately are enumerated here. Lu et al. developed a biosensor that could assess the degree of AIDS progression by monitoring Gp4 protein. 49 Similarly, Shafifiee et al. devised an optical biosensor that could detect HIV‐1 virus from biological samples. 50 The use of a biosensor in the detection of hepatitis B was investigated by Tam et al. by using an SPR biosensor. 51 Kaushik et al. fabricated an EC biosensor for ZKV protein sensing. 52 Riedel et al. innovated a new biosensor platform based on the SPR system for the identification of Epstein–Barr virus at varied stages in clinical samples. 53 Successful detection of Japanese encephalitis virus using molecularly imprinted polymer‐based biosensor was studied by Feng et al. 54 The use of biosensors for detecting human respiratory viruses is not a new concept where EC, optical and thermal biosensors are used for the detection of influenza A, MERS and SARS‐CoV, respectively. 14 Thus, the use of this biosensor technology can be further expanded and could be aptly used for the detection of other viruses also.
5. NANOBIOSENSORS: RECENT PROGRESS IN RAPID DETECTION
Biosensors have been in use for almost 4 decades. For its further improvisation, researchers are now focusing on nanoscience and technology. 55 Work regarding the same has already been initiated where nanomaterials are employed for the construction of biosensors (known as nanobiosensors) mainly for the diagnosis of nucleic acids like RNA or DNA with high sensitivity and selectivity. Thus, nanobiosensors are defined as sensors that are made up of nanomaterials (Figure 3). The use of nanomaterials in biosensors has garnered attention owing to its unique EC, mechanical, optical, catalytic, biological, magnetic and surface properties. 24 Although nanobiosensors work on a similar principle as that of biosensors, however, in the former, the transducers are constructed by combining with nanomaterials. 56 Nanobiosensors have various advantages over conventional methods like they are reusable and very fast, suitable for mass production with less power consumption, placement of enzymes and so on. 26 Moreover, the use of nano‐sized components in nanobiosensors increases their sensitivity which makes it possible to engineer smaller sized sensors that can be used to minimise the laboratory space necessities. Finally, construction expenses of these nanobiosensors can be reduced by employing advanced technology and this can, in turn, reduce clinical healthcare costs for the detection and diagnosis of different diseases.
FIGURE 3.
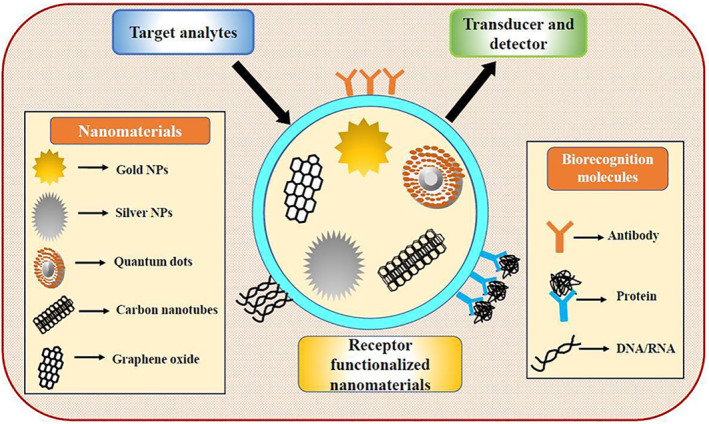
Schematic representation of a nanobiosensor
These enormous advantages of nanobiosensors make them more attractive technology for the improvement in disease detection. Nanobiosensors can aid in the early detection of HIV‐1 and human T‐cell lymphotropic virus‐1 (HTLV‐1). Such nanobiosensors‐based diagnoses are more accurate, rapid, sensitive and cost‐effective. Genosensors were designed with nanomaterials recently for the diagnosis of HIV‐1 and HTLV‐1 based on EC and optical methods, enabling improvements in clinical therapy and preventing virus propagation. 57 For Ebola virus detection, a novel DNA biosensor based on an EC method was fabricated. It was fabricated by altering the gold electrode and single‐strand DNA (ssDNA) was used as a capture probe and hybridised with biotinylated target nucleic acids. The hybridisation is measured using EC detection. This method can also be employed for detecting the microbes in environmental pollution. 9 A peptide‐functionalised polydiacetylene nanosensor was developed for the diagnosis of the H1N1 virus. This is prepared by the nano‐precipitation method. It showed unique chromatic properties. The colour change from blue to red is noted. This nanobiosensor can be applied for developing commercially available kits in the diagnosis of H1N1 virus. 58 A DNA biosensor based on gold nanotubes in label‐free detection was materialised for detecting the human papilloma virus. By the electrodeposition method, gold nanotubes decorated nanoporous polycarbonate was fabricated and used as a template. The ssDNA probe was immobilised on the electrode and hybridisation of target sequences with this probe can be observed by electrochemical impedance spectroscopy (EIS). It is rapid, stable and reproducible. 59
5.1. Types of nanobiosensors
Different types of nanomaterials are used in biosensors in recent years to increase their selectivity and accuracy. Some of the important types of nanobiosensors are discussed in the following sections.
5.1.1. Carbon‐based nanomaterials
Carbon nanotubes
Spherical carbon nanoparticles are used in medicine because of their simple geometry, uniform surface chemistry, non‐immunogenicity and biocompatibility. However, their importance is low because of fewer biomedical applications. 60 In this context, carbon in the form of carbon nanotubes (CNTs) is more explored in nanobiosensors since 1991 owing to its high electrical conductivity, chemical stability, high surface area, fast heterogeneous and long‐range electron transfer, excellent biocompatibility and mechanical strength. 61 CNTs are widely used in the last decade for their advantages like high surface area, fast heterogeneous and long‐range electron transfer. These CNTs electrostatically adsorb nucleic acids or other biomolecules and attach them to functional groups on the modified CNTs and thus help in rapid and sensitive label‐free bioelectronic detection.
For viral disease detection, modified CNT‐based electrodes containing metallic nanoparticles like gold nanoparticles (AuNPs) were prepared. To deposit metallic nanoparticles on a single‐walled CNT (SWCNTs), electrical impedance was used. The nucleic acid of a particular virus can be captured by probe DNA and immobilised on SWCNTs/AuNPs. This helps in the diagnosis of diseases in the early stages. 12
Wiriyachaiporn et al. used carbon nanotag‐based lateral flow assay for the detection of influenza A virus. Carbon nanoparticles act as receptors in the form of nanostrings and the different strains of the influenza virus can be detected under optimal conditions. 62 Another study was reported by Li et al. in which 4,4′‐diaminoazobenzene and multiwalled carbon nanotube‐modified glassy carbon electrode was used to detect HBV using EC approach. 63
Graphene‐based nanobiosensors
This is another well‐known carbon‐based nanomaterial that has unique physical properties. The graphene oxide (GO) reduction method is used to produce graphene‐based nanobiosensors. Graphene from GO reduction is also called functionalised graphene sheets or chemically reduced GO and has advantages to be used in nanobiosensors like intrinsically high surface‐to‐volume ratio and high electron transferability. However, its optical properties are not explored to date. The main application of graphene is the EC detection of small biomolecules. Like CNTs, graphite is also decorated with metallic nanoparticles for the detection of viruses which is more accurate than conventional methods. Optical biosensing techniques have been employed for the detection of many special viruses. 64
As an example, Afsagi et al. developed a portable graphene‐based biosensor for detecting the ZKV. Zika antigens in human serum were measured using field effect biosensing with monoclonal antibodies which were covalently linked to graphene nanoparticles. 65 Navakul et al. presented a new technique for fast detection of dengue virus based on EIS using GO nanoparticles as nanobiosensors. 66
5.1.2. Metal‐based nanobiosensors
Gold nanoparticles
AuNPs have unique properties like simple and rapid synthesis, large surface area, strong adsorption ability and facile conjugation to biomolecules. Because of these properties, they are studied on EC and optical techniques based on nanobiosensors for various viral detection. AuNPs act as electroactive and catalytic tags for the detection of viruses in EC assays. 67 Laderman et al. described the development of lateral‐flow immunochromatographic assay for the detection of herpes simplex virus type 2 based on colloidal gold nanoparticles. 68 Shawky et al. utilised the unique properties of optical and physicochemical properties of gold nanoparticles and developed an assay for sensing and quantifying HCV. 69
Silver nanoparticles
Silver nanoparticles (AgNPs) are the most widely used metallic nanoparticles, particularly in biological detection. They are used in biosensors techniques like EC and SPR because of their physicochemical properties, strong adsorption and good electric conducting properties. 70 In this regard, Zou et al. successfully developed DNA‐stabilised silver nanoclusters‐ based label‐free fluorescent platform to detect two human immunodeficiency virus oligonucleotides (HIV DNAs) simultaneously. 71 Another research conducted by Sepunaru et al. shows that the influenza virus can be rapidly detected using a single virus tagged with AgNPs in real time. 72
5.1.3. Semiconductors
Semiconductor‐based nanobiosensors have wide application in the detection of analytes because of their surface potential and tunable fluorescence properties. 73 They also have unique photophysical, optical, catalytic and electronic properties which help in biorecognition processes. The most commonly used semiconductors in the application of nanobiosensors include zinc oxide (ZnO) and titanium dioxide. Nanostructures such as nanorods, nanobelts, nanodisks, nanoparticles, nanosheets, nanoporous and radial nanowire assay were synthesised from these semiconductors. 74
Also, QDs, having intrinsic electronic and optical properties, are used in developing biosensors. QDs are colloidal nanocrystalline semiconductors and are composed of a core containing group II–VI elements or group III–V elements or shells made up of another semiconductor, covering the core to increase its optical properties. 75
Low et al. synthesised pure graphene and composed it with ZnO nanoparticles using a hydrothermal process. An EC DNA sensor was fabricated based on graphene/ZnO nanocomposite for the detection of Avian Influenza H5 gene. 76 In another study, Wang et al. reported that QDs‐DNA nanosensor can be used for detecting single mismatch and the target DNA in HBV gene – based on fluorescence resonance energy transfer. It is a simple and efficient method for detecting target DNA and mutants. 2
5.1.4. Electrospun nanofibers
Electrospinning is a widely used technique in nanotechnology in which energy is applied in the form of electrostatic field force to any polymer solution and it causes charged liquid jet to move in the downward direction towards a collector which is oppositely charged and as a result, fine fibres are deposited. Electrospun nanofibers are used in different applications like drug delivery systems, biosensing, scaffolds for tissue engineering. These are due to its potential advantages like high specific surface area, better sensibility, high porosity and so on. These nanobiosensors are based on sensing principles like electric resistance, vibration frequency, optics, photoelectricity and electric current. 77
Tripathy et al. developed an ultrasensitive EC platform with electrospun semiconducting manganese oxide for the detection of DNA hybridisation. This biosensor works on EC transduction techniques for zeptomolar detection in dengue consensus primer with a 120 × 10−21 M limit of detection. 78
5.1.5. Approaching nanobiosensors towards Covid‐19 detection
During the pandemic, diagnostics is an important weapon as the infected persons can be isolated to prevent further spreading of infection and given treatment. 79 SARS‐CoV‐2 is diagnosed by various techniques like nucleic acid detection, CT scan, immune identification technology and blood culture. However, nucleic acid detection mainly real‐time RT‐PCR that involves in the detection of RNA and immune identification technology mainly enzyme‐linked immune sorbent assay that involves in the detection of antibodies are used in the detection of SARS‐CoV‐2. 28
Although these methods are highly sensitive, they have some disadvantages. They are not cost‐effective and take a long time to complete and so, large‐scale screening is not suitable. Also, the above‐mentioned techniques require skilled technicians for performing the screening and it is not effective for point‐of‐care testing. 80 RT‐PCR has biological safety hazards issue while retention and operation of the samples from the patients and also have sampling error. CT scans have indistinguishability issues from the other viruses. 28
To overcome all these shortcomings, nanotechnology‐based sensors can be preferred for the detection of Covid‐19. It can enable high interactions between target and receptor because of their extremely large surface‐to‐volume ratios and so, they are rapid and produce more reliable results. 79 In this regard, scientists at the National Institute of Animal Biotechnology, India, had devised an indigenous nanobiosensor device (eCovSens) using fluorine‐doped tin oxide electrode (FTO) and AuNPs which were immobilised with nCovid‐19 monoclonal antibody for the detecting spike antigen in saliva samples. The advantage of such a system was its ability to detect the said protein in very low concentrations in the human saliva. Besides portability, the nanobiosensor boasted of its ease of collection of data which was done by either connecting to a computer or a cell phone via bluetooth. 81 Many such nanobiosensors which are being studied to be implemented for SARS‐CoV‐2 detection are described in Figure 4 and discussed below.
FIGURE 4.
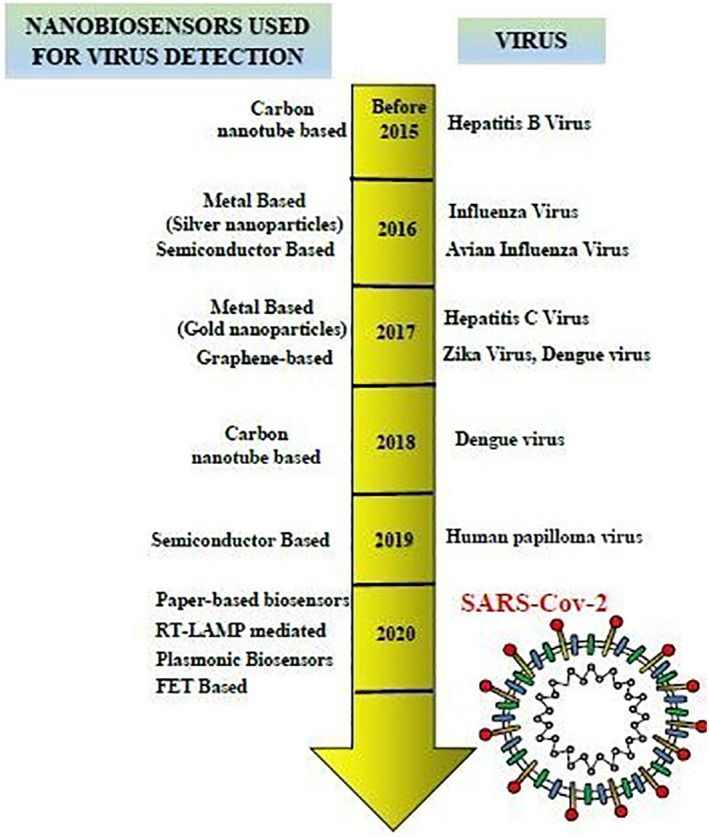
Time line to indicate the use of nanobiosensors for detection of different viruses
5.1.6. Paper‐based biosensors
Paper‐based biosensors are more crucial for use in point‐of‐care testing because of their cost‐effectiveness, ease of fabrication, modification, functionalisation, biodegradability and rapidity. Lateral flow strips are used widely for the detection of Covid‐19. They are used to detect IgG and IgM in either whole blood, serum or plasma samples from the patients. The strips consist of (a) a sample pad for adding samples taken from the patients; (b) a conjugate pad which contains Covid‐19 antigen that is conjugated with gold nanoparticles and gold rabbit IgG; (c) nitrocellulose membrane consisting of a control line that is coated with gold anti‐rabbit IgG, IgG test line that is coated with anti‐human IgG and an IgM test line that is coated with anti‐human IgM; and (d) an absorbent pad to absorb the wastes.
When IgM/IgG is present in the patient sample, they react with antigen conjugated with gold nanoparticles and form a complex and interact with the anti‐IgM/anti‐IgG test lines. At the control line, gold‐rabbit IG and anti‐rabbit IgG reacts and produce a red colour. Primary or acute infection is indicated by either positive IgM and IgG or positive IgM and negative IgG. Secondary or later infection is indicated by positive IgG and negative IgM. 82
5.1.7. RT‐LAMP mediated nanoparticles‐based biosensor
An extremely precise RT‐LAMP‐based test mediated with nanoparticles‐based biosensor has been developed for the detection of SARS‐CoV‐2. In this method, LAMP primer sets, F1ab (opening reading frame 1a/b) and nucleoprotein genes of the virus are amplified and detected simultaneously in one step. A nanoparticle‐based biosensor is used to interpret the detection results. This assay is more accurate and highly sensitive as the sensitivity was 12 copies per reaction. Also, they produce low false‐positive results. The detection method was very fast and was completed within an hour. 83
5.1.8. Plasmonic biosensors
Plasmonic biosensors have been used for the detection of viruses for many years. For the detection of SARS‐CoV‐2, an SPR sensor coated with a peptide monolayer and functionalised with SARS‐CoV‐2 nucleocapsid recombinant protein was devised which detected viral antibodies in human serum in nanomolar range within 15 min of sample/sensor contact. 84 Similarly, as nanobiosensors, dual‐functional plasmonic biosensor has been developed using plasmonic photothermal and LSPR combined effects to diagnose Covid‐19. Complementary DNA receptors are combined with 2D gold nanoislands (AuNIs) and it can detect the selective sequences from SARS‐CoV‐2 using hybridisation with target nucleic acids. The plasmonic heat was generated on the surface of AuNIs for the further enhancement of detecting abilities. This biosensor provides good sensing performance in the detection of the target sequence with a low detection limit of 0.22 pm. 85
5.1.9. FET‐based biosensor
An FET‐based biosensor is fabricated for detecting SARS‐CoV‐2 in the clinical specimens. In this device, graphene was used to carry out the diagnosis process with high sensitivity. The graphene sheets present in the FET were conjugated with specific antibodies for constructing the biosensor. The nasopharyngeal swab samples were taken from the infected persons and the assay is performed. SARS‐CoV‐2 spike protein is detected by FET biosensor in self‐culture medium with detection limits of 1.6 × 101 plague forming units/ml (pfu/mL) and in nasopharyngeal swab samples with detection limits 2.42 × 102 copies/mL. This device was very sensitive and could detect a small volume of target instantaneously without showing any cross‐reactivity with MERS‐CoV antigen. 86
5.1.10. Limitations of nanobiosensors
Despite their success in the detection of a wide spectrum of viruses, there still exist some limitations which curtail the wide of nanobiosensors in the medical industry. Some of the nanobiosensors are not cost‐effective because of the high cost of raw materials like CNTs. It restricts the use of nanobiosensors in manufacturing on a large scale and commercialisation. Also, there are technical difficulties in the manufacturing of nanobiosensors because of their size and sensitivity to various synthesis techniques.
Further, there is insufficient knowledge about the adverse and cytotoxic effects associated with nanobiosensors. The toxicity of nanomaterials is mostly affected by their composition and physicochemical properties such as size, shape, surface chemistry, texture and protein absorption gradient. So, the toxicity can be revised by the manipulation of several physicochemical properties. 87 Moreover, a basic understanding of the impact of nanobiosensors in biological settings is lacking. It is mandatory to study their biological relevance before designing any nanobiosensor for large‐scale applications. This can improve its impact on humans and the environment. 88
Though many of the diagnoses are available for the detection of SARS‐CoV‐2, the Covid‐19 pandemic has shown the weaknesses of these diagnostic systems in many parts of the world (Laboratory capacity in Covid‐19 diagnosis and the need to enhance molecular testing in Ghana). Particularly, the second wave of this Covid‐19 is spreading at a very faster rate mainly in countries like India. The cases in the second wave are found to be almost double the number of cases reported during the first wave. It is a raising question about tests done to diagnose Covid‐19 affected patients, as some of the tests produce a high rate of false‐positive and false‐negative results. So, the evaluation of performance characteristics of these tests and improvement in diagnostic techniques are important to control the ongoing pandemic. 89
6. FUTURE PERSPECTIVES AND CONCLUSION
The entire world is plagued by the menace of SARS‐CoV‐2 which has infiltrated every nook and corner. Both treatment and diagnosis of the virus remain challenging even though researchers have deciphered many facts about the virus. Correct diagnosis is the first step to initiate the treatment procedures in Covid‐19 patients. The standard procedure adopted for SARS‐CoV‐2 identification involves CT scan, RT‐qPCR and LFICS along with the rapid diagnostic kit and strips implemented in many places. Unfortunately, due to the intense and grim situation in many places, these techniques are not sufficient and adequate to meet the demands of the teeming millions. Hence, designing more dependable, fast, affordable and widely accessible diagnostic tools or sensing strategies is the call of the hour and a challenge to be met by every scientist and researcher.
In this context, ultrasensitive biosensors targeting virus antigen detection have garnered a lot of impetus. Besides being user‐friendly, the ability of combined detection of different biomarkers has helped the biosensors to smoothly make their way into clinics for diagnosis of many diseases. These biosensors are aptly designed and implemented in the early diagnosis of Covid‐19 infection via detecting various virus antigens to appraise the severity of infection. In this context, special emphasis has been levied on EC biosensors, SERS‐based biosensors, FET‐based biosensors and SPR‐based biosensors.
Another important issue, that is, a prerequisite of any biosensor is that it should be portable and reusable besides being highly selective so that it can distinguish viral targets from other elements with ease. The advent of nanotechnology has infused new potentiality for betterment in this arena. 90 , 91 In this regard, the concept of nano‐biosensor has germinated which is based on the binding of a biological species to perturb electrical properties thereby detecting analytes with high speed, fantabulous sensitivity and precision. The attention is principally to harness various nano‐effects like quantum size effect, macro quantum tunnel effect and surface effect which is unique to nanomaterials. The high mutation rates in coronavirus affect the viral detection process. Ultra‐sensitive nanobiosensors owing to specific physicochemical characteristics can detect low viral load thus paving roadmaps towards impending insights in diagnosis. Besides, the dependability and reproducibility of nanobiosensors can be boosted by developing podiums that aid machine learning‐based signal processing and direct readout of results. It is soon anticipated that these nanobiosensors will be incorporated into miniature biochips to make probes which we can carry in our pockets for onsite detection. For asymptomatic patients, such nanobiosensors will be readily available that can substantiate the presence or absence of SARS‐CoV‐2 anywhere. Similarly, smartphone‐based biosensors also have the potential to become the point‐of‐care treatment strategies in the future.
Nevertheless, frequent use of such platforms is hindered owing to some drawbacks. Sometimes the nanobiosensor designed is not able to amplify the signals owing to improper biochemical reactions between the analyte and biological component of the biosensor. This can be due to a lack of detailed knowledge about validated markers which hinders the specificity of nanobiosensors. Even the long‐term safety and efficiency of such platforms need to be assessed impartially before recommending it as the gold standard of diagnosis. False exaggerated optimism regarding such nanobiosensors can harm its use. Thus, due to the unique characteristics of Covid‐19 pathogen, designing portable and smart one‐size‐fits‐all nanobiosensors may revolutionise the field of diagnostics making it much easier for identifying the pathogen even if it is present at a minimum amount.
CONFLICT OF INTEREST
None to declare.
AUTHOR CONTRIBUTION
The text has been written and edited by RM, SA, and SN.
ACKNOWLEDGMENTS
Ranjita Misra is thankful to the Sathyabama Institute of Science and Technology and Sarbari Acharya is thankful to KIIT University for the support provided.
Misra R, Acharya S, Sushmitha N. Nanobiosensor‐based diagnostic tools in viral infections: special emphasis on Covid‐19. Rev Med Virol. 2022;32(2):e2267. 10.1002/rmv.2267
Ranjita Misra and Sarbari Acharya have equal contribution.
Contributor Information
Ranjita Misra, Email: ranjita.biotech@gmail.com.
Sarbari Acharya, Email: sarbariacharya@gmail.com.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Burbelo PD, Ladarola MJ, Chaturvedi A. Emerging technologies for the detection of viral infections. Future Virol. 2019;14:39‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang N, Wang L, Deng X, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92:408‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nasrollahzadeh M, Sajjadi M, Soufi GJ, Iravani S, Varma RS. Nanomaterials and nanotechnology‐associated innovations against viral infections with a focus on coronaviruses. Nanomaterials (Basel). 2020;10:1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Misra R, Kandoi S, Varadaraj S, Vijayalakshmi S, Nanda A, Verma RS. Nanotheranostics: a tactic for cancer stem cells prognosis and management. J Drug Deliv Sci Technol. 2020;55:101457. [Google Scholar]
- 5. Misra R, Das M, Biswas P, Nanda A. EGFR targeted Mn‐doped ZnO fluorescent nanocrystals for cancer theranostic application. Mater Today Commun. 2021;26:102170. [Google Scholar]
- 6. Bhalla N, Pan Y, Yang Z, Payam AF. Opportunities and challenges for biosensors and nanoscale analytical tools for pandemics: COVID‐19. ACS Nano. 2020;14:7783‐7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sin ML, Mach KE, Wong PK, Liao JC. Advances and challenges in biosensor‐based diagnosis of infectious diseases. Expert Rev Mol Diagn. 2014;14:225‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Demeke Teklemariam A, Samaddar M, Alharbi MG, Al‐Hindi RR, Bhunia AK. Biosensor and molecular‐based methods for the detection of human coronaviruses: a review. Mol Cell Probes. 2020;54:101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ilkhani H, Farhad S. A novel electrochemical DNA biosensor for Ebola virus detection. Anal Biochem. 2018;557:151‐155. [DOI] [PubMed] [Google Scholar]
- 10. Singhal C, Dubey A, Mathur A, Pundir CA, Narang J Paper based DNA biosensor for detection of chikungunya virus using gold shells coated magnetic nanocubes. Process Biochem. 2018;74:35‐42. [Google Scholar]
- 11. Malik P, Katyal V, Malik V, Asatkar A, Inwati G, Mukherjee TK. Nanobiosensors: concepts and variations. ISRN Nanomater. 2013;2013:9.1–9. [Google Scholar]
- 12. Mokhtarzadeh A, Eivazzadeh‐Keihan R, Pashazadeh P, et al. Nanomaterial‐based biosensors for detection of pathogenic virus. Trends Analyt Chem. 2017;97:445‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nguyen BT, Koh G, Lim HS, Chua AJ, Ng MM, Toh CS. Membrane‐based electrochemical nanobiosensor for the detection of virus. Anal Chem. 2009;81:7226‐7234. [DOI] [PubMed] [Google Scholar]
- 14. Samson R, Navale GR, Dharne MS. Biosensors: frontiers in rapid detection of COVID‐19. 3 Biotech. 2020;10:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holzinger M, Le Goff A, Cosnier S. Nanomaterials for biosensing applications: a review. Front Chem. 2014;2:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Torabi R, Ranjbar R, Halaji M, Heiat M. Aptamers, the bivalent agents as probes and therapies for coronavirus infections: a systematic review. Mol Cell Probes. 2020;53:101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jadav SS, Kumar A, Ahsan MJ, Jayaprakash V. Ebola virus: current and future perspectives. Infect Disord Drug Targets. 2015;15:20‐31. [DOI] [PubMed] [Google Scholar]
- 18. Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet. 2017;390(10107):2099‐2109. [DOI] [PubMed] [Google Scholar]
- 19. Javed F, Manzoor KN, Ali M, et al. Zika virus: what we need to know? J Basic Microbiol. 2018;58:3‐16. [DOI] [PubMed] [Google Scholar]
- 20. Ehichioya DU, Dellicour S, Pahlmann M, et al. Phylogeography of Lassa virus in Nigeria. J Virol. 2019;93:e00929‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Audi A, AlIbrahim M, Kaddoura M, Hijazi G, Yassine HM, Zaraket H. Seasonality of respiratory viral infections: will COVID‐19 follow suit? Front Public Health. 2020;8:567184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noor R, Maniha SM. A brief outline of respiratory viral disease outbreaks: 1889‐till date on the public health perspectives. Virusdisease. 2020;31:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coronaviridae Study Group of the International Committee on Taxonomy of V . The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Srivastava M, Srivastava N, Mishra PK, Malhotra BD. Prospects of nanomaterials‐enabled biosensors for COVID‐19 detection. Sci Total Environ. 2021.754:142363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323:1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharifi M, Hasan A, Haghighat S, et al. Rapid diagnostics of coronavirus disease 2019 in early stages using nanobiosensors: challenges and opportunities. Talanta. 2021;223:121704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaya SI, Karadurmus L, Ozcelikay G, Bakirhan NK, Ozkan SA. Electrochemical virus detections with nanobiosensors. Nanosensors for Smart Cities 2020;303‐326. [Google Scholar]
- 28. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID‐19. J Pharm Anal. 2020;10:102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a Review. JAMA. 2020;323:1824‐1836. [DOI] [PubMed] [Google Scholar]
- 30. Gewirtz AM. Exogenous and endogenous regulations of human megakaryocytopoiesis. Adv Exp Med Biol. 1988;241:149‐164. [DOI] [PubMed] [Google Scholar]
- 31. Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. J Med Virol. 2020;92:595‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park GS, Ku K, Baek SH, et al. Development of reverse transcription loop‐mediated isothermal amplification assays targeting severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). J Mol Diagn. 2020;22:729‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu L, Wu S, Hao X, et al. Rapid detection of COVID‐19 coronavirus using a reverse transcriptional loop‐mediated isothermal amplification (RT‐LAMP) diagnostic platform. Clin Chem. 2020;66:975‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin Y, Shanshan, W , Xiaowen, H , et al. Rapid colorimetric detection of COVID‐19 coronavirus using a reverse transcriptional loop‐mediated isothermal amplification (RT‐LAMP) diagnostic platform: iLACO. Clin Chem. 2020;66(7):975‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Broughton JP, Deng X, Yu G, et al. CRISPR‐Cas12‐based detection of SARS‐CoV‐2. Nat Biotechnol. 2020;38:870‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russo A, Minichini C, Starace M, et al. Current status of laboratory diagnosis for COVID‐19: a narrative review. Infect Drug Resist. 2020;13:2657‐2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katsarou K, Bardani E, Kallemi P, Kalantidis K. Viral detection: past, present, and future. Bioessays. 2019;41:e1900049. [DOI] [PubMed] [Google Scholar]
- 38. Ribeiro BV, Aparecid T, Cordeiro R, Freitas GRO, Ferreira LF, Franco DL. Biosensors for the detection of respiratory viruses: a review. Talanta Open. 2020;2:100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olson N, Bae J. Biosensors‐publication trends and knowledge domain visualization. Sensors (Basel). 2019;19(11):2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Misra R, Acharya S, Dilnawaz F, Sahoo SK. Sustained antibacterial activity of doxycycline‐loaded poly(D,L‐lactide‐co‐glycolide) and poly(epsilon‐caprolactone) nanoparticles. Nanomedicine (Lond). 2009;4:519‐530. [DOI] [PubMed] [Google Scholar]
- 41. Misra R, Acharya S, Sahoo SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today. 2010;15:842‐850. [DOI] [PubMed] [Google Scholar]
- 42. Misra R, Das M, Sahoo BS, Sahoo SK. Reversal of multidrug resistance in vitro by co‐delivery of MDR1 targeting siRNA and doxorubicin using a novel cationic poly(lactide‐co‐glycolide) nanoformulation. Int J Pharm. 2014;475:372‐384. [DOI] [PubMed] [Google Scholar]
- 43. Saylan Y, Erdem O, Unal S, Denizli A. An alternative medical diagnosis method: biosensors for virus detection. Biosensors (Basel). 2019;9(2):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shrivastav AM, Cvelbar U, Abdulhalim I. A comprehensive review on plasmonic‐based biosensors used in viral diagnostics. Commun Biol. 2021;4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Touhami A. Biosensors and nanobiosensors: design and applications. Nanomedicine. 2014;15:374‐403. [Google Scholar]
- 46. Wu A, Khan WS. Nanobiosensors: From Design to Applications. Wiley‐VCH; 2020. [Google Scholar]
- 47. Krejcova L, Michalek P, Merlos Rodrigo M, et al. Nanoscale virus biosensors: state of the art. Dovepress. 2015;4:47‐66. [Google Scholar]
- 48. Castillo‐Henriquez L, Brenes‐Acuna M, Castro‐Rojas A, Cordero‐Salmeron R, Lopretti‐Correa M, Vega‐Baudrit JR. Biosensors for the detection of bacterial and viral clinical pathogens. Sensors (Basel). 2020;20:6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lu CH, Zhang Y, Tang SF, et al. Sensing HIV related protein using epitope imprinted hydrophilic polymer coated quartz crystal microbalance. Biosens Bioelectron. 2012;31:439‐444. [DOI] [PubMed] [Google Scholar]
- 50. Shafiee H, Lidstone EA, Jahangir M, et al. Nanostructured optical photonic crystal biosensor for HIV viral load measurement. Sci Rep. 2014;4:4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tam YJ, Zeenathul NA, Rezaei MA, et al. Wide dynamic range of surface‐plasmon‐resonance‐based assay for hepatitis B surface antigen antibody optimal detection in comparison with ELISA. Biotechnol Appl Biochem. 2017;64:735‐744. [DOI] [PubMed] [Google Scholar]
- 52. Kaushik A, Yndart A, Kumar S, et al. A sensitive electrochemical immunosensor for label‐free detection of Zika‐virus protein. Sci Rep. 2018;8:9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Riedel T, Rodriguez‐Emmenegger C, De Los Santos Pereira A, et al. Diagnosis of Epstein‐Barr virus infection in clinical serum samples by an SPR biosensor assay. Biosens Bioelectron. 2014;55:278‐284. [DOI] [PubMed] [Google Scholar]
- 54. Feng W, Liang C, Gong H, Cai C. Sensitive detection of Japanese encephalitis virus by surface molecularly imprinted technique based on fluorescent method. New J Chem. 2018;42:3503–3508. [Google Scholar]
- 55. Gdowski A, Ranjan AP, Mukerjee A, Vishwanatha JK. Nanobiosensors: role in cancer detection and diagnosis. Adv Exp Med Biol. 2014;807:33‐58. [DOI] [PubMed] [Google Scholar]
- 56. Singh RP. Prospects of nanobiomaterials for biosensing. Int J Electrochem. 2011;4:30. [Google Scholar]
- 57. Mozhgani SH, Kermani HA, Norouzi M, Arabi M, Soltani S. Nanotechnology based strategies for HIV‐1 and HTLV‐1 retroviruses gene detection. Heliyon. 2020;6:e04048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Song S, Ha K, Guk K, et al. Colorimetric detection of influenza A (H1N1) virus by a peptide‐functionalized polydiacetylene (PEP‐PDA) nanosensor. RSC Adv. 6. 2016.48566–48570. [Google Scholar]
- 59. Shariati M, Ghorbani M, Sasanpour P, Karimizefreh A. An ultrasensitive label free human papilloma virus DNA biosensor using gold nanotubes based on nanoporous polycarbonate in electrical alignment. Anal Chim Acta. 2019;1048:31‐41. [DOI] [PubMed] [Google Scholar]
- 60. Aguilar ZP. Nanomaterials for Medical Applications. Elsevier; 2013. [Google Scholar]
- 61. Shi D. NanoScience in Biomedicine. Springer; 2009. [Google Scholar]
- 62. Wiriyachaiporn N, Sirikett H, Maneeprakorn W, Dharakul T. Carbon nanotag based visual detection of influenza A virus by a lateral flow immunoassay. Microchimica Acta. 2017;184:1827‐1835. [Google Scholar]
- 63. Li XM, Zhan ZM, Ju HQ, Zhang SS. Label‐free electrochemical detection of short sequences related to the hepatitis B virus using 4,4′‐diaminoazobenzene based on multiwalled carbon nanotube‐modified GCE. Oligonucleotides. 2008;18:321‐328. [DOI] [PubMed] [Google Scholar]
- 64. Razavi H, Janfaza S. Medical nanobiosensors: a tutorial review. Nanomed J. 2015;2:74‐87. [Google Scholar]
- 65. Afsahi S, Lerner MB, Goldstein JM, et al. Novel graphene‐based biosensor for early detection of Zika virus infection. Biosens Bioelectron. 2018;100:85‐88. [DOI] [PubMed] [Google Scholar]
- 66. Navakul K, Warakulwit C, Yenchitsomanus PT, Panya A, Lieberzeit PA, Sangma C. A novel method for dengue virus detection and antibody screening using a graphene‐polymer based electrochemical biosensor. Nanomedicine. 2017;13:549‐557. [DOI] [PubMed] [Google Scholar]
- 67. Draz MS, Shafiee H. Applications of gold nanoparticles in virus detection. Theranostics. 2018;8:1985‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Laderman EI, Whitworth E, Dumaual E, et al. Rapid, sensitive, and specific lateral‐flow immunochromatographic point‐of‐care device for detection of herpes simplex virus type 2‐specific immunoglobulin G antibodies in serum and whole blood. Clin Vaccine Immunol. 2008;15:159‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shawky SM, Awad AM, Allam W, Alkordi MH, El‐Khamisy SF. Gold aggregating gold: a novel nanoparticle biosensor approach for the direct quantification of hepatitis C virus RNA in clinical samples. Biosens Bioelectron. 2017;92:349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rai M, Yadav A, Cioffi N. Silver nanoparticles as nano‐antimicrobials: bioactivity, benefits and bottlenecks. In: Cioffi N, Rai M, eds., Nano‐Antimicrobials. Berlin, Heidelberg: Springer‐Verlag; 2012: 211‐224. [Google Scholar]
- 71. Zou R, Zhang F, Chen C, Cai C. DNA‐programming multicolor silver nanoclusters for sensitively simultaneous detection of two HIV DNAs. Sens Actuators B Chem. 2019;296:126608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sepunaru L, Plowman BJ, Sokolov SV, Young NP, Compton RG. Rapid electrochemical detection of single influenza viruses tagged with silver nanoparticles. Chem Sci. 2016;7:3892‐3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang F, Hu S. Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim Acta. 2009;165:1–22. [Google Scholar]
- 74. Xiang XC, Chi Y, Xiang GB, Jiang FS. Nanostructured ZnO for biosensing applications Nano‐Biomedical Optoelectronic Materials and Devices. Chin Sci Bull. 2013;58:2563‐2566. [Google Scholar]
- 75. Frasco MF, Chaniotakis N. Semiconductor quantum dots in chemical sensors and biosensors. Sensors (Basel). 2009;9:7266‐7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Low SS, Tan MT, Loh HS, Khiew PS, Chiu WS. Facile hydrothermal growth graphene/ZnO nanocomposite for development of enhanced biosensor. Anal Chim Acta. 2016;903:131‐141. [DOI] [PubMed] [Google Scholar]
- 77. Liu Y, Hao M, Chen Z, Liu L. A review on recent advances in application of electrospun nanofiber materials as biosensors. Curr Opin Biomed Eng. 2020;13:174‐189. [Google Scholar]
- 78. Tripathy S, Bhandari V, Sharma P, Vanjari SRK, Singh SG. Chemiresistive DNA hybridization sensor with electrospun nanofibers: a method to minimize inter‐device variability. Biosens Bioelectron. 2019;133:24‐31. [DOI] [PubMed] [Google Scholar]
- 79. Talebian S, Wallace GG, Schroeder A, Stellacci F, Conde J. Nanotechnology‐based disinfectants and sensors for SARS‐CoV‐2. Nat Nanotechnol. 2020;15:618‐621. [DOI] [PubMed] [Google Scholar]
- 80. Layqah LA, Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle‐modified carbon electrodes. Mikrochim Acta. 2019;186:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mahari S, Roberts A, Shahdeo D, Gandhi S. eCovSens‐ultrasensitive novel in‐house built printed circuit board based electrochemical device for rapid detection of nCovid‐19 antigen, a spike protein domain 1 of SARS‐CoV‐2. Biorxiv. 2020;24:059204. [Google Scholar]
- 82. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020;92:1518‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhu X, Wang X, Han L, et al. Reverse transcription loop‐mediated isothermal amplification combined with nanoparticles‐based biosensor for diagnosis of COVID‐19. Medrxiv. 2020;17:20037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Djaileb A, Charron B, Jodaylami MH, et al. A rapid and quantitative serum test for SARS‐CoV‐2 antibodies with portable surface plasmon resonance sensing. ChemRXiv. 2020. 10.26434/chemrxiv.12118914 [DOI] [Google Scholar]
- 85. Qiu G, Gai Z, Tao Y, Schmitt J, Kullak‐Ublick GA, Wang J. Dual‐functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14:5268‐5277. [DOI] [PubMed] [Google Scholar]
- 86. Seo G, Lee G, Kim MJ, et al. Rapid detection of COVID‐19 causative virus (SARS‐CoV‐2) in human nasopharyngeal swab specimens using field‐effect transistor‐based biosensor. ACS Nano. 2020;14:5135‐5142. [DOI] [PubMed] [Google Scholar]
- 87. Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M . Toxicity of nanomaterials. Chem Soc Rev. 2012;41:2323‐2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ganguly P, Breen A, Pillai SC. Toxicity of nanomaterials: exposure, pathways, assessment, and recent advances. ACS Biomater Sci Eng. 2018;4:2237‐2275. [DOI] [PubMed] [Google Scholar]
- 89. Axell‐House DB, Lavingia R, Rafferty M, Clark E, Amirian ES, Chiao EY. The estimation of diagnostic accuracy of tests for COVID‐19: a scoping review. J Infect. 2020;81:681‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kumar P, Kandoi S, Misra R, Vijayalakshmi S, Rajagopal K, Verma RS. The mesenchymal stem cell secretome: a new paradigm towards cell‐free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1‐9. [DOI] [PubMed] [Google Scholar]
- 91. Singh A, Misra R, Mohanty C, Sahoo SK. Applications of nanotechnology in vaccine delivery. Int J Green Nanotechnol Biomed. 2010;2:B25‐B45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


