Abstract
Background & aims
Recent studies have verified that the SARS-CoV-2 infection (from December 2019 has affected 123 million people throughout the world and more than 3 million people in Italy), can have medium-term and long-term effects, collectively referred to as “post-Covid syndrome” or “long-Covid” characterized by chronic fatigue, followed by muscle weakness, dyspnea and headache. Chronic fatigue or chronic tiredness is a persistent symptom both in patients who have experienced a severe infection and in those who have experienced a mild form of infection. Studies conducted on both patients discharged from hospital and patients managed at home showed that there was no association between the severity of the Coronavirus disease (Covid-19) and the subsequent chronic fatigue symptom. The aim of this study was to evaluate the ability of a nutritional supplement based on vitamins, minerals, amino acids and plant extracts (Apportal®) intake, to ameliorate the general health status in particular the chronic fatigue symptom in subjects after SARS-CoV-2 negativity.
Methods
Participants were advised to take one sachet daily of Apportal® for 28 consecutive days. At the beginning (T0), after 14 days (T1) and after 28 days (T2) of supplementation, general fatigue, mental fatigue and Quality of Life indexes were evaluated through specific questionnaires. The assessment of quality of life and health status were measured through the EuroQoL-5D questionnaire, chronic fatigue using the FACIT-Fatigue questionnaire and mental fatigue using the modified Chalder questionnaire.
Results
201 subjects were enrolled for the study; results showed a significant improvement in all indexes analyzed after 14 and 28 days of supplementation. The main significant improvement was observed after the first 14 days and it was further confirmed at 28 days as well. The RTE (Relative Treatment Effect) trend about quality of life, health status, FACIT-Fatigue and mental fatigue in the three questionnaires was statistically significant (Wald Statistic, p < 0.0001). The data of FACIT-questionnaire showed an improvement of at least 1 unit in 76.62% of subjects after 14 days and in 90.05% of subjects after 28 days. An improvement of 10-unit was found in about one third of subjects after 14 days and in half of the subjects after 28 days.
Conclusions
This study shows that Apportal® can reduce chronic fatigue and improve quality of life and health status in subjects after SARS-CoV-2 negativity due to the synergistic effect of its components.
Keywords: SARS-CoV-2, Long-Covid, Chronic fatigue, Nutritional supplement
1. Introduction
Since December 2019 to the date of writing of this article, the Coronavirus pandemic (SARS-CoV-2) has affected 123 million people worldwide and more than 3 million people in Italy. It has been observed that after the acute phase of the viral disease some individuals do not completely return to their initial health conditions but continue to manifest previous symptoms. Several studies have verified that the SARS-CoV-2 infection can have medium- and long-term effects, collectively called the “post-Covid syndrome” or “long-Covid”. One of the symptoms most frequently reported by patients with “long-Covid” was fatigue or chronic tiredness, followed by muscle weakness, dyspnea, and headache [1].
Persistent fatigue lasting 6 months or longer can be defined as chronic fatigue syndrome (CFS). The physiopathology of CFS is controversial, but probably there is a relation with alterations of nervous, endocrine and immune systems activities [2]. Studies conducted on patients discharged from hospital and those managed at home showed that there was no association between the severity of Coronavirus (Covid-19) disease and subsequent chronic fatigue [3]. Specifically, a study conducted in the USA states that: (i) one in five out of the population examined among individuals aged 18–34 years without chronic diseases, were unable to regain their initial health conditions after the end of the acute phase; (ii) this prolongation of the disease had a major impact on their quality of life as well as negative implications at a social level [4].
A large cohort study conducted in China involved 1733 patients after hospital discharge and found that after 6 months most patients showed at least one symptom, particularly fatigue or muscle weakness, sleep difficulties, and anxiety or depression [5].
In Italy, as elsewhere in the world, several research teams have addressed the issue of the persistence of symptoms after the SARS-CoV-2 infection. Carfì et al. [6] confirmed that the main symptom found in 53.1% of a group of 143 patients attending the post-Covid outpatient clinic of the Gemelli Hospital in Rome was chronic fatigue, and 44.1% reported a worsening of quality of life.
The consequences of Covid-19 disease on our organism are not yet clear. The fatigue experienced by the patients after SARS-CoV-2 infection was similar to those noted with post-intensive care syndrome and other post-viral syndromes such as Epstein–Barr virus infection [7]. Furthermore, in some studies have been postulated similarities between Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and post-Covid syndrome [8].
In patients with severe Covid-19 disease admitted to intensive care unit (ICU) fatigue symptoms may be improved through rehabilitation programs [9], but, as far as we know, there are no specific solutions for the management of post-covid fatigue in non-ICU patients.
At the same time, is well demonstrated that in other conditions, such as oncology, post-operative or in the elderly, nutritional support with vitamins, minerals or plant extract have been shown to help improving fatigue and quality of life [[10], [11], [12]].
2. Objectives
The aim of this study is to evaluate the response to the intake of a nutritional supplement based on vitamins, minerals, amino acids, and plant extracts (Apportal®, Pharmanutra Spa) in terms of chronic fatigue symptoms improvement, in subjects with persistent fatigue after SARS-CoV-2 negativity, managed at home by the family doctor. The secondary aim is to assess the impact of the supplementation on quality of life and mental fatigue on the study population.
2.1. Methods
2.1.1. Participants
Participants were recruited from all over Italy, with a distribution of 25% in Northern Italy, 25% in Central Italy and 50% in the South and Islands. The territorial distribution was deliberately very varied; there were no specific territorial clusters capable of portraying the entire Italian situation as realistically as possible. The subjects enrolled had contracted Covid-19 and overcome the acute phase of the infection (at least 3 days without fever and no other symptoms except for ageusia and anosmia) but still reported persistent fatigue. Patients who had been admitted to the intensive care unit or had 3 or more chronic diseases were excluded.
2.1.2. Intervention
Participants were advised to take the food supplement Apportal®, which contains 19 nutrients including group B vitamins, minerals in Sucrosomial® forms such as iron, magnesium, zinc and selenium, amino acids such as arginine and carnitine, and plant extracts including Panax ginseng and Eleutherococcus senticosus. Each participant was advised to take one sachet daily of Apportal® for 28 consecutive days. At the beginning (T0), after 14 days (T1) and after 28 days (T2) of supplementation, general fatigue, mental fatigue and Quality of Life indexes were evaluated through specific questionnaires.
2.1.3. Assessment
The assessment of quality of life and health status were measured through the EuroQoL-5D questionnaire, chronic fatigue using the FACIT-Fatigue questionnaire and mental fatigue using the modified Chalder questionnaire.
The EQ-5D questionnaire is widely used as a screening tool in large populations and consists of two sections: the first entails a subjective evaluation of five dimensions (mobility, self-care, daily activities, pain/discomfort and anxiety/depression) and each point foresees graded answers from 1 to 3. Level 1 represents no problems, while level 3 represents extreme limitation. The second section includes a visual analogue scale (VAS) assessment, graphically represented by a graduated scale from 0 (worst possible health status) to 100 (best possible health status), where respondents indicate their perceived level of health. For simplicity, this scale was readjusted into integers ranging from 0 to 10 and the score obtained in the first section was converted into the EQ-5D index which is a score between 0 and 1 where 0 represents very poor quality of life, and 1 perfect health. Therefore, two numerical indices representing the subject's quality of life (EQ-5D index) and health status were derived from the first questionnaire [13].
The FACIT-Fatigue questionnaire which consists of 13 items was originally designed to understand the impact of asthenia on the daily activities of cancer patients, but it has also been validated for numerous other chronic diseases (e.g., rheumatoid arthritis, anemia). Each question has 5 response options depending on the severity ranging from “not at all” to “often”. The total points, calculated according to the official scoring, is on a numerical scale from 0 to 52, in which the higher the score the lesser the fatigue [14].
For assessing mental fatigue, we used the Chalder questionnaire, already cited in other studies on Covid-19 patients, but only using the last 6 questions, which concern mental symptoms, and then readjusting them on a Likert scale from 0 to 4, similar to the one in the FACIT questionnaire. Summing the scores of the individual answers yields a scale ranging from 0 to 24 where the higher score represents greater mental fatigue.
2.2. Sample sizing
The main goal of the statistical analyses was the estimation of the patients fatigue recovery, defined as the proportions of patients who showed an increase of at least 5 units in the FACIT scale. Aiming an increase in recovery of at least 10%, as compared to a hypothesized 60% recovery in a non-treated sample, a power of 80% with alpha equal to 0.05 is guaranteed by a sample of 181 patients. Considering a 10% loss at follow up, the sample required increases to 200 patients.
2.3. Statistical analysis
The variables collected are described as mean ± standard deviation, median and interquartile range, or proportion, depending on their distribution. The longitudinal data are analyzed with a non-parametric method based on ranks [15], calculating the Relative Treatment Effects (RTEs), and using the Wald statistic for comparisons on repeated measures and groups into which the sample is divided. Considering a group within a sample, the relative RTE for that group is an estimate of the probability that the value observed in any one component of the group is greater than the value observed in any other component, selected at random from the entire sample. Being a probability measure, the RTE will have a value ranging between 0 and 1. After one way or two-way repeated measure analysis, Bonferroni correction was used for post-hoc comparisons. A value of p less than 0.05 was considered statistically significant. All statistical analyses were performed with the R open-source software.
3. Results
A total of 201 subjects were enrolled; each of whom signed an informed consent form regarding the study which was carried out in compliance with Good Clinical Practices and in accordance with the ethical principles of the Declaration of Helsinki. Data collected for the 4 indices (Quality of life, Health Status, FACIT-Fatigue and Mental Fatigue) allow to assess the trend in the general population. The RTE (Relative Treatment Effect) was determined, allowing a better understanding of the progression of the parameters analyzed over time. The characteristics of the subjects analyzed are shown in Table 1 .
Table 1.
Sample characteristics.
| Entire sample (n = 201) | |
|---|---|
| Age | 48.11 ± 13.16 |
| Male Gender | 79 (39.3%) |
| Days since the onset of the disease | 37 [30–46] |
| Days of fever | 3 [1–5] |
| Hospitalization | 15 (7.46%) |
| Other chronic diseases | 39 (19.40%) |
| Pneumonia | 19 (9.5%) |
| Smoking | 19 (9.5%) |
3.1. Analysis of the entire sample
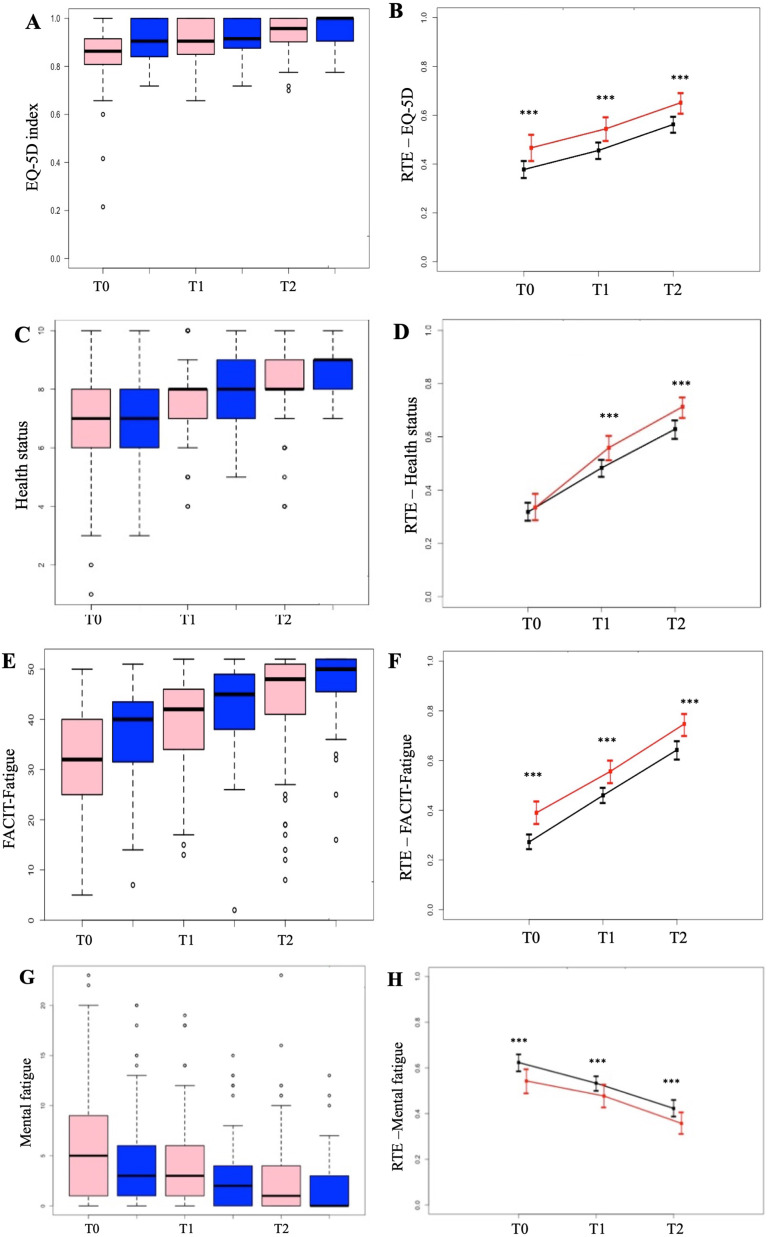
The results of the 4 indices analyzed in the entire sample and relative treatment effects (RTEs) are shown in Fig. 1 .
Fig. 1.
A: results (median) and B: RTE trend for quality of life; C: results (median) and D: RTE trend for health status; E: results (median) and F: RTE trend for FACIT-Fatigue; G: results (median) and H: RTE trend for mental fatigue (∗∗∗p < 0.0001 for all pairs of questionnaires).
The RTE trend concerning (i) quality of life, (ii) health status, (iii) FACIT-Fatigue and (iiii) mental fatigue in the three questionnaires is statistically significant (Wald Statistic, p < 0.0001). Furthermore, the data between the three pairs of possible values were verified for significance and found to be highly significant for all parameters (p01 < 0.0001, p02 < 0.0001, p12 < 0.0001). The increase in the absolute value and the RTEs of the EQ-5D index, health status and the FACIT-Fatigue represent an improvement in the quality of life and a reduced feeling of fatigue in the subjects. The decrease in the absolute value and the RTE of the mental fatigue index represents an improvement of this aspect in the interviewees.
3.2. Chronic fatigue improvement percentage
The results obtained from the analysis of the FACIT-Fatigue questionnaire showed an improving trend for the population analyzed. The percentage of subjects with a reduction in the total score was assessed. By considering the reduction of at least 1 unit in the questionnaire as an improvement, in the period between T0 and T1, 154 subjects improved (76.62%) and between T0 and T2, 181 subjects improved (90.05%). By considering the reduction of at least 5 units in the questionnaire as an improvement, in the period between T0 and T1, 115 subjects improved (57.21%), and between T0 and T3, 150 subjects improved (74.63%). By considering the reduction of at least 10 units in the questionnaire as an improvement, in the period between T0 and T1 65 subjects improved (32.34%), and between T0 and T2, 102 subjects improved (50.75%).
3.3. Analysis according to gender
Based on the recent literature showing a different incidence of chronic fatigue in females compared to males, we extrapolated two subgroups divided by gender from our study population. Therefore, the 4 indices under examination were analyzed in the male subgroup (79 subjects) and female subgroup (122 subjects). The results of the relative treatment effects (RTE) are illustrated in Fig. 2 .
Fig. 2.
A: results (median) and B: RTE trend for quality of life; C: results (median) and D: RTE trend for health status; E: results (median) and F: RTE trend for FACIT-Fatigue; G: results (median) and H: RTE trend for mental fatigue (Significance between genders at all times:∗∗∗p < 0,0001.  Women
Women  Men;
Men;  Women
Women  Men.)
Men.)
Within the same gender, for both male and female, the differences between each pair of questionnaires for all 4 indices analyzed are statistically significant; the parallelism between the trends statistically confirms that the two subgroups improve similarly.
Furthermore, when analyzing the differences between genders (men vs women) at the various times, it can be observed how the differences between genders are always statistically significant (p < 0.0001), except for the RTE of health status at T0. The results of the scores obtained from the individual questionnaires and the RTE trend are shown in Fig. 3 .
Fig. 3.
A: results (median) and B: RTE trend for quality of life; C: results (median) and D: RTE trend for health status; E: results (median) and F: RTE trend for FACIT-Fatigue; G: results (median) and H: RTE trend for mental fatigue (Significance between subgroups: ∗∗∗p < 0,0001). (Young  Elderly
Elderly  ;
;  Young
Young  Elderly).
Elderly).
3.4. Chronic fatigue improvement percentage by gender
Likewise in the men-women subgroups, the number of improved subjects was evaluated as a percentage by assessing various improvement steps in the total score of the FACIT questionnaire. By considering the reduction of at least 1 unit in the questionnaire, in the period between T0 and T1, 98 women (80.33%) and 56 men (70.89%) improved; in the period between T0 and T2 109 women (89.34%) and 72 men (91.13%) improved.
3.5. Analysis according to age
Since the pandemic affected most seriously elderly people, an additional analysis parameter could be the age of the subjects. The average age was 48 and subjects over 80 years of age were excluded; therefore, an analysis was carried out by distinguishing 2 subgroups: subjects aged ≥60 years (28 subjects), for convenience defined as “Elderly” and the others aged <60 years (173 subjects), defined as “Young”. The results of the relative treatment effects (RTEs) are shown in Fig. 3.
Within the same subgroup, both for the young and the elderly, the differences between each pair of questionnaires for the 4 indices analyzed are statistically significant. Between the subgroups, only the differences in the RTE trend of the quality of life are statistically significant; in the case of the other indices, statistical significance is not reached due to the small number of the elderly subgroup, although in the case of the RTE trend of mental fatigue in the elderly subgroup at T1 (14 days) there is a numerically greater improvement than in the young.
3.6. Percentage of improvement in chronic fatigue by age
The number of subjects who improved as a percentage of the total score of the FACIT questionnaire was also evaluated on the young-elderly subgroups. By considering the reduction of at least 1 unit in the questionnaire, in the period between T0 and T1, 24 elderly subjects (85.71%) and 130 young subjects (75.14%) improved; in the period between T0 and T2, 25 elderly subjects (89.29%) and 156 young subjects (90.17%) improved.
4. Special cases
Within the studied sample, two subjects were identified who reported persistent fatigue for much longer than the others, hence it was deemed appropriate to carry out an ad hoc analysis of their progress. The two subjects were a man, 56 years old, (Subject A) and a woman, 29 years old, (Subject B) began the analysis 269 and 266 days respectively after the onset of the SARS-CoV-2 infection (March 2020, “1st pandemic wave”). Table S1 shows the scores from the FACIT-Fatigue and Mental Fatigue questionnaires. The increase in the score of the FACIT-fatigue questionnaire represents an improvement in the feeling of fatigue, while the decrease in the mental fatigue index score represents an improvement in this aspect.
5. Discussion
To the best of our knowledge, there is no other study to date that has associated the intake of a nutritional supplement with the reduction in chronic fatigue or an improvement in the quality of life of patients with post-Covid syndrome.
The subjects were recruited nationwide and included because they reported still feeling tired and fatigued more than one month after the end of the acute phase of the disease. The data obtained from the anamnesis confirm that they are representative of the general situation: the average age (48 years) is in line with the average age of those affected in the October–November 2020 wave, which was 45–49 years according to the report from the Istituto Superiore di Sanità [Italian National Institute of Health] on the Covid-19 epidemic [16]; the higher incidence of chronic fatigue in female subjects confirms the consistency of the data observed also in other studies. Indeed, the study by Venturelli et al. conducted on 767 patients discharged from the hospital of Bergamo (Italy) showed the higher incidence of post-Covid symptoms in women: in fact, women had about twice the incidence of chronic fatigue [5,17].
The results on the whole population show a significant improvement in the questionnaire’s score used to analyze fatigue and quality of life after both 14 and 28 days of supplementation with Apportal®. The improvement observed in patient symptoms already after the first 14 days showed that the positive effect is not solely attributable to the time elapsed since the end of the disease but is due to the supplement as well. The effect of the supplementation is further confirmed by the progressive improvement observed also after 28 days. Moreover, Apportal® ameliorate general condition also in two subjects with persistent fatigue who had experienced the acute phase of the infection almost 9 months before starting the investigation.
The data on the percentage of patients who improved FACIT-questionnaire further confirm the benefit deriving from nutritional treatment, showing an improvement of at least 1 unit in 76.62% of subjects after 14 days and in 90.05% of subjects after 28 days. Even more interesting is the result showing the 10-unit improvement found in about one third of subjects after 14 days and in half of the subjects after 28 days.
The analysis of the subgroups shows that both women and the over-60s start the treatment with a higher degree of initial chronic fatigue. Scores of fatigue and quality of life progressively ameliorate in the same way as men and young people, as evidenced by the relative trend effect (RTE) of the treatment.
The mental fatigue RTE trend for the over-60s subgroup highlights a greater benefit in this population after 14 days of supplementation than in the others, even though it does not reach statistical significance due to the small number of over-60s analyzed in this study. It would be interesting to evaluate whether this greater benefit observed in the mental improvement of the over-60s is confirmed in a larger sample.
Mental symptoms have been evaluated and reported in many post-Covid studies and in the most severe cases include severe headache, myalgia, depression, and sleeping disorder. In other subjects, a “brain fog” is reported, which is a mild cognitive decline that consists of difficulties in concentration, memory and executive abilities [18]. The “brain fog” symptoms are observed in the sample analyzed in this study judging by the answers to the questions concerning the feeling of anxiety, the ability to concentrate, the ability to think clearly and memory. Indeed, the data emerging at the end of the supplementation period show an improvement in anxiety and mental fatigue scores, probably because a correction with nutritional treatment can also act on psychological and not just physical well-being.
The improvement observed following nutritional treatment in subjects with chronic fatigue due to Covid-19 disease may be related to the action of the nutrients contained in Apportal® and their synergies on the same biological functions.
Indeed, it is well known that vitamins and minerals play a key role in counteracting chronic physical and mental fatigue. Group B Vitamins, Vitamin C, Iron and Magnesium are all involved in energy metabolism and when supplemented in the right amounts, are able to stimulate the physiological functions by inducing the body reach a condition of self-sufficiency which has health benefits in terms of physical and mental fatigue [19]. Calder in his review explained the role of nutrition in the SARS-CoV-2 infection and showed that nutrients such as Vitamin D, Zinc and Selenium can improve the immune system response especially at such a stressful time as recovery from SARS-CoV-2 infection [20]. Nonetheless, some of the substances mentioned and contained in Apportal® (Niacin, Vitamin C, Iron, Magnesium) have obtained specific health claims for the reduction of tiredness and fatigue from the European Food Safety Authority (EFSA), based on scientific evidence [21].
Besides, Apportal® contains plant extract like P. ginseng since several studies confirmed its effect in counteracting chronic fatigue in subjects with idiopathic chronic fatigue (ICF) and in chronic fatigue syndrome (CFS). P. ginseng also showed a positive effect in improvement of memory and cognitive performance on healthy subjects [22]. The anti-fatigue effect and the mental fatigue improvement observed after the supplementation could be also obtained thanks to the presence of P. ginseng in the formulation.
As a last point, it should be considered that oxidative stress is also a phenomenon closely related to persistent fatigue. Lee et al., who correlated the serum parameters of oxidative stress in healthy and fatigued subjects, found that parameters such as ROS (Reactive Oxygen Species) or SOD (Superoxide Dismutase) are significantly higher in fatigued subjects than in healthy subjects [23]. Therefore, a positive effect can also be attributed to antioxidant substances, such as Coenzyme Q10, Lycopene, Selenium and Vitamin C.
Nutritional supplements aim to improve the overall well-being of the subjects who take them, they do not have the ambition to “heal” them. Indeed, subjects involved in this study reported feeling better in terms of anxiety, fatigue and ability to concentrate, which means that the goal has been achieved. Furthermore, the supplementation with Apportal® could reduce the use of drugs that are commonly used in these situations, thanks to its ability to restore the physiological conditions of the body.
6. Limitation
This is an open-label observational trial and it has some limitation. First of all, the study lacks a control group that will clarify if the recovery occurs in the same way and at the same time even without supplementation. It may also be useful to design a placebo-controlled RCT and biochemical parameters or objective assessments can also be taken into consideration, not only self-administered questionnaires. Further studies are needed to assess the effect of nutritional supplementation even in patients older and with severe fatigue, especially when these symptoms persist for long time after the end of the infection.
7. Conclusions
In conclusion, the novelty of this study is the demonstration that a combination of nutrients can have a positive synergistic effect on decreasing chronic fatigue in moderately fatigued subjects and improving quality of life. This is the first evidence to offer a solution for speeding up the recovery of subjects with chronic post-Covid fatigue and appears to be effective in almost all subjects (90.05%), regardless of gender, age, and severity of the previous disease.
The condition of chronic fatigue observed in these SARS-CoV-2 infected subjects is comparable to other chronic physical and mental fatigue conditions found in subjects with other chronic diseases. The results obtained from this study show that supplementation with Apportal® can be a quick and easy opportunity to counteract chronic fatigue.
Funding
This work was supported by Pharmanutra Spa, Pisa (Italy).
Author contributions
Maria Sole Rossato: Conceptualization, Validation, Project administration and Writing - Original draft. Elisa Brilli: Conceptualization and Writing - Original draft. Nicola Ferri: Investigation and Writing - Reviewing and Editing. Giulio Giordano: Investigation and Writing - Reviewing and Editing. Germano Tarantino: Conceptualization, Supervision and Writing - Original draft.
Declaration of competing interest
Maria Sole Rossato and Germano Tarantino are Pharmanutra S.p.a. employees. Elisa Brilli is Alesco S.r.l. employee, a company included in Pharmanutra group. The other Authors declare that they have no conflict of interest.
Acknowledgments
Our thanks go to the general practitioners for their valuable contribution in the recruitment of patients, as well as in the collection of informed consent and the questionnaires filled in by the subjects. In alphabetical order: Dr. Agostinelli Marco, Dr. Altieri Giuseppe, Dr. Arimatea Salvatore, Dr. Barone Filippo, Dr. Bartolini Silvio Maria, Dr. Bennici Gaspare, Dr. Bizzini Carmelo, Dr. Bonfirraro Saverio, Dr. Bongiovanni Elisa, Dr. Bovi Michela, Dr. Calzolari Cinzia, Dr. Campanale Maria, Dr. Campo Iole, Dr. Candoli Paolo Mario Martino, Dr. Cardetta Antonio, Dr. Cargnello Sabrina, Dr. Castaldi Domenico, Dr. Cavallo Pasqualino, Dr. Chiarella Gianclaudio, Dr. Cialini Paolo Maria, Dr. Cifali Ermenegildo, Dr. Colella Michele, Dr. Corbi Lina, Dr. Cramarossa Saverio, Dr. Cusumano Mariano, Dr. D'Alessandro Luigi, Dr. D'Amato Mario, Dr. D'Amico Giacomo, Dr. D'Ercole Nello, Dr. De Francesco Pietro, Dr. De Prisco Serena, Dr. Di Martino Gennaro, Dr. Fantozzi Giacomo, Dr. Gaeta Chiara, Dr. Gargiulo Maria Vittoria, Dr. Granito Giuseppe, Dr. Hamoud Abdul Amir, Dr. La Penna Walter, Dr. Leone Serena, Dr. Maiello Giacomo, Dr. Maltese Liberia, Dr. Marasso Sara, Dr. Marinelli Paolo, Dr. Mazzaferro Mauro, Dr. Mazzei Dominique, Dr. Menciotti Anna Maria, Dr. Meneghetti Loris, Dr. Micheli Davide, Dr. Michelini Daniela, Dr. Minuto Maurizio, Dr. Mogavero Paola, Dr. Monaco Pietro Gino, Dr. Pagliero Markus, Dr. Palumbo Rossella, Dr. Pandale Michele, Dr. Patriccioli Stefano, Dr. Petraccaro Agostino, Dr. Pistritto Maurizio, Dr. Polizzotti Nunziella, Dr. Poloni Andrea, Dr. Polvere Rosanna, Dr. Prado Marta, Dr. Putaggio Rosalba, Dr. Richichi Giuseppe, Dr. Rinaldi Francesca, Dr. Russo Sabatino, Dr. Scala Roberto, Dr. Scarabello Francesco, Dr. Sclafani Vito, Dr. Sgherri Giulia, Dr. Solinas Pietro, Dr. Turcato Francesca, Dr. Turino Salvatore, Dr. Ventura Alessandra.
We would also thanks Dr. Andrea Ripoli for the statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2021.08.031.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Dennis A., Wamil M., Alberts J., Oben J., Cuthbertson D., Wootton D., et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klimas N., Broderick G., Fletcher M.A. Biomarkers for chronic fatigue. Brain Behav Immun. 2012;26:1202–1210. doi: 10.1016/j.bbi.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15(11) doi: 10.1371/journal.pone.0240784. Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenforde M.W., Kim S., Lindsell C., Billig Rose E., Shapiro N., Files C., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network— United States, March–June 2020. Morb Mortal Wkly Rep. 2020; Jul 31;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. J Am Med Assoc. 2020; Aug 11;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scordo K.A., Richmond M., Munro N. Post–COVID-19 syndrome: theoretical basis, identification, and management. ACN Advanced Critical Care. 2021;32(2):188–194. doi: 10.4037/aacnacc2021492. [DOI] [PubMed] [Google Scholar]
- 8.Bornstein S.R., Voit-Bak K., Donate T., Rodionov R.N., Gainetdinov R.R., Tselmin S., et al. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: is there a role for extracorporeal apheresis? Mol Psychiatr. 2021; Jun 17:1–4. doi: 10.1038/s41380-021-01148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candan S.A., Elibol N., Abdullahi A. Consideration of prevention and management of long-term consequences of post-acute respiratory distress syndrome in patients with COVID-19. Physiother Theory Pract. 2020;36(6):663–668. doi: 10.1080/09593985.2020.1766181. Jun. [DOI] [PubMed] [Google Scholar]
- 10.Inglis J.E., Lin P.J., Kerns S.L., Kleckner I.R., Kleckner A.S., Castillo D.A., et al. Nutritional interventions for treating cancer-related fatigue: a qualitative review. Nutr Canc. 2019;71(1):21–40. doi: 10.1080/01635581.2018.1513046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira P., Reis A.D., Diniz R.R., Lima F.A., Leite R.D., da Silva M.C.P., et al. Dietary supplements and fatigue in patients with breast cancer: a systematic review. Breast Canc Res Treat. 2018 Oct;171(3):515–526. doi: 10.1007/s10549-018-4857-0. [DOI] [PubMed] [Google Scholar]
- 12.Na W., Kim J., Kim H., Lee Y., Jeong B., Lee S.P., et al. Evaluation of oral nutritional supplementation in the management of frailty among the elderly at facilities of community care for the elderly. Clin Nutr Res. 2021 Jan;10(1):24–35. doi: 10.7762/cnr.2021.10.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balestroni G., Bertolotti G. EuroQoL-5D (EQ-5D): an instrument for measuring quality of life. Monaldi Arch Chest Dis. 2012;78:155–159. doi: 10.4081/monaldi.2012.121. [DOI] [PubMed] [Google Scholar]
- 14.Cella D., Wilson H., Shalhoub H., Revicki D.A., Cappelleri J.C., Bushmakin A.G., et al. Content validity and psychometric evaluation of Functional Assessment of Chronic Illness Therapy-Fatigue in patients with psoriatic arthritis. J Patient Rep Outcomes. 2019; May 20;3(1):30. doi: 10.1186/s41687-019-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner E., Menzel U., Puri M.L. Rank score test in factorial designs with repeated measures. J Multivariate Anal. 1999;70:286–317. [Google Scholar]
- 16.IMPATTO DELL’EPIDEMIA COVID-19 sulla mortalità totale della POPOLAZIONE RESIDENTE PERIODO GENNAIO-novembre 2020. Rapporto ISTAT 30 December 2020.
- 17.Venturelli S., Benatti S.V., Casati M., Binda F., Zuglian G., Imeri G., et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149:e32. doi: 10.1017/S0950268821000145. Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021 Apr;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tardy A.L., Pouteau E., Marquez D., Yilmaz C., Scholey A. Vitamins and minerals for energy, fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients. 2020 Jan 16;12(1):228. doi: 10.3390/nu12010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calder P.C. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020; May 20;3(1):74–92. doi: 10.1136/bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of health claims related to vitamin C, magnesium, iron, niacin and reduction of tiredness and fatigue. EFSA Journal. 2010;8(10):1740-1807–1757-1815. [Google Scholar]
- 22.Ogawa-Ochiai K., Kawasaki K. Panax ginseng for frailty-related disorders: a review. Front Nutr. 2019;5:140. doi: 10.3389/fnut.2018.00140. Jan 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J., Kim H., Lee D., Son C. Oxidative stress is a convincing contributor to idiopathic chronic fatigue. Sci Rep. 2018; Aug 27;8(1):12890. doi: 10.1038/s41598-018-31270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.