Abstract
Background
Coronavirus disease 2019 (COVID‐19) is associated with coagulopathy but the optimal prophylactic anticoagulation therapy remains uncertain and may depend on COVID‐19 severity.
Objective
To compare outcomes in hospitalized adults with severe COVID‐19 treated with standard prophylactic versus intermediate dose enoxaparin.
Methods
We conducted a multi‐center, open‐label, randomized controlled trial comparing standard prophylactic dose versus intermediate dose enoxaparin in adults who were hospitalized with COVID‐19 and admitted to an intensive care unit (ICU) and/or had laboratory evidence of coagulopathy. Patients were randomly assigned in a 1:1 ratio to receive standard prophylactic dose enoxaparin or intermediate weight‐adjusted dose enoxaparin. The primary outcome was all‐cause mortality at 30 days. Secondary outcomes included arterial or venous thromboembolism and major bleeding.
Results
A total of 176 patients (99 males and 77 females) underwent randomization. In the intention‐to‐treat population, all‐cause mortality at 30 days was 15% for intermediate dose enoxaparin and 21% for standard prophylactic dose enoxaparin (odds ratio, 0.66; 95% confidence interval, 0.30–1.45; P = .31 by Chi‐square test). Unadjusted Cox proportional hazards modeling demonstrated no significant difference in mortality between intermediate and standard dose enoxaparin (hazard ratio, 0.67; 95% confidence interval, 0.33–1.37; P = .28). Arterial or venous thrombosis occurred in 13% of patients assigned to intermediate dose enoxaparin and 9% of patients assigned to standard dose enoxaparin. Major bleeding occurred in 2% of patients in each arm.
Conclusion
In hospitalized adults with severe COVID‐19, standard prophylactic dose and intermediate dose enoxaparin did not differ significantly in preventing death or thrombosis at 30 days.
Keywords: anticoagulant, blood coagulation, COVID‐19 disease, enoxaparin, thrombosis
Essentials
-
•
Coronavirus disease 2019 (COVID‐19) is associated with coagulopathy that may contribute to mortality.
-
•
The risks and benefits of anticoagulation may vary depending on the severity of COVID‐19.
-
•
We prospectively compared standard prophylactic and intermediate dose enoxaparin in severe COVID‐19.
-
•
No differences in overall mortality, thrombosis, or bleeding were observed between the two arms.
Alt-text: Unlabelled Box
1. INTRODUCTION
In severe cases, coronavirus disease 2019 (COVID‐19) is characterized by profound systemic inflammation and a coagulopathy with some laboratory features similar to disseminated intravascular coagulation (DIC).1., 2. Abnormal coagulation parameters such as elevated D‐dimer are associated with increased risk of thromboembolism, organ failure, and death in patients hospitalized with COVID‐19.1., 3., 4., 5. Observational studies have suggested that preventive treatment with anticoagulants may improve clinical outcomes.6., 7., 8., 9. Guidance documents issued by professional societies recommend prophylactic anticoagulation with heparin or low molecular weight heparin (LMWH) for hospitalized patients with COVID‐19 while acknowledging that high‐quality evidence is lacking, optimal dosages remain uncertain, and prospective randomized controlled trials comparing different intensities of anticoagulation are needed.10., 11., 12., 13., 14.
A recent report from the prospective INSPIRATION trial suggested no benefit of intermediate dose prophylactic anticoagulation over standard dose prophylactic anticoagulation in preventing thrombosis or death among patients with COVID‐19 who required admission to an intensive care unit (ICU).15 A preliminary report from a collaboration between three prospective trials (ATTACC, REMAP‐CAP, and ACTIV‐4a) suggested that the optimal dosing of prophylactic anticoagulation therapy may depend on the severity of COVID‐19 illness.16 An interim analysis of these trials suggested benefit from therapeutic dose anticoagulation (heparin or LMWH) compared to usual care pharmacologic thromboprophylaxis in patients hospitalized with moderate COVID‐19 but potential harm in patients with more severe COVID‐19 who required ICU‐level care, raising concerns about the risk of bleeding with therapeutic dose anticoagulation.16 Additional prospective data are needed to further evaluate the safety and efficacy of escalated dose prophylactic anticoagulation in patients with a range of COVID‐19 severity.
We conducted an investigator‐initiated, multi‐center, open‐label, randomized controlled trial comparing standard prophylactic and intermediate dosing of the LMWH enoxaparin in adults hospitalized with severe COVID‐19, defined as requiring intensive care or manifested by laboratory criteria for coagulopathy.
2. METHODS
2.1. Trial design
We conducted the trial at three centers in the United States; the University of Iowa, Iowa City, IA (coordinating center); Gunderson Health System, La Crosse, WI; and Louisiana State University Health Shreveport, Shreveport, LA. The trial was designed as a prospective, randomized, open‐label, interventional study to compare the safety and efficacy of two enoxaparin dosing protocols in adult patients hospitalized with confirmed SARS‐CoV‐2 infection and evidence of severe COVID‐19, defined as requiring admission to an ICU and/or having laboratory evidence of coagulopathy. The trial protocol was approved by the local institutional review boards of the participating sites. The trial was opened initially at the University of Iowa in April 2020; Gunderson Health System was added as a second site in June 2020, and Louisiana State University Health Shreveport was added as a third site in October 2020. Written informed consent was obtained from all patients or their legally authorized representatives. The trial was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Full details of the trial protocol, conduct, oversight, and analyses can be found in the Clinical Trial Protocol and Statistical Analysis Plan (available in the supporting information). The trial was registered with ClinicalTrials.gov (NCT04360824).
2.2. Participants
Patients hospitalized with a diagnosis of COVID‐19 were screened for eligibility. Adults 18 years of age or older with SARS‐CoV‐2 infection confirmed by nasopharyngeal swab polymerase chain reaction and requiring hospitalization were eligible if they were admitted to an ICU and/or had a modified ISTH Overt DIC score ≥3 (Table S1 in supporting information).17 D‐dimer was measured in fibrinogen equivalent units. Patients were excluded if there was an indication for full therapeutic dose anticoagulation or they had active major bleeding, severe thrombocytopenia (platelet count <25,000/μL), current pregnancy, a history of acute venous or arterial thrombosis within the prior 3 months, or acute or chronic renal insufficiency with an estimated creatinine clearance <30 ml/min calculated by the modified Cockcroft and Gault formula. The full list of inclusion and exclusion criteria is provided in the protocol.
2.3. Randomization and masking
Using the Research Electronic Data Capture (REDCap) platform,18 patients were randomly assigned in a 1:1 ratio to receive either a standard prophylactic dose or an intermediate dose of enoxaparin. Both the standard and intermediate doses were adjusted for obesity. The standard dose was 40 mg SC daily if the body mass index (BMI) was <30 kg/m2 and either 30 mg SC twice daily or 40 mg SC twice daily if the BMI was ≥30. The choice of 30 mg twice daily or 40 mg twice daily was determined by the treating physician according to the local institutional standard of practice. The intermediate dose was 1 mg/kg SC daily if the BMI was <30 or 0.5 mg/kg SC twice daily if the BMI was ≥30. All doses were rounded up to the nearest unit‐dose syringe. This was an open‐label trial without masking.
2.4. Procedures
Patients received the assigned dose of enoxaparin until hospital discharge or a clinical event occurred requiring either discontinuation of anticoagulation therapy or initiation of full therapeutic dose anticoagulation therapy. Dose reductions were specified for severe thrombocytopenia (platelet count <25,000/μL), hypofibrinogenemia (fibrinogen < 50 mg/dL), or acute kidney injury defined as an estimated creatinine clearance <30 mL/min (see protocol). Some patients received other experimental or approved treatments for COVID‐19 (see Table 2 ). Enrollment in non‐competing clinical trials was allowed.
TABLE 2.
Other treatments for COVID‐19 in the intention‐to‐treat population during the trial perioda
| All patients (N = 173) |
Standard dose (N = 86) |
Intermediate dose (N = 87) |
P‐valueb | |
|---|---|---|---|---|
| Azithromycin—no. (%) | 36 (21) | 11 (13) | 25 (29) | .01 |
| Convalescent plasma—no. (%) | 46 (27) | 23 (27) | 23 (26) | .96 |
| Corticosteroids—no. (%) | 130 (75) | 67 (78) | 63 (72) | .40 |
| Famotidine—no. (%) | 55 (32) | 30 (35) | 25 (29) | .39 |
| Remdesivir—no. (%) | 105 (61) | 54 (63) | 51 (59) | .59 |
The protocol allowed patients to receive other experimental or approved treatments for COVID‐19.
Chi‐square test.
2.5. Outcomes
The primary outcome measure was all‐cause mortality at 30 days. Secondary outcome measures included acute kidney injury, defined as estimated creatinine clearance <30 ml/min, arterial or venous thrombosis confirmed with imaging, major bleeding, and minor bleeding. Major bleeding was defined according to ISTH criteria.19 Minor bleeding was defined as a bleeding event that did not meet ISTH criteria for major bleeding. The outcomes were adjudicated independently by two investigators who were not blinded to the treatment arm. The study protocol also included an exploratory laboratory biomarker component that will be reported separately.
2.6. Statistical analysis
Demographic and clinical measures collected were summarized and tested for differences. Continuous measures are displayed as medians and interquartile ranges. Tests for differences used the Wilcoxon rank sum test. Categorical measures were displayed as counts and percentages. Tests for differences used Pearson's chi‐square and Fisher's exact tests, where appropriate.
Analysis of the primary and secondary outcome measures was performed on the intention‐to‐treat population, defined as all patients who provided informed consent and underwent randomization (N = 173, Figure 1 ). The primary outcome measure was assessed as the 30‐day all‐cause mortality. In an exploratory analysis, we also assessed the time to death with censoring at 30 days. We hypothesized that the intermediate dose enoxaparin group (intervention arm) has a mortality rate below (and time‐to‐death above) the standard prophylactic dose enoxaparin group (standard of care arm). Estimates for the 30‐day mortality odds ratio, confidence interval, and Pearson's chi‐square P‐value, testing for a difference between doses, are provided. For the time‐to‐death analysis, differences between doses were analyzed using Cox proportional hazard modeling and reported as hazard ratios with 95% confidence intervals, along with their P‐values. We used log(−log[survival]) plots to verify the proportional hazards assumption. Raw proportional hazards models were fit on five samples: all patients, patients with BMI < 30, BMI > 30, admitted to ICU, and not admitted to ICU. Additional models were fit in both the intention‐to‐treat and per‐protocol populations, adjusting for age, gender, BMI, and ICU admission. We estimated that the risk of death within 30 days would be 40% in the standard dose enoxaparin group.6 Assuming the risk would be reduced to 20% in the intermediate dose enoxaparin group, we calculated that the assignment of 164 patients with 1:1 randomization would provide 80% power for a two‐sided test to detect such a difference in the primary outcome between the two arms with alpha of 0.05. The presumed effect size was chosen by consensus among the investigators at the coordinating center.
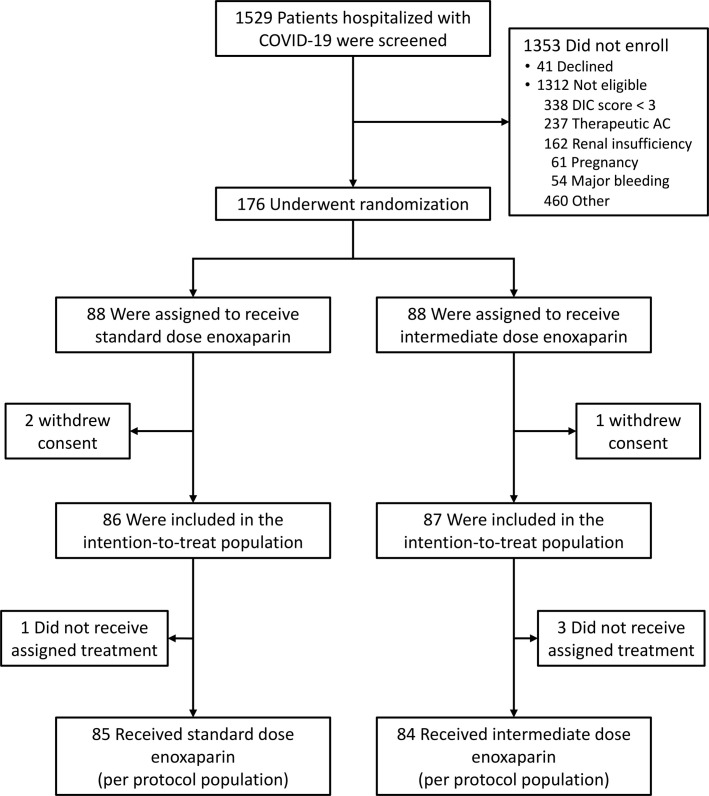
FIGURE 1.
Screening, enrollment, randomization and populations analyzed.
Secondary outcomes included arterial or venous thromboembolism (VTE) and major bleeding. Like the dichotomous primary outcome, secondary outcome comparisons are reported as estimates for the odds ratios, confidence intervals, and P‐values. Additional analyses were performed on the per‐protocol population, defined as all randomized patients who received the assigned treatment, to assess the sensitivity of our results after removing untreated patients.20 SAS 9.4 was used for all calculations and data analysis.
2.7. Role of the funding source
This trial was funded by a Clinical and Translational Science Award from the National Institutes of Health (UL1 TR002537). The funding agency had no role in the study design, data collection, data analysis, or interpretation.
3. RESULTS
3.1. Patients
Between April 26, 2020, and January 6, 2021, 1529 patients hospitalized with COVID‐19 were screened for enrollment. More than 85% of screened patients were not enrolled because they declined to participate or did not meet the eligibility criteria (Figure 1). Reasons for screen failure included declination of consent (3%), a modified ISTH DIC Score <3 (25%), an indication for therapeutic dose anticoagulation (17.5%), renal insufficiency (12%), pregnancy (4.5%), major bleeding (4%), and past COVID infection or failure to meet other eligibility criteria (34%).
A total of 176 patients (99 males and 77 females) were enrolled and underwent randomization (145 patients at the University of Iowa, 26 patients at Gunderson Health System, and 5 patients at Louisiana State University – Shreveport); 88 were assigned to receive standard prophylactic dose enoxaparin and 88 to receive intermediate dose enoxaparin. Three patients withdrew consent prior to the initiation of treatment and were excluded from the intention‐to‐treat population (Figure 1). Four additional patients did not receive the assigned treatment; these patients were included in the intention‐to‐treat population but excluded from the per‐protocol population.
Baseline characteristics of the patients in the intention‐to‐treat population are shown in Table 1 . The median age of the patients was 64 years (range 24–86); 14% were Hispanic, 6% were Black, and 76% were White. More than 60% of the patients had a BMI greater than 30. Pre‐existing medical conditions included hypertension in 60% of the patients, diabetes mellitus in 37%, heart disease in 31%, and lung disease in 23%. Forty‐two percent of the patients were current or former cigarette smokers. At the time of enrollment, 107 patients (62%) were admitted to an ICU and 40 (23%) were receiving invasive mechanical ventilation. The median value for D‐dimer was 1680 ng/mL (interquartile range 920–3980 ng/mL). Similar percentages of patients in the two arms received corticosteroids (75% of patients), remdesivir (61%), convalescent plasma (27%), or famotidine (32%) (Table 2). More patients received azithromycin in the intermediate dose arm (29%) than in the standard dose arm (13%, P = .01). Thirty‐seven patients were enrolled in other non‐competing COVID clinical trials (20 in the standard dose arm and 17 in the intermediate dose arm).
TABLE 1.
Baseline characteristics of the patients in the intention‐to‐treat population
| All patients (N = 173) |
Standard dose (N = 86) |
Intermediate dose (N = 87) |
|
|---|---|---|---|
| Median age—years (range) | 64 (24–86) | 63.5 (30–85) | 65 (24–86) |
| Gender—no. (%) | |||
| Female | 76 (44) | 36 (42) | 40 (46) |
| Male | 97 (56) | 50 (58) | 47 (54) |
| Race or ethnic group—no. (%) | |||
| Black | 10 (6) | 3 (3) | 7 (8) |
| Asian | 4 (2) | 2 (2) | 2 (2) |
| Hispanic | 24 (14) | 15 (17) | 9 (10) |
| White | 131 (76) | 63 (73) | 68 (78) |
| Other | 4 (2) | 3 (3) | 1 (1) |
| Median BMI—kg/m2 (IQR) | 30.5 (25.9–36.2) | 30.7 (27.2–35.8) | 30.0 (24.7–36.6) |
| BMI ≥ 30 kg/m2—no. (%) | 106 (61) | 56 (65) | 50 (57) |
| Median time from positive COVID‐19 test to enrolment—days (IQR) | 5 (1–9) | 4.5 (1–9) | 5 (1–9) |
| Coexisting conditions—no. (%) | |||
| Cancer | 20 (12) | 13 (15) | 7 (8) |
| Diabetes mellitus | 64 (37) | 34 (40) | 30 (34) |
| Heart disease | 54 (31) | 27 (31) | 27 (31) |
| Hypertension | 104 (60) | 53 (62) | 51 (59) |
| Lung disease | 39 (23) | 19 (22) | 20 (23) |
| Obesity | 84 (49) | 39 (45) | 45 (52) |
| Current or former smoker—no. (%) | 73 (42) | 38 (44) | 35 (40) |
| Admitted to ICU—no. (%) | 107 (62) | 54 (63) | 53 (61) |
| Median lab values (IQR) | |||
| D‐dimer (ng/ml FEU) | 1680 (920–3980) | 1900 (870–3980) | 1570 (1030–4050) |
| Prothrombin time (s) | 11 (11–12) | 11 (11–12) | 11 (11–12) |
| Fibrinogen (mg/dl) | 552 (461–666) | 552 (461–654) | 543 (462–670) |
| Platelet count (x 109/L) | 261 (207–358) | 270 (204–358) | 257 (217–332) |
| Absolute lymphocyte count (x 106/L) | 781 (500–1310) | 735 (545–1460) | 800 (490–1230) |
Abbreviation: FEU, fibrinogen equivalent units; ICU, intensive care unit; IQR, interquartile range.
Enoxaparin was continued at the assigned dosage until hospital discharge or death for 145 patients (84%). Enoxaparin was dose adjusted or discontinued in 28 patients (18 patients in the standard dose enoxaparin arm and 10 patients in the intermediate dose enoxaparin arm) for acute kidney injury (12 patients), bleeding (3 patients), an indication for full therapeutic dose anticoagulation due to acute thrombosis (9 patients) or atrial fibrillation (2 patients), transition to end of life goals of care (1 patient), or protocol deviation (1 patient).
A total of 20 patients experienced a thrombotic event, including 8 episodes of arterial thrombosis and 13 episodes of venous thrombosis (one patient had both venous and arterial thrombosis; Table 3 ). Of these, 9 patients discontinued the assigned treatment with enoxaparin at the time of diagnosis of acute thrombosis to be started on therapeutic anticoagulation. The other 11 patients either continued the assigned treatment with enoxaparin because therapeutic anticoagulation was not indicated for arterial thrombosis (1 patient) or had discontinued the assigned treatment due to acute kidney injury (5 patients) or hospital discharge (5 patients) prior to the diagnosis of thrombosis.
TABLE 3.
Primary and secondary outcome measures at 30 daysa
| Standard dose (N = 86) |
Intermediate dose (N = 87) |
Odds ratio (95% CI) |
P‐value | |
|---|---|---|---|---|
| Primary outcome | ||||
| All‐cause mortality—no. (%)b | 18 (21) | 13 (15) | 0.66 (0.30–1.45) | .31 |
| Secondary outcomes | ||||
| Acute kidney injury—no. (%)b | 15 (17) | 11 (13) | 0.68 (0.29–1.59) | .38 |
| Arterial thrombosis—no. (%)c | 3 (3) | 5 (6) | 1.69 (0.39–7.29) | .72 |
| Venous thrombosis—no. (%)c | 6 (7) | 7 (8) | 1.79 (0.51–6.25) | >.99 |
| Major bleeding—no. (%)c | 2 (2) | 2 (2) | 0.99 (0.14–7.14) | >.99 |
| Minor bleeding – no. (%)c | 6 (7) | 6 (7) | 0.99 (0.31–3.23) | >.99 |
Abbreviation: CI, confidence interval.
Intention‐to‐treat population (N = 173).
Chi‐square test.
Fisher's exact test.
3.2. Primary outcome
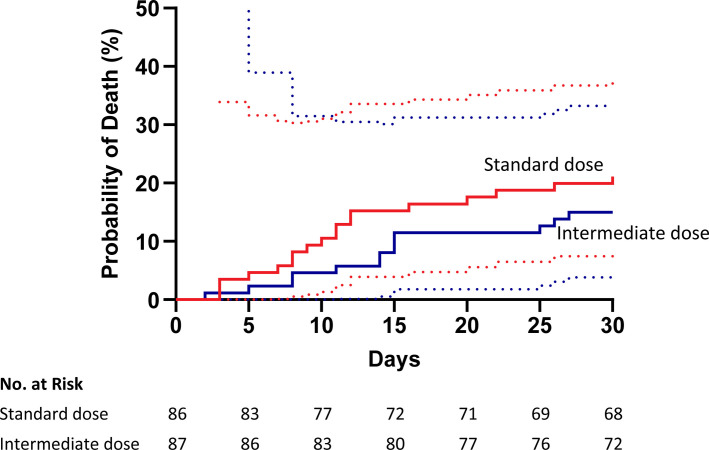
A total of 31 of the 173 patients (18%) in the intention‐to‐treat population died within 30 days of enrollment. All‐cause mortality at 30 days was 15% for those assigned to receive intermediate dose enoxaparin and 21% for those assigned to receive standard dose enoxaparin (P = .31 by a Chi‐square test; Table 3). Cumulative incidence plots of the probability of death for all patients in the two arms are shown in Figure 2 . In an unadjusted Cox proportional hazard model, the hazard ratio for mortality in the intermediate dose group compared to the standard dose group was 0.67 (95% confidence interval [CI], 0.33–1.37; P = .28). After adjustment for age, gender, BMI, and ICU admission, the hazard ratio for mortality was 0.57 (95% CI, 0.28–1.17, P = .12) in the intention‐to‐treat population and 0.53 (95% CI, 0.24–1.13, P = .10) in the per‐protocol population (Table 4 ). The cumulative incidence of death did not differ significantly between the two arms among the 107 patients admitted to an ICU at the time of enrollment (hazard ratio [HR], 0.47; 95% CI, 0.19–1.15, P = .10) or the 66 patients not admitted to an ICU at the time of enrollment (HR, 1.41; 95% CI, 0.40–4.98, P = .60). Three patients died after discharge from the hospital within 30 days of enrollment, two in the standard dose arm and one in the intermediate dose arm (Table S2 in supporting information).
FIGURE 2.
Time to event (cumulative incidence) plot of the probability of death for all patients in the intention‐to‐treat population. In an unadjusted Cox proportional hazard model, the hazard ratio for mortality in the intermediate dose group compared with the standard dose group was 0.67; 95% confidence interval, 0.33 to 1.37, P = 0.28. The dotted lines indicate the 95% confidence interval bands.
TABLE 4.
Hazard ratios for intermediate versus standard dose enoxaparin adjusted for control variablesa
| Model | Hazard ratio | 95% CI | P‐value |
|---|---|---|---|
| Intention‐to‐treat population | |||
| Unadjusted | 0.67 | 0.33–1.37 | .276 |
| Adjusted for age, gender, BMI, and ICU admission | 0.57 | 0.28–1.17 | .123 |
| Per‐protocol population | |||
| Unadjusted | 0.61 | 0.29–1.31 | .208 |
| Adjusted for age, gender, BMI, and ICU admission | 0.53 | 0.24–1.13 | .100 |
Abbreviations: CI, confidence interval; BMI, body mass index; ICU, intensive care unit.
Cox proportional hazard model (PHREG).
Because the prespecified dosage of enoxaparin was determined by the BMI, we performed a post hoc analysis of the primary outcome in subgroups of patients with and without obesity. Among the 67 patients with a BMI less than 30, the hazard ratio for all‐cause mortality in the intermediate dose group compared to the standard dose group was 0.55 (95% CI, 0.19–1.59, P = .27) (Figure S1A in supporting information) compared to 0.75 (95% CI, 0.29–1.98, P = .57) for the 106 patients with a BMI > 30 (Figure S1B). Figure S2A in supporting information summarizes the assigned dosage of enoxaparin in the intention‐to‐treat population. The relationship between the daily dosage of enoxaparin (mg per kg body weight) and mortality is illustrated in Figure S2B. Within each subgroup, the daily dosage of enoxaparin did not differ between patients who were alive or deceased on day 30.
3.3. Secondary outcomes
Acute kidney injury, defined as an estimated creatinine clearance less than 30 mL/min, occurred in 17% of patients in the standard dose enoxaparin arm and 13% of patients in the intermediate dose enoxaparin arm (P = .38; Table 3). Arterial thrombosis was diagnosed in 3 of 86 patients (3%) in the standard dose enoxaparin arm and 5 of 87 patients (6%) in the intermediate dose enoxaparin arm (P = .72). Three patients had ischemic stroke, four patients had myocardial infarction, and one patient had both ischemic stroke and myocardial infarction. Of the five patients who had myocardial infarction, one patient in the standard dose enoxaparin arm had an inferior wall ST‐segment elevation myocardial infarction and underwent percutaneous coronary intervention with placement of a drug‐eluting stent in the right coronary artery. The other four patients were diagnosed with type 2 non‐ST segment elevation myocardial infarction based on ECG changes and serial troponin measurements, consistent with demand ischemia (two patients in the standard dose arm and two patients in the intermediate dose arm). Venous thrombosis was diagnosed in 7% of patients in the standard dose arm and 8% of patients in the intermediate dose arm (P > .99). Eleven patients had pulmonary embolism, one patient had inferior vena cava thrombosis, and one patient had both pulmonary embolism and deep vein thrombosis. Five of the eleven patients with pulmonary embolism were diagnosed following discharge from the hospital, all in the intermediate dose arm (Table S2 in supporting information). Major and minor bleeding were infrequent, occurring in 2% and 7%, respectively, of patients in both arms.
4. DISCUSSION
The optimal approach to prophylactic anticoagulation therapy in patients with COVID‐19 is under active investigation and remains a challenging clinical conundrum.1., 2., 4., 21. In a multi‐center, open‐label, randomized controlled trial of hospitalized adults with severe COVID‐19, we found no significant differences in the safety or efficacy of standard prophylactic dose versus weight‐adjusted intermediate dose enoxaparin in preventing death or thrombosis at 30 days. We did not observe excess major or minor bleeding in patients treated with intermediate dose enoxaparin in this population of hospitalized patients with high acuity COVID‐19. Our findings in ICU patients are in agreement with the recently reported results of the INSPIRATION trial.15
One limitation of our study is that the trial design was based on data available in early 2020 that suggested a mortality of up to 40% in hospitalized patients with severe COVID‐19 who were treated with standard prophylactic dose LMWH.6 Studies performed later in the pandemic suggested a lower in‐hospital mortality of 15 to 20%,7., 22. which is in agreement with our finding of 18% all‐cause mortality at 30 days. Another limitation is that the results of our study cannot be extrapolated to all patients hospitalized with COVID‐19, because more than 85% of screened patients did not meet the eligibility criteria. The most frequent reasons for screen failure were renal insufficiency, a clinical indication for therapeutic dose anticoagulation, or lack of laboratory evidence for coagulopathy. Both ICU and non‐ICU patients were eligible, so our results should not be compared directly to other trials limited only to critically ill ICU patients. Another potential difference between our trial and other trials of preventive anticoagulation therapy in COVID‐19 patients is the dosing of enoxaparin in obese patients. Standard prophylactic dosing of LWMH is typically adjusted for obesity, usually defined as a BMI greater than or equal to 30, although protocols for dose adjustment for weight are not standardized.34 Our protocol allowed obese patients in the standard dose arm to receive either 30 mg or 40 mg of enoxaparin twice daily per dosing guidelines at the participating institutions.35 In the intermediate dose arm, all obese patients were assigned to receive 0.5 mg/kg twice daily. To facilitate comparison with other trials, we have provided the total daily dosages of enoxaparin received by obese and non‐obese patients in both arms in Figure S2. Finally, our study was not designed to examine outcomes beyond 30 days. Therefore, additional studies are needed to confirm our findings and assess the impact of anticoagulation therapy on the long‐term effects of COVID‐19. Several other trials investigating a variety of different antithrombotic strategies, including intermediate dose LMWH, are currently ongoing.21., 23.
A hallmark of COVID‐19 is its wide range of severity, from asymptomatic infection to life‐threatening illness.24 Criteria for COVID‐19 severity are not standardized, but the baseline characteristics suggest that the patient population studied in this trial represented a group of patients at high risk for thrombotic complications of COVID‐19.4 All of the patients were hospitalized and had severe COVID‐19, which we defined as requiring admission to an ICU and/or having laboratory evidence of coagulopathy. Because initial reports suggested that the coagulopathy of COVID‐19 resembles DIC,1 we used a modified ISTH Overt DIC score17 to assess eligibility. In the intention‐to‐treat population, 62% of patients were admitted to an ICU, 34% required mechanical ventilation, and 77% had laboratory evidence for coagulopathy (modified DIC score ≥3). Similar to other reports of patients with severe COVID‐19,3 we found that the main driver of the DIC score was the plasma D‐dimer concentration.
It has been apparent since the early days of the COVID‐19 pandemic that coagulopathy and thromboembolism are highly prevalent among hospitalized and critically ill patients.3., 25., 26. A systematic review estimated a pooled incidence of VTE of 17% among hospitalized patients with COVID‐19.5 Coagulopathy with elevated plasma concentrations of D‐dimer is associated with COVID‐19 mortality even in the absence of a clinical diagnosis of thromboembolism.4 Post mortem findings from patients with COVID‐19 have demonstrated a high frequency of pulmonary microvascular platelet‐fibrin thrombi,7., 27., 28., 29. which suggests that coagulopathy may contribute to respiratory failure and death in COVID‐19 even in the absence of a clinical diagnosis of thromboembolism. Several potential mechanisms of COVID‐19‐‐driven coagulopathy have been proposed, including platelet hyperactivation,30., 31. prothrombotic antiphospholipid antibodies,32 and neutrophil extracellular traps.33 The contribution of coagulopathy to the adverse short‐term and long‐term clinical outcomes of COVID‐19 remains to be defined, however. Our protocol included an exploratory laboratory biomarker component in which blood samples from patients were collected for assessment of prothrombotic mechanisms and correlative studies. These studies are ongoing and will be reported separately.
In summary, in a multi‐center, open‐label randomized controlled trial, weight‐adjusted intermediate dose enoxaparin was not more effective than standard dose enoxaparin in preventing death or thrombosis in a population of hospitalized adults with severe COVID‐19. These prospective data will need to be interpreted in the context of other trials investigating strategies for thromboprophylaxis in patients with a range of severities of COVID‐19.15., 16., 34. Understanding the risks and benefits of escalated dose anticoagulation in critically ill COVID‐19 patients receiving intensive care is particularly challenging, with some conflicting evidence emerging from different trials.16., 34., 35. An international pooled analysis of trials investigating different dose regimens of anticoagulant interventions in hospitalized patients with COVID‐19 is planned.23
CONFLICTS OF INTEREST
S. R. Lentz has served as a paid consultant for Novo Nordisk, UniQure, and Argenx, all outside the scope of the submitted work. S. R. Bailey has served as an editor or committee member for Wiley, Abbott, and Boston Scientific Corporation. All other authors declare no competing interests.
AUTHOR CONTRIBUTIONS
The trial was conceived and designed by USP, IC, SD, and SRL. These authors also contributed to data collection, data analysis, and data interpretation. The manuscript was written by USP and SRL with input and revision by all of the authors. AW, GS, SRB, and LJR contributed to data collection and analysis. PTE and CW performed statistical analyses and contributed to writing and revision of the manuscript. USP, SRB, and LJR supervised trial activities at the three trial sites. All authors read and approved submission of the manuscript.
ACKNOWLEDGMENTS
The authors thank Tracy Peters, Cassidy Bowen, Christine Meyer, Monicah Jepkemboi, and Aakash Sheth, MD for study coordination and technical support. This trial was funded by the University of Iowa Section of Hematology and The University of Iowa Clinical and Translational Science Award granted with funds from the NIH (UL1 TR002537).
National Center for Advancing Translational SciencesUL1 TR002537
Footnotes
Manuscript handled by: Saskia Middeldorp
Supporting Information
Supplementary Material
Supplementary Material
REFERENCES
- 1.Miesbach W., Makris M. COVID‐19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connors J.M., Levy J.H. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al‐Samkari H., Karp Leaf R.S., Dzik W.H., et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez D., Garcia‐Sanchez A., Rali P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta‐analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadkarni G.N., Lala A., Bagiella E., et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rentsch C.T., Beckman J.A., Tomlinson L., et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughn V.M., Yost M., Abshire C., et al. Trends in venous thromboembolism anticoagulation in patients hospitalized with COVID‐19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thachil J., Juffermans N.P., Ranucci M., et al. ISTH DIC subcommittee communication on anticoagulation in COVID‐19. J Thromb Haemost. 2020;18:2138–2144. doi: 10.1111/jth.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spyropoulos A.C., Levy J.H., Ageno W., et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes G.D., Burnett A., Allen A., et al. Thromboembolism and anticoagulant therapy during the COVID‐19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuker A., Tseng E.K., Nieuwlaat R., et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID‐19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaczyk A., Rosovsky R.P., Reed C.T., Bankhead‐Kendall B.K., Bittner E.A., Chang M.G. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations. Crit Care. 2020;24:559. doi: 10.1186/s13054-020-03273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.INSPIRATION Investigators. Sadeghipour P., Talasaz A.H., et al. Effect of intermediate‐dose vs standard‐dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID‐19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ATTACC. ACTIV‐4a & REMAP‐CAP multiplatform RCT: results of interim analysis. National Heart, Lung, and Blood Institute. Published January 28, 2021.
- 17.Toh C.H., Hoots W.K., SSC on DIC of the ISTH The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5‐year overview. J Thromb Haemost. 2007;5:604–606. doi: 10.1111/j.1538-7836.2007.02313.x. [DOI] [PubMed] [Google Scholar]
- 18.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulman S., Kearon C., Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 20.Schulz K.F., Altman D.G., Moher D., Group C CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340 doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talasaz A.H., Sadeghipour P., Kakavand H., et al. Recent randomized trials of antithrombotic therapy for patients with COVID‐19: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;77:1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID‐19 is high and associated with a higher risk of mortality: A systematic review and meta‐analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tritschler T., Mathieu M.E., Skeith L., et al. International Network of VTCRNI‐VTE. Anticoagulant interventions in hospitalized patients with COVID‐19: A scoping review of randomized controlled trials and call for international collaboration. J Thromb Haemost. 2020;18:2958–2967. doi: 10.1111/jth.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 25.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolhnikoff M., Duarte‐Neto A.N., de Almeida Monteiro R.A., et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemost. 2020;18:1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manne B.K., Denorme F., Middleton E.A., et al. Platelet gene expression and function in patients with COVID‐19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hottz E.D., Azevedo‐Quintanilha I.G., Palhinha L., et al. Platelet activation and platelet‐monocyte aggregate formation trigger tissue factor expression in patients with severe COVID‐19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuo Y., Estes S.K., Ali R.A., et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID‐19. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo Y., Yalavarthi S., Shi H., et al. Neutrophil extracellular traps in COVID‐19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes R.D., de Barros E.S.P.G.M., Furtado R.H.M., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID‐19 and elevated D‐dimer concentration (ACTION): an open‐label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger J.S., Connors J.M. Anticoagulation in COVID‐19: reaction to the ACTION trial. Lancet. 2021;397:2226–2228. doi: 10.1016/S0140-6736(21)01291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material