Abstract
Background
During COVID‐19 outbreak, Italy was the first country in Europe to be heavily affected with an intensive care unit mortality of 26%. In order to reduce this percentage, physicians should establish clear and objective criteria to stratify COVID‐19 patients at high risk of in‐hospital death. Thus, the aim has been to test a large spectrum of variables ranging from clinical evaluation to laboratory biomarkers to identify which parameter would best predict all‐cause in‐hospital mortality in COVID‐19 patients.
Design
observational study.
Results
Multivariate Cox regression analysis showed that each 5 years of increase in age corresponded to a hazard ratio (HR) of 1.28 (95% CI 1.00‐1.65, P = .050); each increment of 803 ng/L of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) corresponded to a HR of 1.24 (95% CI 1.11‐1.39, P < .001); each increment of 58 ng/L of interleukin (IL)‐6 corresponded to a HR of 1.23 (95% CI 1.09‐1.40, P < .001), and each increment of 250 U/L of lactate dehydrogenase (LDH) corresponded to a HR of 1.23 (95% CI 1.10‐1.37, P < .001). According to the calculated cut‐points for age (≥70 years), NT‐proBNP (≥803 ng/L), IL‐6 (≥58 ng/L) and LDH (≥371 U/L) when 2 out of these 4 were overcome, the HR was 2.96 (95% CI 1.97‐4.45, P < .001).
Conclusion
In COVID‐19 patients, besides age, the evaluation of three biochemical parameters, available in few hours after hospital admission can predict in‐hospital mortality regardless of other comorbidities.
Keywords: COVID‐19, in‐hospital mortality, interleukin 6, lactate dehydrogenase, NT‐proBNP, PCSK9, Tn‐T
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic caused by severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) has recently caused high rates of morbidity and mortality worldwide.1 Italy was the first country in Europe to be heavily affected, being Lombardy the region with the highest burden of infections and deaths. As of April 2020, among SARS‐CoV‐2 infection confirmed individuals, the majority were older men, a large proportion requiring mechanical ventilation and high levels of positive end‐expiratory pressure, with an intensive care unit (ICU) mortality of 26%.2 In line with this study, analysis of a database including 592 acute care hospitals in the United States showed that risk factors most strongly associated with in‐hospital mortality were older age, male sex and white ethnicity.3
Given the high commitments of resources in ICU care, the goal of physicians and hospitals during the pandemic has been to establish clear and objective criteria to stratify COVID‐19 patients at high risk to death already from the admission at low‐ and high‐dependency units.4 Hence, we tried to build a prognostic model to optimize an early identification and intervention of COVID‐19 patients at risk of poor prognosis. Based on the clinical experience of this research group and to pursue the objective of the present study, namely to identify which clinical, biological and physiological parameter would best predict all‐cause in‐hospital mortality in COVID‐19 patients, a large spectrum of variables, ranging from clinical evaluation to laboratory biomarkers, has been considered. These variables were: (a) hypertension, which associates with respiratory failure,5 (b) complete blood count, which predicts in‐hospital mortality,6 (c) thrombo‐inflammatory biomarkers, such as D‐dimer, C‐reactive protein (CRP), ferritin and interleukin 6 (IL‐6) which are associated with increased severity and mortality,7 (d) levels of lactate dehydrogenase (LDH) indicating disseminate tissue injuries,8 (e) cardiovascular biomarkers, such as N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), and troponin (Tn‐T), which reflect haemodynamic cardiac stress and are frequently elevated during the disease and predict poor prognosis,9, 10 (f) circulating angiotensin‐converting enzyme 2 (ACE2) levels that could foresee disease progression or clinical worsening11 and (g) development of hypolipidaemia, which begins in patients with mild symptoms to become worse in an association with the disease severity.12 Among lipids, lipoprotein(a), composed of an LDL particle bound to apolipoprotein(a), seems to inhibit the activation of plasminogen to plasmin by endogenous tissue plasminogen activators, thus compromising clot lysis.13 Finally, proprotein convertase subtilisin/kexin type 9 (PCSK9) may inhibit cellular interferon expression, an interesting evidence considering that carriers of inborn errors of interferon‐dependent immunity are at high risk of severe COVID‐19.14
2. MATERIALS AND METHODS
2.1. Population
One‐hundred and eighteen consecutive patients hospitalized at the Cardiorespiratory COVID‐19 Unit of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico of Milan were enrolled in the study between February and June 2020. All patients were diagnosed to have SARS‐CoV‐2 infection through a positive RT‐PCR assay of nasopharyngeal swabs. In order to collect clinical data and biobanking, all of patients signed an informed consent and approved their participation to NETwork Registry (advice n. 241_2020, protocol n. 101389). The present study was approved as retrospective observational protocol by local ethical committee (protocol n. 107162). The study conforms to broad EQUATOR guidelines.15
Once admitted to the Cardiorespiratory Unit, patients were followed according to the standardized clinical protocol. All patients were hospitalized within 24 hours from positive diagnostic swab test for COVID‐19. Respiratory failure was defined through arterial gas analysis and expressed by partial pressure of oxygen to inspiratory fraction of oxygen ratio (PaO2/FiO2) and alveolar‐arterial oxygen gradient (ΔA‐aO2). Tachypnoea was defined by a respiratory rate above 20 breaths per minute. An elevated body temperature was defined as a temperature of ≥37.5℃.
Concomitant systolic and diastolic blood pressure (BP) were recorded, and then, mean BP was calculated as 1/3 sBP + 2/3 dBP. The following comorbidities were considered in the analysis: arterial hypertension, coronary artery disease, heart failure, peripheral vasculopathy, diabetes, chronic obstructive pulmonary disease, renal failure (defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2 calculated using the MDRD equation) and obesity (defined by a body mass index ≥30 kg/m2).
Specific in‐hospital treatments were antiviral therapy (lopinavir/ritonavir), hydroxychloroquine, enoxaparin, methylprednisolone and tocilizumab. If needed, patients were treated with antibiotics and anti‐hypertensive agents. Pre‐existent cardiovascular active treatments including ACE inhibitors (ACE‐i), angiotensin receptors antagonists, beta‐blockers, statins, antiplatelet agents and mineralocorticoid receptor antagonists (MRA) were recorded.
Oxygen support consisted of low‐ (nasal cannula) and high‐flow (Venturi and reservoir masks, nasal high flow) oxygen, helmet continuous positive airway pressure (CPAP) or noninvasive ventilation (NIV). The choice for the oxygen support was determined by rapid deterioration of PaO2/FiO2 ratio and upgraded if a further worsening after treatment was detected, according to internal protocol. During follow‐up, all‐cause in‐hospital death was considered as primary outcome, whereas successful treatment was defined as patient's hospital discharge.
2.2. Biochemical assessment
Blood samples were collected into EDTA tubes at rest, in supine position, within 24 hours after admission at the Cardiorespiratory Unit. CRP, D‐dimer, ferritin, LDH, NT‐proBNP and high‐sensitive Tn‐T were determined by blood sample collection during routine laboratory analyses performed at the Central Lab through turbidimetric assays. IL‐6 was determined by electro‐chemiluminescence binding assay (ECLIA). Normal levels of these markers were set according to laboratory reference values, that is CRP <0.5 mg/dL, D‐dimer <500 μg/L, IL‐6 < 6 ng/L, NT‐proBNP <800 ng/L, and Tn‐T < 14 ng/L, and LDH between 135 and 225 U/L. Neutrophilia has been defined as 6.5 × 103/µL.
Triacylglycerol, cholesterol, low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol, apolipoprotein (apo)B and lipoprotein(a) were evaluated by using appropriate calibrators and the binding site optilite automated, bench‐top turbidimeter (Optilite). Non‐HDL‐C was calculated as the difference between TC and HDL‐C. Plasma proprotein convertase subtilisin/kexin type 9 (PCSK9) concentrations were measured by a commercial ELISA kit (R&D Systems). Samples were diluted 1:20 and incubated onto a microplate pre‐coated with a monoclonal human‐PCSK9‐specific antibody. Sample concentrations were obtained by a four‐parameter logistic curve fit, with a minimum detectable PCSK9 concentration of 0.219 ng/mL. Intra‐ and inter‐assay CVs were 3.2% and 5.1%, respectively. ACE2 concentrations were measured by a commercial ELISA kit (Novus biologicals, US). Samples were diluted 1:10 and incubated onto a microplate pre‐coated with a biotinylated detection antibody specific for human ACE2. Sample concentrations were obtained by a four‐parameter logistic curve fit, with a detection range between 0.39 and 25 ng/mL and a sensitivity of 0.23 ng/mL. Intra‐ and inter‐assay CVs were 4.8% and 6.5%, respectively.
2.3. Statistical methods
Demographic and clinical characteristic data were expressed as mean and standard deviation when normally distributed, otherwise by median and first‐third quartiles. Categorical variables were expressed as frequencies and percentages. Survival time was calculated starting at the date of hospitalization till the date of all‐cause in‐hospital mortality. Data were censored at the date of hospital discharge for patients who have not experienced the event during their follow‐up.
Univariate and multivariate Cox proportional hazard (PH) models were used to evaluate the estimate of the hazard ratios (HR) and 95% confidence intervals (CI) for the association between all variables and in‐hospital mortality. The assumption of a PH was checked with the Log[‐log(survival)] plot and by the time‐dependent covariate test. Variables that were associated with survival (two‐sided P‐value < .05) in univariate analysis were included in multivariate Cox PH models. HRs in univariate analysis were calculated per 1 standard deviation increase in each predictor variable and for selected values in the multivariate analysis.
We categorized the continuous variables that were significantly associated with in‐hospital mortality choosing the most discriminative cut‐off point basing on the ROC analysis and using Youden's index which maximizes both sensitivity and specificity. Cut‐off points were also used to resize the HR of multiple cox model, apart from age that was reported for each 5 years of increase, basing on its distribution in our patients. Categorized variables were included in the risk score calculation assigning equal unitary weights to each variable. Individual risk score was based on the sum of weighted scores for each variable. We then compute the score for each patient. The score ranges between 0 and 4 with a high score indicating high risk of mortality.
We performed operating characteristic curve analysis and computed the area under the ROC curve to assess the discriminant performance of the risk score.
The model was internally validated by using threefold cross‐validation. We first randomly divided all data into three equal‐size subsamples. The aim is to use two subsamples for training and the remaining on for testing, over all possible permutations. The analysis is then repeated three times, with each of the three subsamples used once as the validation data. We calculated the AUC for the three analyses, using only the respective test data, and the three AUC statistics are then aggregated into means with 95% confidence intervals.
We compared survival curves of the four categories of the score using Kaplan‐Meier estimates, testing statistical significance using the log‐rank test. Statistical analyses were performed with SAS software (version 9.4) and R software (version 3.6.3).
3. RESULTS
3.1. Population characteristics
Out of 118 patients, 21 were excluded due to incomplete data and 97 were considered in the analysis. As reported in Table 1, 70% were male and the median period from symptoms to diagnosis was of 7 (4‐10) days. Mean age was 61 ± 13 years, with 23% presenting with obesity (mean BMI = 28 ± 4 kg/m2). Hypertension represented the most common comorbidity (41%) with a total of 23 patients given renin‐angiotensin‐aldosterone system (RAAS) inhibitors (13 ACE‐i and 10 ARBs), 16 β‐blockers, 10 statins, 16 acetylsalicylic acid and none MRA. According to the semi‐intensive care level of our Cardiorespiratory Unit, all patients were treated with helmet CPAP in acute phase.
TABLE 1.
Clinical characteristics of COVID‐19 patients at hospital admission
| Overall population (n 97) | |
|---|---|
| Sex, m (%) | 68 (70%) |
| Age, years, mean ± SD | 61 ± 13 |
| Obesity, n (%) | 22 (23%) |
| BMI, kg/m2, mean ± SD | 28 ± 4 |
| COPD, n (%) | 7 (8%) |
| Diabetes, n (%) | 14 (14%) |
| Hypertension, n (%) | 40 (41%) |
| Coronary artery disease, n (%) | 12 (12.4%) |
| Peripheral vasculopathy, n (%) | 11 (11.3%) |
| Heart failure, n (%) | 4 (4.1%) |
| Renal failure, n (%) | 9 (15%) |
| ΔA‐aO2, mm Hg, median (Q1‐Q3) | 204 (138‐240) |
| PaO2/FiO2, mm Hg, median (Q1‐Q3) | 221 (172‐283) |
| Systolic BP, mm Hg, mean ±SD | 133 ± 18 |
| Diastolic BP, mm Hg, mean ±SD | 77 ± 11 |
| Mean BP, mm Hg, mean ±SD | 96 ± 12 |
| WBC, ×103/µL, median (Q1‐Q3) | 7.34 (5.33‐9.85) |
| Neutrophils, ×103/µL, median (Q1‐Q3) | 5.84 (3.79‐8.09) |
| Lymphocytes, ×103/µL, median (Q1‐Q3) | 0.83 (0.63‐1.22) |
| Monocytes, ×103/µL, median (Q1‐Q3) | 0.41 (0.27‐0.60) |
| Eosinophil, ×103/µL, median (Q1‐Q3) | 0.01 (0.00‐0.06) |
| Basophil, ×103/µL, median (Q1‐Q3) | 0.01 (0.01‐0.02) |
| CRP, mg/dL, mean ± SD | 11 ± 8 |
| D‐dimer, µg/L, median (Q1‐Q3) | 1270 (682‐1977) |
| LDH, U/L, median (Q1‐Q3) | 311 (250‐424) |
| Ferritin, mg/L, median (Q1‐Q3) | 1111 (514‐2478) |
| NT‐proBNP, ng/L, median (Q1‐Q3) | 271 (75‐893) |
| Tn‐T, ng/L, median (Q1‐Q3) | 12 (6‐23) |
| IL‐6, ng/L, median (Q1‐Q3) | 40 (12‐97) |
| Triglycerides, mg/dL, median (Q1‐Q3) | 151 (123‐193) |
| Total cholesterol, mg/dL, median (Q1‐Q3) | 134 (107‐171) |
| HDL‐C, mg/dL, mean ± SD | 28 ± 10 |
| Non‐HDL‐C, mg/dL, median (Q1‐Q3) | 109 (85‐137) |
| PCSK9, ng/mL, median (Q1‐Q3) | 339 (251‐430) |
| Apolipoprotein B, g/L, mean ± SD | 0.7 ± 0.5 |
| Lipoprotein(a), nmol/L, median (Q1‐Q3) | 21 (11‐44) |
| ACE2, ng/mL, median (Q1‐Q3) | 68.2 (48‐91) |
| Lopinavir/ritonavir, n (%) | 97 (100%) |
| Hydroxychloroquine, n (%) | 97 (100%) |
| Low‐molecular‐weight heparin (LMWH), n (%) | 97 (100%) |
| Methylprednisolon, n (%) | 34 (35%) |
| Tocilizumab, n(%) | 3 (3%) |
| Disease duration, days, median (Q1‐Q3) | 12 (8‐19) |
| Overall mortality, %, (n. of deaths) | 20.6% (20) |
Data are expressed as mean ± SD (standard deviation) or median (first (Q1) and third (Q3) quartiles).
Abbreviations: ACE2, angiotensin‐converting enzyme 2; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; HDL‐C, high‐density lipoprotein cholesterol; IL‐6, interleukin 6; LDH, lactate dehydrogenase; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; PaO2/FiO2, pressure of arterial oxygen to fractional inspired oxygen concentration; PCSK9, proprotein convertase subtilisin/kexin type 9; Tn‐T, troponin T; WBC, white blood cells; ΔA‐aO2, alveolar‐arterial oxygen gradient (ΔA‐aO2).
Mild‐to‐moderate respiratory failure was diagnosed in all patients (median PaO2/FiO2 221 mm Hg and ΔA‐aO2 204 mm Hg), and 52 had respiratory rate ≥20 breaths per minute. NT‐proBNP and Tn‐T were 271 and 12 ng/L, respectively. Twenty‐nine patients had NT‐proBNP >800 ng/L, and 37 had Tn‐T > 14 ng/L. Concerning inflammation markers, median IL‐6 was 40 ng/L and mean CRP 11 ± 8 mg/dL. IL‐6 and CRP were superior to normal cut‐off in 67 and in 63 patients, respectively. Median values of ferritin and D‐dimer were 1111 mg/L and 1270 μg/L, respectively, and neutrophilia was present in 62% of patients. Relative to LDH, median values were 311 U/L.
The median follow‐up period was 17 days with an overall mortality of 20.6%. The first death was observed within 2 days after admission for advanced respiratory failure and the last one after 34 days of follow‐up for severe complications during hospitalization in a male aged 79.
3.2. Logistic univariate and ROC analysis
Age, ΔA‐aO2, Tn‐T, IL‐6, NT‐proBNP, apolipoprotein B, LDH and neutrophils obtained statistical significance at univariate logistic regression (Table S1). Moreover, arterial hypertension, coronary artery disease, heart failure, peripheral vasculopathy, renal failure with glomerular filtration rate <60 mL/min/1.73 m2 and obesity (defined as body mass index ≥30 kg/m2) were significantly associated with in‐hospital mortality at univariate logistic regression. When using these potential predictors in multivariate logistic regression analysis as categories, only age, IL‐6, NT‐proBNP and LDH remained independently associated with in‐hospital mortality. ROC analysis defined the optimal cut‐points for each of these four parameters. As shown in Table 2, age (with a cut‐off of 70 years) was the most specific predictor of in‐hospital death with sensibility and specificity of 65 and 82 (AUC = 0.805) and an OR of 6.3 (95% CI 1.7, 23.9). NT‐proBNP threshold was consistent with the reference value (below or above 803 ng/L) being sensibility and specificity 70 and 81 (AUC = 0.771); OR was 3.9 (95% CI 1.1, 14.0). The cut‐point for IL‐6 was 58 ng/L with an equal sensibility and specificity of 70 (AUC = 0.729) and an OR of 4.9 (95% CI 1.4, 17.7). The cut‐point for LDH was 371 U/L with a sensibility of 74 and a specificity of 71 (AUC = 0.804) and an OR of 3.8 (95% CI 1.5, 9.6). ROC analysis of combined parameters obtained by age, NT‐proBNP, IL‐6 and LDH showed an AUC of 0.878 (Figure S1). When 2 out of 4 parameters were overcome, sensibility was 89, specificity 71 and OR for in‐hospital mortality 13.9 (95% CI 3.2‐60.2) .
TABLE 2.
Sensitivity and specificity of predictive variables associated with all‐cause in‐hospital mortality
| Predictive parameter | Sensibility (%) | Specificity (%) | PPV | NPV | Threshold value | AUC (95% CI) | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Age (y) | 65 | 82 | 50 | 90 | ≥70 | 0.805 (0.708, 0.906) | 6.3 (1.7, 23.9) |
| NT‐proBNP (ng/L) | 70 | 81 | 50 | 91 | ≥803 | 0.771 (0.639, 0.902) | 3.9 (1.1, 14.0) |
| IL‐6 (ng/L) | 70 | 70 | 39 | 90 | ≥58 | 0.729 (0.606, 0.852) | 4.9 (1.4, 17.7) |
| LDH (U/L) | 74 | 71 | 39 | 92 | ≥371 | 0.804 (0.710, 0.898) | 3.8 (1.5, 9.6) |
|
Age (y) NT‐proBNP (ng/L) IL‐6 (ng/L) LDH (U/L) |
89 | 71 | 0.44 | 0.96 | ≥2 | 0.878 (0.710, 0.951) | 13.9 (3.2, 60.2) |
Abbreviations: AUC, area under the curve; IL‐6, interleukin 6; LDH, lactate dehydrogenase; NPV, negative predictive value; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; OR, odds ratio; PPV, positive predictive value; y, years.
3.3. COX analysis, score and Kaplan‐Meier curves
Age, ΔA‐aO2, NT‐proBNP, Tn‐T, IL‐6 and LDH were significant at univariate Cox regression analysis (Table 3). Relative to all‐cause in‐hospital mortality, multivariate Cox regression analysis showed that each 5 years of increase in age corresponded to a HR of 1.28 (95% CI 1.00‐1.65, P = .050); each 803 ng/L increment of NT‐proBNP corresponded to a HR of 1.24 (95% CI 1.11‐1.39, P < .001); each 58 ng/L increment of IL‐6 corresponded to a HR of 1.23 (95% CI 1.09‐1.40, P < .001); and each 250 U/L increment of LDH corresponded to a HR of 1.23 (95% CI 1.10‐1.37, P < .001). According to the calculated cut‐points for age, NT‐proBNP, IL‐6 and LDH when 2 out of these 4 were overcome, the HR was 2.96 (95% CI 1.97‐4.45, P < .001) (Table 4 and Figure S2). Figure 1 shows Kaplan‐Meier curves of overall survival probability and the impact of the score according to the presence of high‐risk criteria for in‐hospital mortality (0, 1, 2, 3 or 4 criteria). According to the score, the deaths were 89% (8 out of 9) when 4 criteria were met; 29% (2 out of 7) when 3 criteria were met; 26% (6 out 23) when 2 criteria were met; and 8% (2 out of 24) when a single criterion was met. No deaths were recorded among patients who did not meet any of these criteria.
TABLE 3.
Univariate Cox proportional hazard models to evaluate the estimate of the hazard ratios (HR) and 95% confidence intervals (CI) for the association among all variables and all‐cause in‐hospital mortality
| Parameter | OR | Lower CI | Upper CI | P‐value |
|---|---|---|---|---|
| Age, y | 1.0773 | 1.0313 | 1.1254 | .001 |
| Obesity | 1.0211 | 0.3697 | 2.8205 | .967 |
| COPD | 2.822 | 0.881 | 9.038 | .081 |
| Diabetes | 1.398 | 0.321 | 6.082 | .655 |
| Hypertension | 2.884 | 1.149 | 7.236 | .024 |
| Coronary artery disease | 2.732 | 1.027 | 7.267 | .044 |
| Peripheral vasculopathy | 4.683 | 1.789 | 12.258 | .002 |
| Heart failure | 3.065 | 0.787 | 11.941 | .106 |
| Renal failure | 3.169 | 1.102 | 9.115 | .032 |
| ΔA‐aO2, mm Hg | 1.0063 | 1.0019 | 1.0107 | .005 |
| NT‐proBNP, ng/L | 1.0003 | 1.0002 | 1.0004 | <.001 |
| Tn‐T, ng/L | 1.0036 | 1.0017 | 1.0054 | <.001 |
| IL‐6, ng/L | 1.0033 | 1.0014 | 1.0052 | .001 |
| LDH, U/L | 1.0008 | 1.0003 | 1.0012 | <.001 |
| PaO2/FiO2, mm Hg | 0.9965 | 0.9899 | 1.0031 | .296 |
| Systolic BP, mm Hg | 1.0227 | 0.9946 | 1.0516 | .114 |
| Diastolic BP, mm Hg | 0.9941 | 0.9489 | 1.0415 | .805 |
| Mean BP, mm Hg | 1.0135 | 0.9720 | 1.0568 | .529 |
| WBC, ×103/µL | 1.0001 | 1.0000 | 1.0002 | .210 |
| Neutrophils, ×103/µL | 1.0001 | 1.0000 | 1.0002 | .142 |
| Lymphocytes, ×103/µL | 1.0000 | 0.9998 | 1.0002 | .977 |
| Monocytes, ×103/µL | 0.9997 | 0.9980 | 1.0014 | .715 |
| Eosinophil, ×103/µL | 0.9955 | 0.9869 | 1.0041 | .304 |
| Basophil, ×103/µL | 0.9959 | 0.9744 | 1.0178 | .710 |
| CRP, mg/dL | 1.0214 | 0.9676 | 1.0782 | .443 |
| D‐dimer, µg/L | 1.0000 | 1.0000 | 1.0001 | .255 |
| Ferritin, mg/L | 1.0002 | 1.0000 | 1.0005 | .090 |
| Triglycerides, mg/dL | 0.9959 | 0.9879 | 1.0041 | .327 |
| Total cholesterol, mg/dL | 0.9928 | 0.9819 | 1.0039 | .205 |
| HDL‐C, mg/dL | 1.0060 | 0.9719 | 1.0413 | .732 |
| Non‐HDL‐C, mg/dL | 0.9900 | 0.9771 | 1.0030 | .130 |
| PCSK9, ng/mL | 1.0017 | 0.9985 | 1.0048 | .302 |
| Apolipoprotein B, g/L | 0.1273 | 0.0141 | 1.1527 | .067 |
| Lipoprotein(a), nmol/L | 1.0000 | 0.9898 | 1.0102 | .995 |
| ACE2, ng/mL | 0.9922 | 0.9765 | 1.0082 | .338 |
OR is reported for 1 unit of increment in each parameter. Values in bold are statistically significant.
Abbreviations: ACE2, angiotensin‐converting enzyme 2; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; HDL‐C, high‐density lipoprotein cholesterol; IL‐6, interleukin 6; LDH, lactate dehydrogenase; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; PaO2/FiO2, pressure of arterial oxygen to fractional inspired oxygen concentration; PCSK9, proprotein convertase subtilisin/kexin type 9; Tn‐T, troponin T; WBC, white blood cells; ΔA‐aO2, alveolar‐arterial oxygen gradient (ΔA‐aO2).
TABLE 4.
Multivariable Cox proportional hazard estimations for in‐hospital mortality
| Increase | HR (95% CI) | P‐value | |
|---|---|---|---|
| Age, y | 5 y | HR 1.28 (1.00, 1.65) | .050 |
| NT‐proBNP, ng/L | 803 ng/mL | HR 1.24 (1.11, 1.39) | <.001 |
| IL‐6, ng/L | 58 ng/L | HR 1.23 (1.09, 1.40) | <.001 |
| LDH, U/L | 250 U/L | HR 1.23 (1.10, 1.37) | <.001 |
| Score | HR 2.96 (1.97, 4.45) | <.001 |
Abbreviations: HR, hazard ratio; IL‐6, interleukin 6; LDH, lactate dehydrogenase; NT‐proBNP, N‐terminal pro–brain natriuretic peptide.
FIGURE 1.
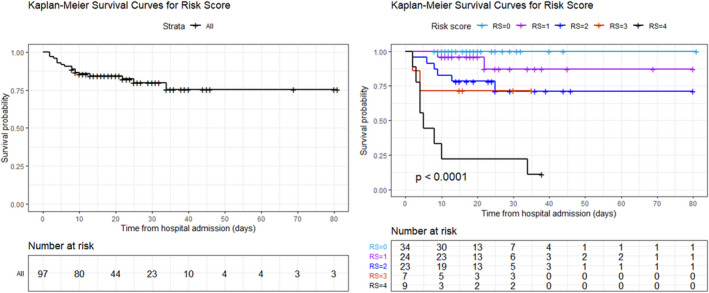
Kaplan‐Meier survival curves. (Panel A) shows overall in‐hospital mortality; (panel B) shows in‐hospital mortality according to the risk score classes. The light blue line refers to patients with score = 0; the purple line refers to patients with score = 1; the blue line refers to patients with score = 2; the red line refers to patients with score 3; and the black line refers to patients with score 4. RS, risk score
4. DISCUSSION
This retrospective monocentric study showed that the application of a score including a composite of four objective and independent variables (ie age, NT‐proBNP, IL‐6 and LDH) predicted all‐cause in‐hospital mortality in patients with COVID‐19. Biochemical evaluation of NT‐proBNP, IL‐6 and LDH is usually available within few hours after hospitalization. As elsewhere reported,16 the ability to determine the risk of adverse outcomes in people with COVID‐19 admitted to hospital highlights the need for more intensive observation and earlier medical intervention.
In the present score, the impact of age was expected, being an exponential risk factor of an adverse outcome. Among 516 patients consecutively admitted for COVID‐19 to two Italian tertiary hospitals of Northern and Central Italy, compared to patients younger than 62 , the risk of death was roughly 3 times higher in individuals between 62 and 74 and 8 times higher in those older than 75.17 The highly negative impact of age suggests that, in the absence of specifically effective drug therapy and delay in vaccination, social isolation could be a relevant issue in individuals aged 70‐75 and over.18 According to our results, despite the low clinical risk of in‐hospital mortality in patients younger than 70 , the concomitant combined presence of 2 out 4 altered NT‐proBNP, IL‐6 and LDH tripled the risk. In addition, our data advise priority programme in vaccination of individuals older than 65 .
Besides causing respiratory disease, SARS‐CoV‐2 infection may also lead to cardiac injury, a feature associated with higher risk of in‐hospital mortality.19 Among 397 consecutive COVID‐19 patients hospitalized in Lombardy region, a rise in the levels of cardiac biomarkers at admission was associated with higher rates of all‐cause mortality.20 In particular, NT‐proBNP was as an independent risk factor for death in patients with severe COVID‐19.21 NT‐proBNP was frequently elevated in patients with SARS‐CoV‐2 infection, although only a minority of them fulfilled clinical criteria for the diagnosis of heart failure. This supports the hypothesis that natriuretic peptides are highly associated with the prognosis of COVID‐19 patients.22 Our data are in line with a meta‐analysis of observational studies supporting the hypothesis that the assessment of NT‐proBNP could improve the discrimination of high‐risk patients.23 A possible mediator of this rise is the inflammation, for example among 5481 participants of Multi‐Ethnic Study of Atherosclerosis (MESA) study, 1‐unit higher baseline natural log IL‐6 level associated with 6% higher NT‐proBNP level.24 In addition, NT‐proBNP inhibits the sympathetic nervous system and the activities of several other hormone systems, including the RAAS.25 This fits with the activation of RAAS during COVID‐19 and its correlation with a disease progression.5 In this frame, a rise in NT‐proBNP could be an indirect marker of endothelial damage leading to diffuse vascular vasoconstriction. The role played by NT‐proBNP has been apparently clear also in the present cohort since patients with higher levels than the cut‐off one (803 ng/L) showed a worsening picture in terms of cardiorespiratory health (eg PaO2/FiO2 and ΔA‐aO2) and inflammatory milieu (eg Tn‐T, D‐dimer, IL‐6, CRP) (Table S2). The cardiovascular COVID‐19 syndrome is also characterized by increased levels of Tn‐T. In the present study, Tn‐T significantly estimated the HR of all‐cause in‐hospital mortality at univariate analysis: each unit increment in Tn‐T corresponded to a HR of 1.0036 (95% CI 1.0017‐1.0054; P < .001). This confirms that the myocardial injury is one of the main adverse events of COVID‐19 mediated by several mechanisms, including inflammation, endothelial dysfunction, and vasoconstriction.26
The cytokine storm syndrome is a phenomenon explaining the reason why some patients exposed to SARS‐CoV‐2 become critically ill with acute respiratory distress syndrome, multiorgan failure and death.27 Although so far there are no standard diagnostic criteria defining the onset of cytokine storm, in SARS‐CoV‐2‐infected individuals, IL‐6 surges during illness and decline during recovery.28 IL‐6 seems superior to CRP, ferritin and liver enzymes for predicting clinical outcomes, such as respiratory failure and death.29 Moreover, in line with our findings, a previous study showed that elevated levels of IL‐6 (above 100 pg/mL) potentially identified COVID‐19 patients who can benefit the most by tocilizumab treatment to reduce in‐hospital mortality.30 Early studies between February and April 2020 showed that elevated levels of IL‐6 before intubation had the strongest association with the need for mechanical intubation.31 Among 1484 patients followed up to 41 days after hospitalization, IL‐6 was one of the most robust prognostic biomarkers of survival, eclipsing or outperforming CRP, D‐dimer and ferritin.32 However, it is worth mentioning that higher levels of D‐dimer have been observed in the ICU patients compared with non‐ICU patients.33
Raised levels of LDH have been associated with poor prognosis in patients with COVID‐19,34 with statistically significant higher levels in ICU vs. non‐ICU patients35 and in those who died.36 Altogether, these findings are in line with ours. LDH is an intracellular enzyme involved in energy production by conversion of lactate to pyruvate and it is present in almost all body cells. Thus, it cannot be a tissue‐specific marker, while it indicates a multiorgan damage. Moreover, LDH elevation may reflect more severe microvascular dysfunction which could lead to myocardial and multiple organ injuries in COVID‐19 patients.37
With the exception of age, NT‐proBNP, IL‐6 and LDH, we did not find any further significant association with the primary outcome. Although a meta‐analysis of 20 studies showed that higher ferritin levels were associated with more frequent hospitalization in ICU,38 our findings are in line with the ones of a cohort of 17 000 patients. No associations stand between the acute phase reactant ferritin and mortality despite severe illness is characterized by hyperferritinaemia.37 Although a decreased ACE2 activity due to SARS‐CoV‐2 infection can be one of the pathways sustaining arterial hypertension,5 the suspected or perceived association of COVID‐19 and hypertension could reflect the older age of patients who did not survive.39 Finally, lessons learned from COVID‐19 support the notion that associated cardiac injury and mortality is higher in patients with dyslipidaemia.40 Lipid parameters often fall in cytokine‐mediated inflammation as a consequence of the acute phase response rather than having a causative or pathological contribution towards infection.41 Overall, cholesterol in the cell membrane and viral envelope may contribute to coronavirus replication by acting as a key component in viral entry. In this context, following the hypothesis that PCSK9 may suppress the expression of interferon type I,14, 42 we measured this protein without finding any association with in‐hospital mortality. Same conclusions were reached for lipoprotein(a), in line with findings reporting that high lipoprotein(a) concentrations do not increase the susceptibility either to SARS‐CoV‐2 infections or to thromboembolic events43.
Our data have to be interpreted in a frame of limitations. First of all, the present study represents a retrospective and single‐centre study. Secondly, although our conclusions are based on 97 patients, this sample size produced a two‐sided 95% confidence interval ranging from 13% to 29% when the prevalence of in‐hospital mortality is 21%. Third, no causal conclusions could be drawn from our algorithm. Fourth, despite we performed an internal validation with an AUC of 0.815 (95% CI 0.660‐0.969), the study missed an external validation that should be applied on multinational data set.
In conclusion, in COVID‐19 patients, besides age, the evaluation of three biochemical parameters, available in few hours after hospital admission, can predict in‐hospital mortality regardless of other comorbidities. Whether this model could be generalizable to outpatients and individuals with different geographic backgrounds would require further validation.
CONFLICT OF INTEREST
All the authors have nothing to disclose relative to the content of the present review article.
Supporting information
Fig S1
Fig S2
Supplementary Material
Supplementa1 Tables
Ruscica M, Macchi C, Iodice S, et al. Prognostic parameters of in‐hospital mortality in COVID‐19 patients—An Italian experience. Eur J Clin Invest. 2021;51:e13629. 10.1111/eci.13629
Contributor Information
Valentina Bollati, Email: valentina.bollati@unimi.it.
Marco Vicenzi, Email: marco.vicenzi@unimi.it.
REFERENCES
- 1.Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in‐hospital mortality in a US national sample of patients with COVID‐19. JAMA Netw Open. 2020;3(12):e2029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliberti S, Amati F, Pappalettera M, et al. COVID‐19 multidisciplinary high dependency unit: the Milan model. Respir Res. 2020;21(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vicenzi M, Di Cosola R, Ruscica M, et al. The liaison between respiratory failure and high blood pressure: evidence from COVID‐19 patients. Eur Respir J. 2020;56(1):2001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Chen J, Yang Q, et al. Development and validation of a risk score using complete blood count to predict in‐hospital mortality in COVID‐19 patients. Med. 2021;2(4):435‐447.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary R, Garg J, Houghton DE, et al. Thrombo‐inflammatory biomarkers in COVID‐19: systematic review and meta‐analysis of 17,052 patients. Mayo Clin Proc Innov Qual Outcomes. 2021;5(2):388‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia de Guadiana‐Romualdo L, Morell‐Garcia D, Rodriguez‐Fraga O, et al. Cardiac troponin and COVID‐19 severity: results from BIOCOVID study. Eur J Clin Invest. 2021:e13532. 51(6):e13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller C, Giannitsis E, Jaffe AS, et al. Cardiovascular biomarkers in patients with COVID‐19. Eur Heart J Acute Cardiovasc Care. 2021;10(3):310‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy B, Fejes Z, Szentkereszty Z, et al. A dramatic rise in serum ACE2 activity in a critically ill COVID‐19 patient. Int J Infect Dis. 2021;103:412‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei X, Zeng W, Su J, et al. Hypolipidemia is associated with the severity of COVID‐19. J Clin Lipidol. 2020;14(3):297‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriarty PM, Gorby LK, Stroes ES, Kastelein JP, Davidson M, Tsimikas S. Lipoprotein(a) and its potential association with thrombosis and inflammation in COVID‐19: a testable hypothesis. Curr Atheroscler Rep. 2020;22(9):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vuorio A, Kovanen PT. PCSK9 inhibitors for COVID‐19: an opportunity to enhance the antiviral action of interferon in patients with hypercholesterolaemia. J Intern Med. 2021;289(5):749‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 16.Gue YX, Tennyson M, Gao J, Ren S, Kanji R, Gorog DA. Development of a novel risk score to predict mortality in patients admitted to hospital with COVID‐19. Sci Rep. 2020;10(1):21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fumagalli C, Rozzini R, Vannini M, et al. Clinical risk score to predict in‐hospital mortality in COVID‐19 patients: a retrospective cohort study. BMJ Open. 2020;10(9):e040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koff WC, Williams MA. Covid‐19 and immunity in aging populations – a new research agenda. N Engl J Med. 2020;383(9):804‐805. [DOI] [PubMed] [Google Scholar]
- 19.Shi S, Qin MU, Shen BO, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefanini GG, Chiarito M, Ferrante G, et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID‐19. Heart. 2020;106(19):1512‐1518. [DOI] [PubMed] [Google Scholar]
- 21.Gao L, Jiang D, Wen X‐S, et al. Prognostic value of NT‐proBNP in patients with severe COVID‐19. Respir Res. 2020;21(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caro‐Codón J, Rey JR, Buño A, et al. Characterization of NT‐PROBNP in a large cohort of COVID‐19 patients. Eur J Heart Fail. 2021;23(3):456‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J‐W, Han T‐W, Woodward M, et al. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta‐analysis. Prog Cardiovasc Dis. 2020;63(4):518‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fish‐Trotter H, Ferguson JF, Patel N, et al. Inflammation and circulating natriuretic peptide levels. Circ Heart Fail. 2020;13(7):e006570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein BC, Levin RI. Natriuretic peptides: physiology, therapeutic potential, and risk stratification in ischemic heart disease. Am Heart J. 1998;135(5 Pt 1):914‐923. [DOI] [PubMed] [Google Scholar]
- 26.Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail. 2020;26(6):470‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weatherhead JE, Clark E, Vogel TP, Atmar RL, Kulkarni PA. Inflammatory syndromes associated with SARS‐CoV‐2 infection: dysregulation of the immune response across the age spectrum. J Clin Invest. 2020;130(12):6194‐6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen SF, Ho YC. SARS‐CoV‐2: a storm is raging. J Clin Invest. 2020;130(5):2202‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Confronting the controversy: interleukin‐6 and the COVID‐19 cytokine storm syndrome. Eur Respir J. 2020;56(4):2003006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flisiak R, Jaroszewicz J, Rogalska M, et al. Tocilizumab improves the prognosis of COVID‐19 in patients with high IL‐6. J Clin Med. 2021;10(8):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL‐6 and CRP predict the need for mechanical ventilation in COVID‐19. J Allergy Clin Immunol. 2020;146(1):128‐136 e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Valle DM, Kim‐Schulze S, Huang H‐H, et al. An inflammatory cytokine signature predicts COVID‐19 severity and survival. Nat Med. 2020;26(10):1636‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruetzler K, Szarpak Ł, Ładny JR, et al. D‐dimer levels predict COVID‐19 severity and mortality. Kardiol Pol. 2021;79(2):217‐218. [DOI] [PubMed] [Google Scholar]
- 34.Martha JW, Wibowo A, Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID‐19: a systematic review and meta‐analysis. Postgrad Med J. 2021; Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Szarpak L, Ruetzler K, Safiejko K, et al. Lactate dehydrogenase level as a COVID‐19 severity marker. Am J Emerg Med. 2020; Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheth A, Modi M, Dawson D, Dominic P. Prognostic value of cardiac biomarkers in COVID‐19 infection. Sci Rep. 2021;11(1):4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Incerti D, Rizzo S, Li X, et al. Prognostic model to identify and quantify risk factors for mortality among hospitalised patients with COVID‐19 in the USA. BMJ Open. 2021;11(4):e047121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szarpak L, Zaczynski A, Kosior D, et al. Evidence of diagnostic value of ferritin in patients with COVID‐19. Cardiol J. 2020;27(6):886‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iaccarino G, Grassi G, Borghi C, et al. Age and multimorbidity predict death among COVID‐19 patients: results of the SARS‐RAS study of the Italian Society of hypertension. Hypertension. 2020;76(2):366‐372. [DOI] [PubMed] [Google Scholar]
- 40.Mechanick JI, Rosenson RS, Pinney SP, Mancini DM, Narula J, Fuster V. Coronavirus and cardiometabolic syndrome: JACC focus seminar. J Am Coll Cardiol. 2020;76(17):2024‐2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbert KE, Erridge C. Regulation of low‐density lipoprotein cholesterol by intestinal inflammation and the acute phase response. Cardiovasc Res. 2018;114(2):226‐232. [DOI] [PubMed] [Google Scholar]
- 42.Gan ES, Tan HC, Le DHT, et al. Dengue virus induces PCSK9 expression to alter antiviral responses and disease outcomes. J Clin Invest. 2020;130(10):5223‐5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maio SD, Lamina C, Coassin S, Forer L, Würzner R, Schönherr S, Kronenberg F. Lipoprotein(a) and SARS‐CoV‐2 infections: susceptibility to infections, ischemic heart disease and thromboembolic events. J Intern Med. 2021; 10.1111/joim.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Supplementary Material
Supplementa1 Tables


