AUTHOR CONTRIBUTION
Siddhartha Dash: Literature search, data acquisition, manuscript preparation, manuscript editing. Biswanath Behera: Concepts, design, definition of intellectual content, manuscript review, guarantor. Madhusmita Sethy: Definition of intellectual content, data acquisition, manuscript review. Jeebanjyoti Mishra: Data acquisition, manuscript preparation. Sonika Garg: Data acquisition, manuscript preparation.
Dear Editor,
Urticarial vasculitis (UV) is characterized by persistent urticarial rashes that last for more than 24 h and heals with hyperpigmentation. It can be idiopathic or due to drugs, infections, malignancy, autoimmune or autoinflammatory diseases. 1 Rarely, vaccines have been implicated in the causation of UV. 2 , 3
A 27‐year‐old man developed multiple itchy reddish, elevated lesions following one after the second dose of COVID‐19 vaccine (whole virion inactivated coronavirus vaccine). It was associated with fever and bilateral knee and ankle joint pain. He received the 1st dose of vaccine 1 month before the 2nd dose. There was no prior history of a similar disease, and the patient denied intake of any other form of medications. Cutaneous examination showed multiple erythematous urticarial plaques over the trunk and extremities (Figure 1A). In most of the lesions, the erythema was non‐blanchable in nature, and the plaques were persistent for more than 1 day. Few lesions had healed with post‐inflammatory hyperpigmentation. Dermoscopy under polarized mode revealed red dots and globules on a reddish background. Other mucocutaneous, general, and systemic examinations were within normal limits. The biochemical and hematological parameters were normal, except for raised C‐reactive protein. Histopathology revealed disruption of the walls of the small vessels, endothelial swelling, erythrocyte extravasation, and mild perivascular infiltration comprising neutrophils, eosinophils, and lymphocytes (Figure 1B,C). Direct immunofluorescence did not reveal any immune deposition. A diagnosis of UV was made, and the patient was treated with oral indomethacin 75 mg once daily, topical calamine lotion, and tab levocetirizine 5 mg once daily. At 1 week, all the lesions had subsided with hyperpigmentation (Figure 1D).
FIGURE 1.
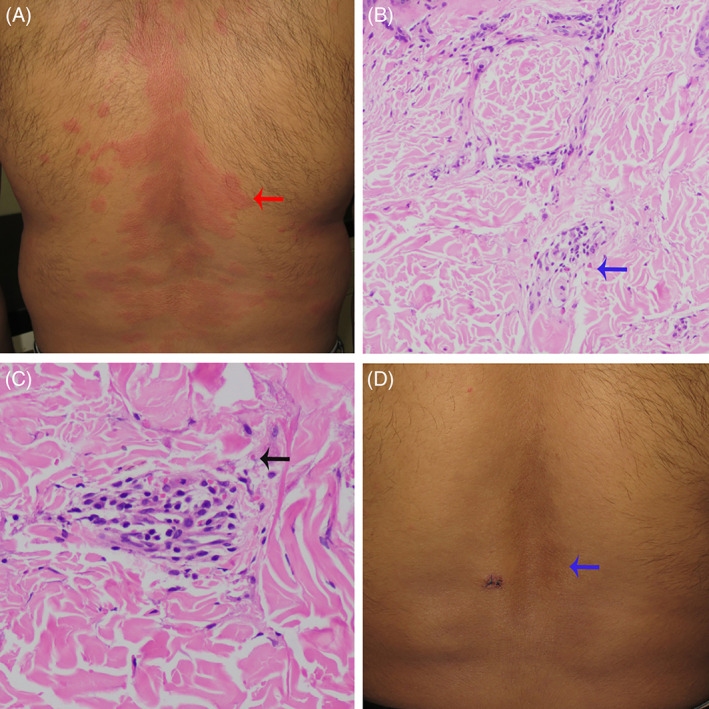
(A) Multiple urticarial plaques (red arrow). (B) Histology shows mild perivascular infiltration comprising neutrophils, eosinophils (blue arrow), and lymphocytes (H & E, ×100). (C) Histology shows vessel wall disruption, erythrocyte extravasation (black arrow) (H & E, ×400). (D) Postinflammatory hyperpigmentation (blue arrow)
The following cutaneous reactions have been attributed to the COVID‐19 vaccine (Moderna and Pfizer): injection site reactions, delayed large local reaction, urticarial eruptions, morbilliform eruptions, pernio/chilblains, cosmetic filler reactions, herpes zoster, herpes simplex flares, and pityriasis rosea‐like reactions. 4
Baiu et al. demonstrated the role of Th1 response and suggested that interferon‐gamma is critically required for initiation of vascular inflammation. 5 The whole‐virion inactivated SARS‐CoV‐2 vaccine induces primarily a Th1 biased response, which could have led to induction of inflammatory response in the vessel wall. 6
Drug‐induced vasculitis has been defined to be having clinical signs and symptoms of vasculitis and having a temporal association with drug, anti‐neutrophil cytoplasmic antibody (ANCA) association, and exclusion of other conditions (especially infections and malignancy). 7 In the index case, the patient had developed lesions after 1 day of second dose vaccination. Gomes et al. reported IgA vasculitis following the second dose of the human papillomavirus vaccine. 8 We hypothesize that, after 2nd dose of vaccination, the patient developed an appreciable amount of inflammatory response leading to vascular inflammation. In concurrence with our hypothesis, an incubation period of as long as 6 months have been described in drug‐induced vasculitis. 7
Radic et al. emphasized histopathological findings as the mainstay of diagnosis and immune complexes deposition to be a rarity in drug‐induced vasculitis, as in the index case. 7 In our case, we avoided immunosuppressives and treated the patient with oral indomethacin for the following reasons: there were no systemic complications, and we wanted to maintain the immunogenicity of the vaccine.
To the best of our knowledge, we are reporting the first case of COVID‐19 vaccine‐induced urticarial vasculitis. This report will create awareness among medical professionals about this exceedingly rare complication of the COVID‐19 vaccine.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kolkhir P, Grakhova M, Bonnekoh H, Krause K, Maurer M. Treatment of urticarial vasculitis: a systematic review. J Allergy Clin Immunol. 2019;143:458‐466. [DOI] [PubMed] [Google Scholar]
- 2. Hughes R, Lacour JP, Baldin B, Reverte M, Ortonne JP, Passeron T. Urticarial vasculitis secondary to H1N1 vaccination. Acta Derm Venereol. 2010;90:651‐652. [DOI] [PubMed] [Google Scholar]
- 3. Fatah S, Cousen P. Urticarial vasculitis after influenza vaccination. BMJ. 2015;351:h5072. [Google Scholar]
- 4. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85:46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baiu DC, Sandor M, Hart M. CD4+ T cells sensitized by vascular smooth muscle induce vasculitis, and interferon gamma is critical for the initiation of vascular pathology. Am J Pathol. 2010;177:3215‐3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBV152: a double‐blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Radić M, Martinović Kaliterna D, Radić J. Drug‐induced vasculitis: a clinical and pathological review. Neth J Med. 2012;70:12‐17. [PubMed] [Google Scholar]
- 8. Melo Gomes S, Glover M, Malone M, Brogan P. Vasculitis following HPV immunization. Rheumatology (Oxford). 2013;52:581‐582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


