Summary
Coronavirus disease 2019 (COVID‐19) caused by the novel severe acute respiratory syndrome coronavirus 2 continues to grow and spread throughout the world since being declared a pandemic. Despite extensive scientific research globally including repurposing of several existing drugs, there is no effective or proven therapy for this enigmatic disease which is still largely managed empirically This systematic review evaluated the role of hydroxychloroquine (HCQ) in the treatment of COVID‐19 infection and was conducted using Cochrane methodology for systematic reviews of interventional studies including risk of bias assessment and grading of the quality of evidence. Only prospective clinical trials randomly assigning COVID‐19 patients to HCQ plus standard of care therapy (test arm) versus placebo/standard of care (control arm) were included. Data were pooled using the random‐effects model and expressed as risk ratio (RR) with 95% confidence interval (CI). A total of 10,492 patients from 19 randomised controlled trials were included. The use of HCQ was not associated with higher rates of clinical improvement (RR = 1.00, 95% CI: 0.96–1.03, p = 0.79) or reduction in all‐cause mortality by Day14 (RR = 1.07, 95% CI: 0.97–1.19, p = 0.19) or Day28 (RR = 1.08, 95% CI: 0.99–1.19, p = 0.09) compared to placebo/standard of care. There was no significant difference in serious adverse events between the two arms (RR = 1.01, 95% CI: 0.85–1.19, p = 0.95). There is low‐to‐moderate certainty evidence that HCQ therapy is generally safe but does not reduce mortality or enhance recovery in patients with COVID‐19 infection.
Keywords: coronavirus, hydroxychloroquine, toxicity
Abbreviations
- ARDS
acute respiratory distress syndrome
- CI
confidence interval
- CIR
clinical improvement rate
- COVID‐19
coronavirus disease 2019
- CQ
chloroquine
- EUA
emergency use authorisation
- FAERS
FDA Adverse Events Reporting System
- GRADE
Grades of Recommendation, Assessment, Development, and Evaluation
- HCQ
hydroxychloroquine
- ICTRP
International Clinical Trials Registry Platform
- OR
odds ratio
- PRISMA
Preferred Reporting of Systematic Reviews and Meta‐Analyses
- RCTs
randomised controlled trials
- RR
risk ratio
- RT‐PCR
reverse transcriptase polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TTCI
time‐to‐clinical improvement
- US‐FDA
United States Food and Drug Administration
- WHO
World Health Organization
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) continues to grow and spread throughout the world since being declared a pandemic 1 , 2 by the World Health Organization (WHO) in March 2020 with over 180 million confirmed cases and nearly 4 million deaths by the time of this report. Although lungs remain the primary target organ of SARS‐CoV‐2, the disease can affect multiple organ systems and elicit highly variable inflammatory response in the host with resultant heterogeneous clinical spectrum. 3 , 4 Despite extensive scientific research globally including repurposing of several existing drugs, there is no effective or proven therapy for this enigmatic disease which is still largely managed empirically. 4 , 5 A plethora of medical and pharmacological therapies including antivirals, antibiotics, anti‐malarials, anti‐parasitic agents, non‐steroidal anti‐inflammatory drugs, corticosteroids and immunomodulators are currently being investigated in over a thousand randomised controlled trials (RCTs) across the world with an aim to generate high‐quality evidence to inform and guide clinical practice during the ongoing pandemic. 6 , 7 , 8 , 9 , 10 , 11 Various anti‐viral drug groups such as fusion inhibitors (umifenovir), protease inhibitors (lopinavir/ritonavir), neuraminidase inhibitors (oseltamivir) and nucleotide reverse transcriptase inhibitors (remdesivir and favipiravir) have been tested in multiple prospective studies since the outbreak of the pandemic. 12 , 13 Only about 9 months ago, remdesivir became the first drug to receive United States Food and Drug Administration (US‐FDA) approval for treatment of hospitalised COVID‐19 patients. 14 Hydroxychloroquine (HCQ), a less toxic derivative of chloroquine (CQ) is a widely used anti‐parasitic agent for malaria and immunomodulatory drug for rheumatologic diseases. In the lab, HCQ has demonstrated impressive activity against COVID‐19 by blocking the entry of SARS‐CoV‐2 into cells by inhibiting glycosylation of cell‐surface receptors, interfering with proteolytic processing and increasing endosomal pH to limit endosome‐mediated viral entry and late‐stage viral replication. 15 , 16 In addition, HCQ reduces the production of several pro‐inflammatory cytokines potentially involved in the development and progression to acute respiratory distress syndrome in patients with COVID‐19 infection. Based on promising in vitro activity and favourable clinical experience in observational studies 17 , 18 during early phase of the pandemic, HCQ received emergency use authorisation (EUA) from US‐FDA in March 2020, which was revoked later due to concerns regarding cardiac toxicity. 19 , 20
Over a year and half into the pandemic, several RCTs have investigated and reported on the safety and efficacy of HCQ as a prophylactic and therapeutic agent in COVID‐19 infection. Multiple systematic reviews and meta‐analyses of HCQ treatment in COVID‐19 have also been reported with conflicting and contradictory results. A meta‐review 21 of systematic reviews and updated meta‐analysis concluded that treatment with HCQ and CQ with or without azithromycin did not reduce mortality in COVID‐19 infection but was associated with a higher risk of adverse events. However, despite the lack of clear evidence of efficacy and presence of valid concerns regarding safety, HCQ continues to be used widely in the management of COVID‐19 infection.
2. AIMS
The aim of this rapid, updated, structured systematic review and direct comparison meta‐analysis was to evaluate the safety and efficacy of HCQ in the treatment of COVID‐19 for generating high‐quality evidence to inform and guide therapeutic decision‐making.
3. METHODS
This systematic review was conducted using Cochrane methodology for systematic reviews of interventional studies 22 and reported in accordance with the Preferred Reporting of Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 23 Data analysis included risk of bias assessment using Cochrane Risk of Bias tool 24 and grading of the quality of evidence and strength of recommendation based on the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach. 25
3.1. Literature search strategy
The priority sources of relevant studies for this rapid systematic review included PubMed (https://pubmed.ncbi.nlm.nih.gov) with its curated version LitCOVID and medRxiv (https://www.medrxiv.org). In addition, the National Library of Medicine database of clinical studies (https://clinicaltrials.gov), WHO International Clinical Trials Registry Platform (https://www.who.int/ictrp/en/), Cochrane living registry of COVID‐19 studies (http://covid‐19.cochrane.org) and Living mapping and living systematic review of Covid‐19 studies (http://covid‐nma.com) were also queried. A systematic search of the medical literature (online supplementary file S1) without any language restrictions was conducted on 25 September 2020 and later updated periodically till 28 February 2021 in accordance with international guidelines for living systematic reviews. 26
3.2. Study eligibility
Only prospective clinical trials randomly assigning patients with suspected and/or proven COVID‐19 infection to HCQ plus standard of care therapy (test arm) versus placebo/standard of care (control arm) were included in this review. Given the lack of globally accepted standard of care therapy, this could be variable across trials but had to be similar in both arms within individual studies and not contain CQ or HCQ therapy in the control arm. Trials comparing CQ versus placebo/standard of care were not considered eligible. Multi‐arm trials were eligible, if they directly compared HCQ versus placebo/standard of care therapy, with only the relevant arms being pooled in the meta‐analysis. Trials allowing co‐enrolment of patients across multiple studies were also eligible provided the concurrent medical therapy was similar in each of the randomised arms. Trials randomly comparing different schedules (dose and/or duration) of HCQ were not included in this review. Quasi‐randomised trials, propensity matched analyses, non‐randomised comparative studies or observational studies were also excluded. Trials comparing HCQ against complementary and alternative medicines, traditional Chinese medicine, nutraceuticals, phytoceuticals and herbal formulations were considered ineligible. Preventive trials using HCQ for pre‐ or post‐exposure prophylaxis were also not considered under the purview of this review.
3.3. Outcome measures
The primary outcome of interest was clinical benefit as measured on the WHO or similar ordinal scale and all‐cause mortality. Clinical improvement was defined as reaching a score of 1 or 2 on the ordinal scale (becoming asymptomatic and/or getting discharged). Relevant endpoints included clinical improvement rate (CIR) on specified days (defined as proportion of patients with clinical improvement by Day7, Day14, Day28 of randomisation) and time‐to‐clinical improvement (TTCI). Death due to any cause within 14 days or 28 days of randomisation was defined as the event of interest for early (Day14) or late (Day28) mortality, respectively. Secondary endpoints included viral negativity rate on specified days (defined as proportion of patients with viral negativity on D7, D14 of randomisation) and time to viral clearance based on a negative COVID‐19 reverse transcriptase polymerase chain reaction test. Safety outcomes included comparison serious adverse events (grade 3 or worse toxicity) between the two arms.
3.4. Data extraction and analyses
Two reviewers (Babusha Kalra and Prafulla Thakkar) independently read each pre‐print, publication, protocol or any other available study report and extracted relevant data from individual studies. Discrepancy was resolved by consensus through interpretation by a third reviewer (Tejpal Gupta). Extracted data included study characteristics (first author and journal), number of participants randomised, patient characteristics (severity of clinical presentation), intervention details (class and type of treatment) and outcome measures. For all dichotomous outcomes (mortality, CIR, viral negativity rate and adverse event rate), the number of events of interest and the number of participants in each study arm were extracted per outcome. Data were pooled using the random‐effects model and expressed as risk ratio (RR) with 95% confidence interval (CI). For continuous outcomes (TTCI and time to viral clearance), mean/median values with their dispersion as reported in individual studies were extracted and expressed as difference in median time (in days) with 95% CI. Any p‐value <0.05 was considered as statistically significant. Sensitivity analysis, subgroup analysis and publication bias were also assessed as appropriate. All analyses were done using Review Manager (RevMan) version 5.3 and GRADE profiler (GRADEpro) version 3.6.1 (The Nordic Cochrane Centre, Cochrane Collaboration, 2008), Stata 14.0 (StataCorp LP) and R Studio. No funding was involved in the study and its protocol is registered with the International Platform of Registered Systematic Reviews and Meta‐analysis Protocols (INPLASY202090092).
4. RESULTS
The flow diagram of study selection and inclusion in the meta‐analysis as per the PRISMA guidelines is depicted in Figure 1. Detailed PRISMA checklist is also provided as an online supplementary file S2. Systematic search of PubMed/LitCOVID identified 1517 records with an additional 344 records being retrieved through supplementary search of other sources. After removing duplicates (159 records) and excluding irrelevant/inappropriate records (n = 1596) through rigorous screening all titles/abstracts, a total of 106 full‐text articles (including pre‐prints) were assessed for eligibility, of which 19 RCTs 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 were finally included and pooled in this systematic review and meta‐analysis.
FIGURE 1.
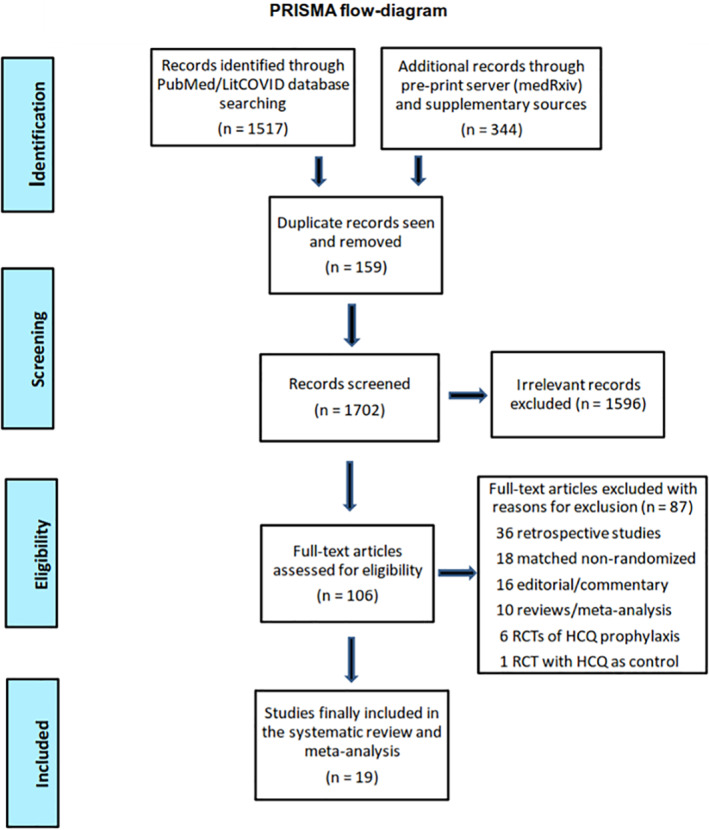
Flow diagram of study selection and inclusion in the meta‐analysis as per Preferred Reporting of Systematic Reviews and Meta‐Analyses (PRISMA) guidelines
4.1. Description of included studies
Patient characteristics, treatment details and relevant outcomes of all the 19 RCTs randomly assigning COVID‐19 patients to HCQ plus standard of care versus placebo/standard of care therapy are briefly summarised in online supplementary files S3 and S4, respectively. Most of the included studies were open‐label trials (excepting few which were placebo‐controlled) conducted between January 2020 and September 2020 in almost all parts of the world ensuring good geo‐ethnic representation. Most trials enrolled only hospitalised patients (excepting two which were done in outpatient setting) with varying degree of disease severity. Primary endpoints in the included RCTs were variable, but they included measures of both efficacy and safety. The dose and duration of HCQ therapy was somewhat variable across different studies based on prevalent local/national guidelines. The standard of care though different in various trials was in keeping with institutional protocols and national guidelines dictated by the best available evidence at the time and comprised of antivirals (oseltamivir, lopinavir/ritonavir and remdesivir), broad‐spectrum antibiotics (azithromycin and doxycycline), immunomodulators (steroids, tocilizumab and anakinra), traditional herbal medicines and supportive care (oxygen inhalation and ventilatory support) as appropriate.
4.2. Data synthesis and meta‐analyses
There was modest methodologic heterogeneity across the 19 included studies prompting the use of random‐effects model for statistical pooling of data.
Clinical efficacy: There were no significant differences in rates of clinical improvement (Figure 2) between HCQ (test arm) versus placebo/standard of care therapy (control arm) in terms of either overall CIR (RR = 1.00, 95% CI: 0.96–1.03, p = 0.79) or CIR on Day7 (RR = 0.98, 95% CI: 0.85–1.11, p = 0.71), D14 (RR = 0.99, 95% CI: 0.96–1.03, p = 0.58) and Day28 (RR = 1.01, 95% CI: 0.96–1.07, p = 0.62), respectively. Similarly, there was no significant difference in TTCI between the two arms (Figure 3) with a median difference of 0.41 days (95%CI: −0.12 to +0.95 days) in favour of HCQ treatment. The use of HCQ was not associated with reduction in all‐cause mortality (Figure 4). There was no significant difference in early (D14) mortality (RR = 1.07, 95% CI: 0.97–1.19, p = 0.19) or late (D28) mortality (RR = 1.08, 95% CI: 0.99–1.19, p = 0.09) between HCQ and placebo/standard of care therapy.
FIGURE 2.
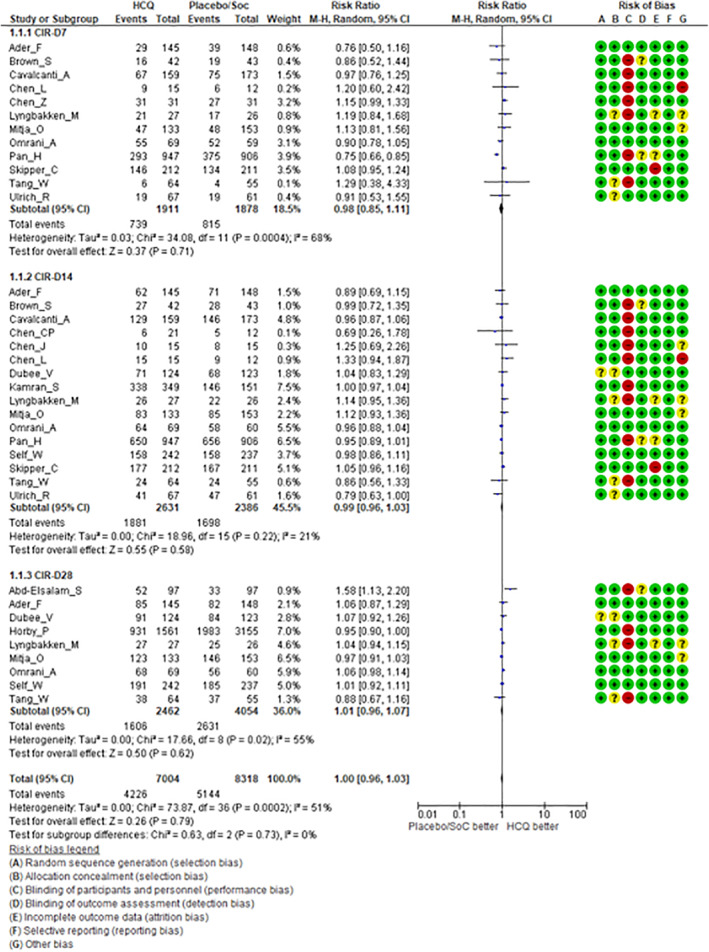
Forest plots including risk of bias in individual studies comparing hydroxychloroquine versus placebo/standard of care therapy for clinical improvement rate (CIR) on specified days from randomisation (Day7, Day14, and Day28)
FIGURE 3.
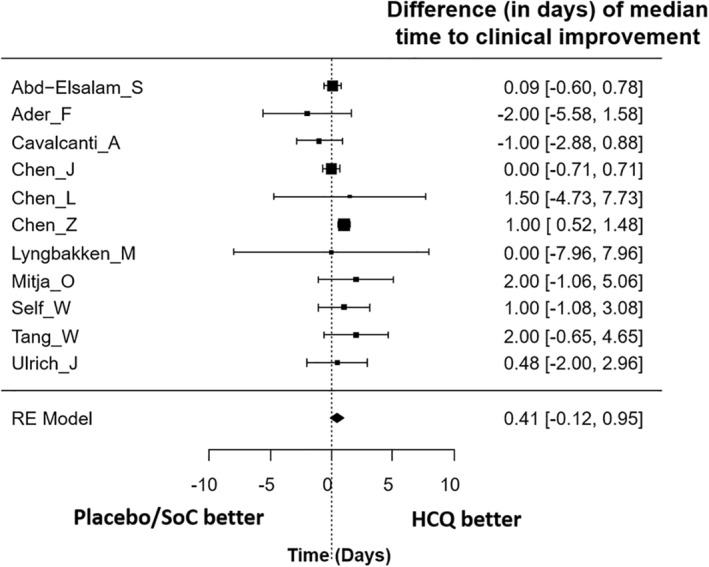
Median difference (in days) in time‐to‐clinical improvement (TTCI) between hydroxychloroquine versus placebo/standard of care therapy in coronavirus disease 2019 (COVID‐19)
FIGURE 4.
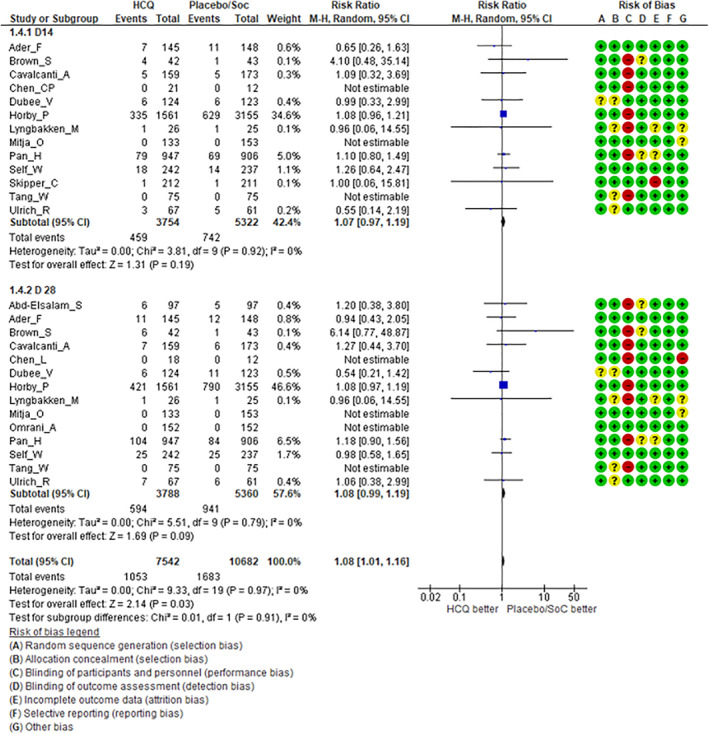
Forest plots including risk of bias in individual studies comparing hydroxychloroquine versus placebo/standard of care therapy for early (Day14) and late (Day28) all‐cause mortality in coronavirus disease 2019 (COVID‐19)
Virologic clearance: Viral negativity rates were also similar between the two arms, both overall (RR = 1.00, 95% CI: 0.92–1.09, p = 0.93) and on Day7 (RR = 1.06, 95% CI: 0.87–1.28, p = 0.56) and Day14 (RR = 0.96, 95% CI: 0.89–1.04, p = 0.34), respectively (online supplementary file S5). The use of HCQ was not associated with significantly faster viral clearance with a median difference of 1.38 days (95% CI: −0.52 to +3.28 days) favouring the HCQ arm (online supplementary file S6).
Safety analysis: Reassuringly, HCQ therapy was generally safe with no significant increase in the rates of serious adverse events (grade 3 or worse toxicity) compared to placebo/standard of care therapy (RR = 1.01, 95% CI: 0.85–1.19, p = 0.95) (Figure 5).
FIGURE 5.
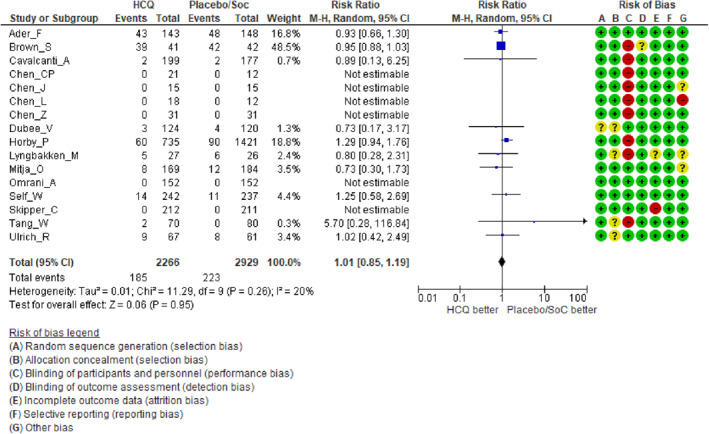
Forest plots including risk of bias in individual studies comparing hydroxychloroquine versus placebo/standard of care therapy for serious adverse events in COVID‐19
Sensitivity analysis: Sensitivity analysis showed that no single trial was driving the results of the analysis (online supplementary file S7).
Publication bias: There was mild asymmetry in the funnel plot suggesting the presence of publication bias (online supplementary file S8).
Subgroup analysis: Subgroup analysis for all‐cause mortality by severity of disease, dose of HCQ, trial setting, sample size and study design could not identify any significant difference in the overall results, inferences and conclusions of the meta‐analysis (data not shown).
Strength of recommendation: All 19 included RCTs 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 were of moderate to good quality with low risk of bias for most domains for the relevant outcomes of interest. However, there was high risk of performance and detection bias due to open‐label nature of most studies without any placebo‐controls and lack of blinding of patients and/or physicians. Based on the present analysis, there is low to moderate certainty evidence that HCQ is not associated with significant clinical benefit or harm in patients with COVID‐19 infection (Table 1).
TABLE 1.
Summary of findings for the safety and efficacy of hydroxychloroquine compared to controls (placebo/standard of care therapy) in COVID‐19 infection including the quality of evidence with grade of recommendation
| HCQ for COVID 19 | |||||
|---|---|---|---|---|---|
| Outcome of interest | No of participants (studies) | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with control | Risk difference with HCQ (95% CI) | ||||
| Clinical improvement rate | 14,443 (18) | ⊕⊕⊝⊝ | RR 1.00 (0.96–1.03) | Study population | |
| 629 per 1000 | 0 fewer per 1000 (from 25 fewer to 19 more) | ||||
| LOW a , b due to risk of bias, imprecision | |||||
| Moderate | |||||
| 660 per 1000 | 0 fewer per 1000 (from 26 fewer to 20 more) | ||||
| All‐cause mortality | 17,638 (15) | ⊕⊕⊝⊝ | RR 1.08 (1.01–1.16) | Study population | |
| 160 per 1000 | 13 more per 1000 (from 0 more to 26 more) | ||||
| LOW a , c due to risk of bias, imprecision | |||||
| Moderate | |||||
| 40 per 1000 | 3 more per 1000 (from 0 more to 7 more) | ||||
| Viral negativity rate | 2425 (7) | ⊕⊕⊕⊝ | RR 1.01 (0.92–1.12) | Study population | |
| 435 per 1000 | 4 more per 1000 (from 35 fewer to 52 more) | ||||
| MODERATE a due to risk of bias | |||||
| Moderate | |||||
| 396 per 1000 | 4 more per 1000 (from 32 fewer to 48 more) | ||||
| Serious adverse events | 4904 (15) | ⊕⊝⊝⊝ | RR 1.03 (0.76–1.4) | Study population | |
| 63 per 1000 | 2 fewer per 1000 (from 15 fewer to 25 more) | ||||
| VERY LOW a , d , e due to risk of bias, inconsistency, imprecision | |||||
| Moderate | |||||
| 11 per 1000 | 0 fewer per 1000 (from 3 fewer to 4 more) | ||||
Note: The basis for the assumed risk (the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
Abbreviations: CI, confidence interval; HCQ, hydroxychloroquine; GRADE, Grades of Recommendation, Assessment, Development, and Evaluation; RR, risk ratio.
Most studies were open labelled without placebo control or blinding.
A significant number of studies straddle the line of unity with RR increase or decrease by >25%.
All studies straddle the line of unity with RR increase or decrease by >25%.
The direction of effect is opposite to one another in individual studies.
All studies straddle the line of unity with RR increase or decrease by >25%.
5. DISCUSSION
The outbreak of COVID‐19 pandemic prompted the scientific and medical community not only to develop new and/or novel medical therapies but also to explore the possibility of repurposing existing drugs with promising anti‐viral activity against SARS‐CoV‐2. 2 , 5 A widely used anti‐malarial and anti‐rheumatic drug CQ and its less toxic congener HCQ were among the first such repurposed agents that showed remarkable in vitro activity against COVID‐19 in the lab 15 , 16 and combined with promising results in early observational studies 17 , 18 received EUA in March 2020 by the US‐FDA to facilitate widespread adoption during the ongoing pandemic. A large‐scale multinational registry analysis reported a significant increase in risk of mortality with CQ/HCQ treatment in COVID‐19 infection related to cardiotoxicity which was subsequently retracted due to concerns regarding authenticity and integrity of data. 46 The FDA authorisation for HCQ was later revoked in June 2020 based on emerging data on the lack of efficacy and valid concerns regarding cardiac safety. 19 , 20
Early evidence for the efficacy of HCQ in COVID‐19 was initially reported by Gautret et al. in a small, prospective cohort, open‐label, non‐randomised study. 17 Subsequently, multiple large retrospective cohorts 47 , 48 , 49 have found the use of HCQ to be associated with significantly decreased risk of prolonged hospitalisation, need for intensive care or death and shorter duration of viral shedding with marginally increased risk of cardiac events compared to other regimens. This apparent clinical benefit of HCQ in observational studies, however, did not stand the scrutiny and rigour of randomised trials. The most robust data demonstrating lack of effectiveness of HCQ in COVID‐19 come from the largest two RCTs, RECOVERY and Solidarity both of which failed to demonstrate any significant clinical benefit and terminated accrual to the HCQ arm on the first interim analysis. Critics argue that both of the RCTs (RECOVERY and Solidarity) initiated treatment with HCQ quite late in the course of the disease (median of 9 days from symptom onset), whereas HCQ appears to be consistently effective 50 if provided much earlier (within 48 h of symptom onset) on outpatient basis and is generally safe when used responsibly. A rapid meta‐analysis 51 involving 10,012 patients in 26 ongoing, completed or discontinued RCTs reported a significant increase in all‐cause mortality with HCQ in patients with COVID‐19 infection with an odds ratio (OR) of 1.11 (95% CI: 1.02–1.20, p = 0.02) compared to placebo/standard of care therapy. More recently, a Cochrane review 52 concluded that treatment with HCQ for COVID‐19 makes little or no difference to death due to any cause (RR = 1.09, 95% CI: 0.99–1.19, 8208 patients in nine trials, high‐certainty evidence). On the contrary, HCQ probably results in nearly threefold increase in the risk of adverse events (RR = 2.90, 95% CI: 1.49–5.64, 1394 patients in six trials, moderate‐certainty evidence), although the risk of serious adverse events is not significantly increased (RR = 0.82, 95% CI: 0.37–1.79, 1004 patients in six trials, low‐certainty evidence). A systematic review of the effects of CQ/HCQ on non‐SARS‐CoV2 viral infections 53 also does not support the use of these agents in COVID‐19 due to lack of efficacy and potential for harm.
Increasing concerns regarding cardiac safety of CQ/HCQ prompted a global review of pharmacovigilance database to identify the prevalence, severity and type of cardiotoxicity. The French Pharmacovigilance network database 54 evaluating postmarketing adverse cardiac adverse drug reactions associated with HCQ reported a significant increase in repolarisation, ventricular rhythm disorders and sinus bradycardia in patients exposed to off‐label, empirical HCQ in COVID‐19 compared to its usage in approved indications (lupus and rheumatoid arthritis) in the pre‐COVID era. Similar conclusions were drawn by a large‐scale disproportionality analysis of the FDA Adverse Events Reporting System database 55 which demonstrated that HCQ was associated with higher reporting of ventricular hypertrophy, diastolic dysfunction, pericarditis, cardiomyopathy, atrio‐ventricular block, torsades de pointes and QT prolongation, the last two of which are most relevant to COVID regimens (higher doses over short periods) and represent the most common HCQ‐associated cardiac adverse events.
The conflicting comparative evidence between big data and the real world 56 on the effectiveness of HCQ in COVID‐19 has led to divided opinion within the medical community. Big data observational studies are associated with conflicts of interest, lack details on HCQ dosage and duration, and have shown the absence of efficacy. On the other hand, real‐world data from clinical studies have reported favourable clinical and virological outcomes with HCQ including trend towards reduction in mortality. 56 A comparison of the mortality estimates from COVID‐19 between developing countries (manly from Asia and Far East) that adopted CQ/HCQ early versus those that expressed concerns regarding its usage (developed countries mainly in Europe and America) shows a striking inverse relationship between widespread CQ/HCQ usage and deaths per million population, which warrants more in‐depth analysis. 57 The current systematic review and meta‐analysis (including both RECOVERY and Solidarity) thus provides the largest (over 10,000 randomised patients) and most updated contemporary evidence regarding the safety and efficacy of HCQ in the treatment of COVID‐19 infection. The addition of HCQ to current standard of care therapy is not associated with statistically significant or clinically meaningful benefit (reduction in mortality or improvements in clinical recovery and/or virological clearance). Reassuringly, HCQ is neither associated with serious harm compared to placebo/standard of care therapy allaying fears regarding its safety.
An analysis of the WHO‐ICTRP database 58 in April 2020 had identified a total of 51 registered RCTs evaluating CQ or HCQ, either alone or in combination against other treatments in COVID‐19. The median (inter‐quartile range) sample size was 262 (100–520) patients with 34 (67%) trials proposing clinical outcome, 12 (24%) trials a surrogate outcome and 5 (10%) trials a combination of clinical and surrogate outcome as primary endpoints. Twenty‐four (47%) RCTs did not describe plans to assess safety outcomes, prompting the conclusion that RCTs investigating CQ/HCQ during the early stages of the COVID‐19 pandemic included heterogeneous and insufficient approaches to measure efficacy/effectiveness and safety relevant to patients and clinical practice. Since the early days of the pandemic, over a thousand studies have been launched to test repurposed medicines and newer drugs as potential therapeutics for COVID‐19. Current global clinical research activity in COVID‐19 appears fragmented with research agenda being driven by anecdote and hype rather than informativeness and societal value. Substantial heterogeneity of study design and diversity of outcome measures has rendered meaningful comparisons difficult. Despite the availability of several large multi‐arm trials, evidence on comparative effectiveness of potential therapeutic alternatives has not been forthcoming. Determining the comparative effectiveness of drugs requires selection of appropriate treatments to be tested in large clinical trials, streamlining trial design, analysis and reporting and timely sharing of individual participant data with greater collaboration and coordination among trialists, meta‐analysts, guideline developers and other stakeholders to facilitate producing trustworthy comparative evidence and guidance. 59 , 60
5.1. Strengths and limitations
Despite being the largest data set derived only from RCTs and pooled using modern meta‐analytic methods, certain caveats and limitations remain. Large majority of studies including the two largest trials (RECOVERY and Solidarity) were open‐label without any placebo‐controls with significant potential for performance and detection bias. The standard of care therapy (control arm) as well as dose and duration of HCQ therapy (test arm) was variable across studies further confounding the analyses and inferences. Finally, unpublished data from completed or prematurely terminated trials were not available for pooling in the meta‐analysis. However, given the number of randomised patients included in the analysis, it is highly unlikely that any further updated pooled analysis would substantially enhance the certainty of evidence or improve the strength of recommendation.
6. CONCLUSIONS
There is low to moderate certainty evidence that HCQ therapy is generally safe but does not reduce mortality or enhance clinical/virological recovery compared to placebo/standard of care therapy in patients with COVID‐19 infection.
CONFLICTS OF INTEREST
None of the authors have any conflicts of interest to declare.
ETHICAL STATEMENT
Not applicable.
AUTHOR CONTRIBUTIONS
Tejpal Gupta: Conceptualization, Methodology, Analysis, and Writing ‐ original draft; Prafulla Thakkar: Data curation and Writing ‐ review & editing; Babusha Kalra: Data curation and Writing ‐ review & editing; Sadhana Kannan: Methodology, Literature search strategy, and Statistical analysis.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
No financial support was involved in the study.
Gupta T, Thakkar P, Kalra B, Kannan S. Hydroxychloroquine in the treatment of coronavirus disease 2019: rapid updated systematic review and meta‐analysis. Rev Med Virol. 2022;32(2):e2276. 10.1002/rmv.2276
REFERENCES
- 1. Mahase E. Covid‐19: WHO declares pandemic because of ‘alarming levels’ of spread, severity, and inaction. BMJ. 2020;368:m1036. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO). A coordinated global research roadmap: 2019 Novel coronavirus [Internet] March 2020 https://www.who.int/blueprint/priority‐diseases/key‐action/Coronavirus_Roadmap_V9.pdf. Accessed September 25, 2020.
- 3. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutierrez‐Ocampo E, et al. Clinical, laboratory, and imaging features of COVID‐19: a systematic review and meta‐analysis. Trav Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID‐19): a literature review. J Infect Public Health. 2020;13(5):667‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan MM, Noor A, Madni A, Shafiq M. Emergence of novel coronavirus and progress toward treatment and vaccine. Rev Med Virol. 2020;30(4):e2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giovane RA, Rezai S. Cleland E, Henderson CE. Current pharmacological modalities for management of novel coronavirus 2019 (COVID‐19) and the rationale for their utilization: a review. Rev Med Virol. 2020;30(5):e2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keam S, Megawati D, Patel SK, et al. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol. 2020;30(5):e2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hariyanto TI, Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol. 2021;93(3):1832‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hariyanto TI, Halim DA, Jodhinata C, Yanto TA, Kurniawan A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. Clin Exp Pharmacol Physiol. 2021;48(6):823‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hariyanto TI, Halim DA, Rosalind J, Gunawan C, Kurniawan A. Ivermectin and outcomes from Covid‐19 pneumonia: a systematic review and meta‐analysis of randomized clinical trial studies. [published online ahead of print June 06, 2021]. Rev Med Virol. 10.1002/rmv.2265 [DOI] [Google Scholar]
- 11. Gupta T, Kannan S, Kalra B, Thakkar P. Systematic review and meta‐analysis of randomized controlled trials testing the safety and efficacy of convalescent plasma in the treatment of coronavirus disease 2019 (COVID‐19): evidence‐base for practice and implications for research. [Published online ahead of print June 29]. Transfus Med. 10.1111/tme.12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frediansyah A, Tiwari R, Sharun K, Dhama K, Harapan H. Antivirals for COVID‐19: a critical review. Clin Epidemiol Glob Health. 2021;9:90‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frediansyah A, Nainu F, Dhama K, Mudatsir M, Harapan H. Remdesivir and its antiviral activity against COVID‐19: a systematic review. Clin Epidemiol Glob Health. 2021;9:123‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. FDA's approval of Veklury (remdesivir) for the treatment of COVID‐19 – the Science of Safety and Effectiveness October 2020 [Internet] https://www.fda.gov/drugs/drug‐safety‐and‐availability/fdas‐approval‐veklury‐remdesivir‐treatment‐covid‐19‐science‐safety‐and‐effectiveness. Accessed October 24, 2020. [Google Scholar]
- 15. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov. 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label, non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID‐19. Int J Infect Dis. 2020;97:396‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS‐CoV‐2 infection. CMAJ. 2020;192(17):E450‐E453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coronavirus (COVID‐19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. June 2020. [Internet] https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐fda‐revokes‐emergency‐use‐authorization‐chloroquine‐and. Accessed September 25, 2020. [Google Scholar]
- 21. Chivese T, Musa OAH, Hindy G, et al. A meta‐review of systematic reviews and updated meta‐analysis on the efficacy of chloroquine and hydroxychloroquine in treating COVID19 infection. medRxiv. Preprint. 10.1101/2020.07.28.20164012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. www.handbook.cochrane.org. Accessed September 25, 2020. [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383‐394. [DOI] [PubMed] [Google Scholar]
- 26. Elliott JH, Turner T, Classivi O, et al. Living systematic reviews: an emerging opportunity to narrow the evidence‐practice gap. PLoS Med. 2014;11(2):e1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abd‐Elsalam S, Esmail ES, Khalaf M, et al. Hydroxychloroquine in the treatment of COVID‐19: a multicentre randomized controlled study. Am J Trop Med Hyg. 2020;103(4):1635‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Ader F, Peiffer‐Smadja N, Poissy J, et al. An open‐label randomized controlled trial of the effect of lopinovir/ritonavir, lopinavir/ritonavir plus IFN‐β‐1a and hydroxychloroquine in hospitalized patients with COVID‐19. [Published online ahead of print May 25, 2021]. Clin Microbiol Infect. 10.1016/j.cmi.2021.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown SM, Peltan I, Kumar N, et al. Hydroxychloroquine vs azithromycin for hospitalized patients with COVID‐19 (HAHPS): results of a randomized active comparator trial. Ann Am Thorac Soc. 2020;18(4):590‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild‐to‐moderate covid‐19. N Engl J Med. 2020;383(21):2041‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen CP, Lin YC, Chen TC, et al. A multicentre, randomized, open‐label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID‐19). PLoS One. 2020;15(12):e0242763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen J, Danping L, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease‐19 (COVID‐19). J Zhejiang Univ. 2020;49(2):215‐219. 10.3785/j.issn.1008-9292.2020.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen L, Zhang ZY, Fu JG, et al. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID‐19: a prospective open‐label randomized controlled study. medRxiv. Preprint. 10.1101/2020.06.19.20136093 [DOI] [Google Scholar]
- 34. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. medRxiv. Preprint. 10.1101/2020.03.22.20040758 [DOI] [Google Scholar]
- 35. Dubee V, Roy PM, Vielle B, et al. Hydroxychloroquine in mild‐to‐moderate COVID‐19: a placebo‐controlled double blind trial. [Published online ahead of print April 1, 2021]. Clin Microbiol Infect. 10.1016/j.cmi.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID‐19. N Engl J Med. 2020;383(21):2030‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamran SM, Moeed H, Mirza Z, et al. Clearing the fog: is hydroxychloroquine effective in reducing coronavirus disease‐2019 progression? a randomized controlled trial. Cureus. 2021;13(3):e14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lyngbakken NM, Berdal JE, Eskesen A, et al. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun. 2020;11(1):5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitja O, Corbacho‐Monne M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild COVID‐19: a randomized‐controlled trial. [Published online ahead of print July 16, 2020]. Clin Infect Dis. 10.1093/cid/ciaa1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Omrani AS, Pathan SA, Thomas SA, et al. Randomized double‐blinded placebo‐controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non‐severe Covid‐19. EClinicalMedicine. 2020;29:100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan H, Peto R, Henao‐Restrepo AM, et al. Repurposed antiviral drugs for COVID‐19 – interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Self WH, Semler MW, Leither LM, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID‐19: a randomized clinical trial. J Am Med Assoc. 2020;324(21):2165‐2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in non‐hospitalized adults with early COVID‐19: a randomized trial. Ann Int Med. 2020;173(8):623‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomized controlled trial. BMJ. 2020;369:m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ulrich RJ, Troxel AB, Carmody E, et al. Treating COVID‐19 with hydroxychloroquine (TEACH): a multicentre, double‐blind, randomized controlled trial in hospitalized patients. Open Forum Infect Dis. 2020;7(10):ofaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehra MR, Ruschitzka F, Patel AN. Retraction – hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: a multinational registry analysis. Lancet. 2020;395(10240):1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lagier JC, Million M, Gautret P, et al. Outcomes of 3,737 COVID‐19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Trav Med Infect Dis. 2020;36:101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Di Castelnuovo A, Costanzo S, Antinori A, et al. Use of hydroxychloroquine in hospitalized COVID‐19 patients is associated with reduced mortality: findings from the observational multicentre Italian CORIST study. Eur J Int Med. 2020;82:38‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Catteau L, Dauby N, Montourcy M, et al. Low‐dose hydroxychloroquine therapy and mortality in hospitlaized patients with COVID‐19: a nationwide observational study of 8075 participants. Int J Antimicrob Agents. 2020;56(4):106144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prodromos C, Rumschlag T. Hydroxychloroquine is effective, and consistently so when provided early, for COVID‐19: a systematic review. New Microbe New Infect. 2020;38:100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Axfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID‐19 from an international collaborative meta‐analysis of randomized trials. Nat Commun. 2021;12(1):2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singh B, Ryan H, Kredo T, et al. Chloroquine or hydroxychloroquine for prevention and treatment of COVID‐19. Cochrane Database Syst Rev. 2021;2(2):CD013587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cui X, Sun J, Minkove SJ, et al. Effects of chloroquine or hydroxychloroquine treatment on non‐SARS‐CoV2 viral infections: a systematic review and meta‐analysis. [Published online ahead of print 11 March 2021]. Rev Med Virol. 10.1002/rmv.2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Romani S, Gerard A, Fresse A, et al. Insights on the evidence of cardiotoxicity of hydroxychloroquine prior and during COVID‐19 pandemic. Clin Transl Sci. 2021;14(1):163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Singh AP, Tousif S, Umbarkar P, Lal H. A pharmacovigilance study of hydroxychloroquine cardiac safety profile: potential implication in COVID‐19 mitigation. J Clin Med. 2020;9(6):1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Million M, Gautret P, Colson P, et al. Clinical efficacy of chloroquine derivatives in COVID‐19 infection: comparative meta‐analysis between the big data and the real world. New Microbe and New Infect. 2020;38:100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roussel Y, Raoult D. First evaluation of hydroxychloroquine recommendations in treating SARS‐CoV‐2. New Microbe and New Infect. 2020;38:100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Junqueira DR, Rowe BH. Efficacy and safety outcomes of proposed randomized controlled trials investigating hydroxychloroquine and chloroquine during the early stages of the COVID‐19 pandemic. Br J Clin Pharmacol. 2021;87(4):1758‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nguyen VT, Riviere P, Ripoll P, et al. Research response to COVID‐19 needed better coordination and collaboration: a living mapping of registered trials. J Clin Epidemiol. 2020;130:107‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. He Z, Erdengasileng A, Luo X, et al. How the clinical research community responded to the COVID‐19 pandemic: an analysis of the COVID‐19 clinical studies in ClinicalTrials.gov. JAMIA Open. 2021;4(2):ooab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


