The Coronavirus Disease‐2019 (COVID‐19) outbreak is still keeping the world in suspense and challenges humanity on a new level. Infection with the acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) impairs oxygen diffusion capacity of the lung and in consequence causes hypoxemia. In 20%‐40% of COVID‐19 cases, patients have alarmingly low oxygen (O2) saturation of the blood without being dyspneic, a condition called “silent hypoxia”. 1 However, hypoxemia leads to severe injuries in diverse organs, particularly those of high oxygen dependency such as the brain, heart or kidney. 1 Tissue hypoxia results from an imbalance between O2 consumption and O2 delivery. 2 Insufficient oxygen supply forces cells to adapt via multiple physiological processes as has been extensively reported in Acta Physiologica (Oxf.). 3 , 4 , 5 In general, but also in the context of COVID‐19, the basic understanding of cellular O2 availability represents a major topic. 6 , 7 , 8 The final amount of oxygen reaching the cell, or more precisely the mitochondria for oxidative metabolism, is mainly attributed to i) the oxygen carrying capacity of the blood as determined by the haemoglobin (Hb) level, and ii) the ability to release O2 to the tissue as determined by the Hb‐O2 affinity. The latter may belong to the physiological knowledge that is underestimated in clinical routine, as indicated by only few investigations in this regard. However, because of the risk of thrombotic events, the issue of Hb‐O2 affinity might be of exceptional relevance in COVID‐19 and silent hypoxia. Thus, we are wondering whether the modulation of oxygen affinity to haemoglobin may help to improve cellular oxygen availability.
Hb‐O2 affinity is displayed by the oxygen dissociation curve (ODC), showing the O2 saturation of the blood (sO2) at changing partial pressures of O2 (pO2). The S shaped ODC indicates two crucial characteristics: First, its position at the half‐saturated pO2, defined as p50, which is used to describe the Hb‐O2 affinity and secondly, its slope (n in the logarithmic Hill plot). 9 Hb‐O2 affinity is modulated by pCO2, pH (H+ level), temperature and organic phosphates, most importantly 2,3‐bisphosphoglycerate (2,3‐BPG, formerly 2,3‐DPG). The standard p50 in humans is 26.9 mmHg at pH 7.4 and 37°C. What are the pros and cons of left and right shifting, which indicate increased or decreased Hb‐O2 affinity respectively?
A leftward shift of ODC (lower p50) is associated with improved oxygen loading of Hb in the pulmonary system. For instance, at high altitude respiratory alkalosis causes increased Hb‐O2 affinity and, thus, enhanced oxygen upload. Nevertheless, a leftward shift of ODC is also associated with lowered oxygen release to the tissue. A rightward shift of ODC improves cellular oxygen availability and occurs, for example, under conditions of elevated muscle workload. 10 Here, increased pCO2, H+ level and temperature provoke a rightward shift of ODC, ensuring that more oxygen is released under conditions of higher oxygen needs. Furthermore, pregnant women show high 2,3‐BPG levels and can more easily release oxygen to the foetus. Foetal haemoglobin, on the other side, is not susceptible to 2,3‐BPG and, because of the high Hb‐O2 affinity, can well uptake the maternal oxygen. By the way, a high capillary density compensates for the high Hb‐O2 affinity in the foetus. A rightward shift of ODC also occurs in anaemia, a compensatory mechanism to support cellular oxygen availability. However, both left‐ as well as rightward shift of ODC might be beneficial depending on the circumstances. In COVID‐19, we are faced with hindered oxygen uptake and one would expect that higher Hb‐O2 affinity could be helpful. 9
Notably, in a cohort of 60 young and healthy people, the p50 showed a strikingly high diversity, ranging from 24.87 mmHg to 31.8 mmHg, which correlated with lower or higher 2,3‐BPG levels in the blood respectively. 11 This grade of basal range is comparable to the grade of right shift from rest to maximum physical activity. Thus, the high p50 diversity observed in the human population (see Figure 1) represents a crucial feature that should be taken into account. Women, on average, showed lower Hb‐O2 affinity than men. However, the cellular oxygen availability is expected to be similar, as men compensate high Hb‐O2 affinity with a high oxygen carrying capacity (Hb level). Nevertheless, such an unexpected individual range in base‐line Hb‐O2 affinity may be linked to the risk of tissue hypoxia, especially in case of acute hypoxemia as in COVID‐19. Low oxygen in the blood combined with impeded release of oxygen to the tissue would be expected to be disastrous.
FIGURE 1.
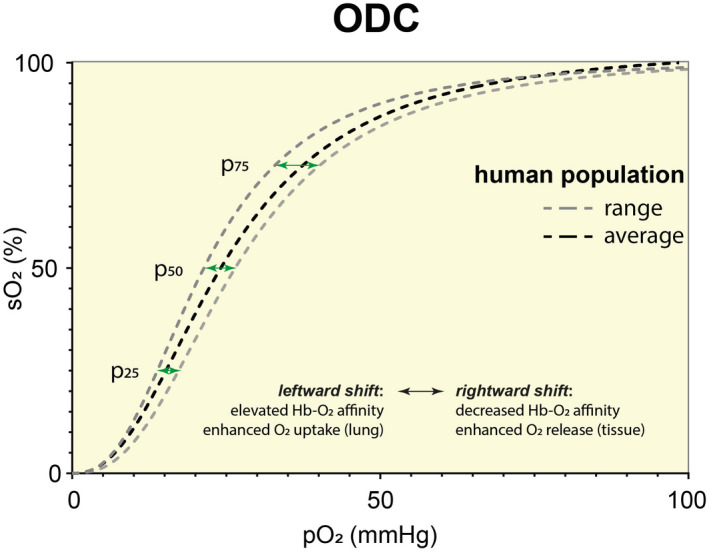
Oxygen Dissociation Curve (ODC): Range in human population. Data were obtained from tonometric measurements of 60 young and healthy volunteers participating in an endurance exercise study as described in Balcerek et al. 11 The average ODC represents the mean of all 60 participants. To indicate the range in a human population, the ODCs of the participant with lowest and highest p50 values were chosen. The higher distance of pO2 at 75% O2 saturation (p75) compared to p50 and p25 (green arrows) indicate that the slope is steeper when ODC is shifting to the left
As various diseases are associated with hypoxia, 12 , 13 , 14 , 15 it could be beneficial to modify the baseline Hb‐O2 affinity. As mentioned above, the Hb‐O2 affinity can be modulated through various endogenous effectors. Hydrogen ions, pCO2 and temperature are crucial determinants of acute adaptation in Hb‐O2 affinity during elevated workload, but are strictly regulated by other mechanisms and cannot serve for adaptation in the long‐term. 11 , 16 , 17 , 18 The most important modifier of Hb‐O2 affinity at rest is 2,3‐BPG that binds and stabilizes the T (tense)‐state of Hb. 2,3‐BPG binding to Hb results in a decrease in Hb‐O2 affinity (rightward shift of ODC) and increase in the delivery of oxygen to the tissue. 17 2,3‐BPG is synthesized by the enzyme 2,3‐Bisphosphoglycerate mutase (BPGM) and its activity is regulated by pH: acidosis lowers and alkalosis elevates BPGM activity. Although assumed for a long time, there is no long‐term effect on the Hb‐O2 affinity (increased p50) through endurance exercise. 11 The main difference seems to be a sex difference, while individual diversity is remarkable. At present, no trans‐acting factor (neither transcription factor, RNA‐binding protein nor micro‐RNA) has been described to regulate BPGM expression. Interestingly, although anaemia is a result of COVID‐19, the few studies published so far indicated no alteration in p50 as would be expected (excellently reviewed in Böning, Kuebler & Bloch 2021). 9 Thus, the question arises whether we can provoke artificial modulation of Hb‐O2 affinity.
In this regard, more than 50 years ago scientists began to search for synthetic modifiers of Hb‐O2 affinity, especially for the treatment of Sickle Cell Disease (SCD). One of the first therapies was the use of oral sodium cyanate. Cyanate interacts with both alpha and beta chains of the amino terminal valine residues in sickle Hb called carbamylation, which prevents sickling of the erythrocytes. Treated patients showed a decrease in haemolytic rate, bilirubin and lactate dehydrogenase levels as well as an increase in Hb and erythrocyte survival. 19 However, further trials on patients were stopped because of the high toxicity of cyanate. 19 To avoid such side effects, Deiderich et al developed a method of extracorporeal carbamylation. 20 Indeed, sickled haemoglobin (HbS) was decreased by 65%, without toxic side effects of cyanate. The p50 shifted to the left from 33 to 26 mmHg but, surprisingly, because of increased Hb levels in the patients, the available O2 capacity increased as well. Overall, this technique is very complex and difficult to administrate, thus, further investigations were canceled. 20 Over the years, many more drugs were developed to interact with Hb and shift the ODC to the left, like 5‐hydroxymethyl‐2‐furfural (5‐HMF or AES‐103) and Voxelotor (GBT440). 19 Both substances have been designed for the treatment of SCD. 5‐HMF binds by Schiff‐base interaction to the N‐terminal αVal1 from the α chain of the oxygenated R2 structure of Hb and thereby increases the Hb‐O2 affinity. Voxelotor acts similar to 5‐HMF and increases the O2 affinity of Hb by stabilizing the R (relaxed) state. 17
On the other hand, to treat ischemic related diseases the research on synthetic Hb modulators shifting the ODC to the right is ongoing. 17 For instance, Efaproxiral or RSR‐13 (2‐[4‐[[(3,5‐dimethylanilino)carbonyl]methyl]phenoxyl]‐2‐methylpropionic acid) acts as an allosteric effector that non‐covalently binds to the middle of the central water cavity of deoxygenated Hb resulting in a decrease in Hb‐O2 affinity. 21 The allosteric effects of RSR‐13 complement those of 2,3‐BPG because both compounds have different Hb binding sites. 17 It was shown that RSR‐13 leads to improved outcomes in diseases characterized by hypoxia and ischemia. Thus, there are arguments for both left vs right shifted ODC and recent studies and opinions still do not answer the question whether an alteration of Hb‐O2 affinity would be beneficial in severely ill COVID‐19 patients.
What are our perspectives? First, it would be of interest to know whether a high risk of severe COVID‐19 course correlates with Hb‐O2 affinity. Another point is the Hill coefficient that describes the cooperativity of oxygen binding, meaning the steepness of the slope of the ODC. As a leftward shift is associated with higher steepness of ODC, 11 the effect of elevated Hb‐O2 affinity is stronger at high sO2 (lung) compared to lower sO2 (tissue). Thus, one would expect that in hypoxemia the beneficial effect of improved oxygen uptake would excel the accompanied lowered oxygen release to the tissue. However, when enhancing Hb‐O2 affinity by synthetic modifiers, tissue oxygenation must be ensured. Maybe, it is neither a left‐ nor a rightward shift we need. When coming back to physiological adaptation to high altitude we might find the goldilocks condition. At high altitude, hyperventilation causes hypocapnic alkalosis that increases Hb‐O2 affinity and improves oxygen uptake. Moreover, the activity of BPGM is elevated in alkalosis leading to enhanced 2,3‐BPG production that lowers Hb‐O2 affinity. Under these circumstances, the slope of ODC might be very steep and enables for improved oxygen uptake and delivery. Of course, we would not provoke hypocapnia and alkalosis in patients, however, 5‐HMF or Voxelotor shift the ODC to the left, too. Simultaneously, we have to provoke an elevation of 2,3‐BPG, which probably will be a challenge. But maybe this is the way it works. At least at high altitude, nature obviously found a way to improve both, pulmonary oxygen loading and cellular oxygen unloading.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Rahman A, Tabassum T, Araf Y, Al Nahid A, Ullah MA, Hosen MJ. Silent hypoxia in COVID‐19: pathomechanism and possible management strategy. Mol Biol Rep. 2021;48:3863‐3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niendorf T, Seeliger E, Cantow K, Flemming B, Waiczies S, Pohlmann A. Probing renal blood volume with magnetic resonance imaging. Acta Physiol (Oxf). 2020;228:e13435. [DOI] [PubMed] [Google Scholar]
- 3. Ivy CM, Sprenger RJ, Bennett NC, et al. The hypoxia tolerance of eight related African mole‐rat species rivals that of naked mole‐rats, despite divergent ventilatory and metabolic strategies in severe hypoxia. Acta Physiol (Oxf). 2020;228:e13436. [DOI] [PubMed] [Google Scholar]
- 4. Olson KR, Gao Y, DeLeon ER, et al. Extended hypoxia‐mediated H2 S production provides for long‐term oxygen sensing. Acta Physiol (Oxf). 2020;228:e13368. [DOI] [PubMed] [Google Scholar]
- 5. Strielkov I, Krause NC, Knoepp F, et al. Cytochrome P450 epoxygenase‐derived 5,6‐epoxyeicosatrienoic acid relaxes pulmonary arteries in normoxia but promotes sustained pulmonary vasoconstriction in hypoxia. Acta Physiol (Oxf). 2020;230:e13521. [DOI] [PubMed] [Google Scholar]
- 6. Larsen FJ, Schiffer TA, Zinner C, et al. Mitochondrial oxygen affinity increases after sprint interval training and is related to the improvement in peak oxygen uptake. Acta Physiol (Oxf). 2020;229:e13463. [DOI] [PubMed] [Google Scholar]
- 7. Pospelov AS, Puskarjov M, Kaila K, Voipio J. Endogenous brain‐sparing responses in brain pH and PO2 in a rodent model of birth asphyxia. Acta Physiol (Oxf). 2020;229:e13467. [DOI] [PubMed] [Google Scholar]
- 8. Harrell JW, Peltonen GL, Schrage WG. Reactive oxygen species and cyclooxygenase products explain the majority of hypoxic cerebral vasodilation in healthy humans. Acta Physiol (Oxf). 2019;226:e13288. [DOI] [PubMed] [Google Scholar]
- 9. Boning D, Kuebler WM, Bloch W. The oxygen dissociation curve of blood in COVID‐19. Am J Physiol Lung Cell Mol Physiol. 2021. 10.1152/ajplung.00079.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skattebo O, Calbet JAL, Rud B, Capelli C, Hallen J. Contribution of oxygen extraction fraction to maximal oxygen uptake in healthy young men. Acta Physiol (Oxf). 2020;230:e13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balcerek B, Steinach M, Lichti J, et al. A broad diversity in oxygen affinity to haemoglobin. Sci Rep. 2020;10:16920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laouafa S, Roussel D, Marcouiller F, et al. Roles of oestradiol receptor alpha and beta against hypertension and brain mitochondrial dysfunction under intermittent hypoxia in female rats. Acta Physiol (Oxf). 2019;226:e13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lempesis IG, van Meijel RLJ, Manolopoulos KN, Goossens GH. Oxygenation of adipose tissue: A human perspective. Acta Physiol (Oxf). 2020;228:e13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ngo JP, Lankadeva YR, Zhu MZL, et al. Factors that confound the prediction of renal medullary oxygenation and risk of acute kidney injury from measurement of bladder urine oxygen tension. Acta Physiol (Oxf). 2019;227:e13294. [DOI] [PubMed] [Google Scholar]
- 15. Gardiner BS, Smith DW, Lee CJ, Ngo JP, Evans RG. Renal oxygenation: From data to insight. Acta Physiol (Oxf). 2020;228:e13450. [DOI] [PubMed] [Google Scholar]
- 16. Nikinmaa M, Berenbrink M, Brauner CJ. Regulation of erythrocyte function: Multiple evolutionary solutions for respiratory gas transport and its regulation in fish. Acta Physiol (Oxf). 2019;227:e13299. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed MH, Ghatge MS, Safo MK. Hemoglobin: Structure, Function and Allostery. Subcell Biochem. 2020;94:345‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damsgaard C, Baliga VB, Bates E, et al. Evolutionary and cardio‐respiratory physiology of air‐breathing and amphibious fishes. Acta Physiol (Oxf). 2020;228:e13406. [DOI] [PubMed] [Google Scholar]
- 19. Ataga KI, Stocker J. The trials and hopes for drug development in sickle cell disease. Br J Haematol. 2015;170:768‐780. [DOI] [PubMed] [Google Scholar]
- 20. Deiderich DA, Trueworthy RC, Gill P, Cader AM, Larsen WE. Hematologic and clinical responses in patients with sickle cell anemia after chronic extracorporeal red cell carbamylation. J Clin Invest. 1976;58:642‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breidbach A, Catlin DH. RSR13, a potential athletic performance enhancement agent: detection in urine by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:2379‐2382. [DOI] [PubMed] [Google Scholar]


