Abstract
Aims of the study
To describe the experience of six hospitals in the management of COVID‐19 patients in rural areas through an assessment of proportions, types and clinical outcomes of remote clinical interventions.
Methods
This was a prospective observational study conducted in six Egyptian hospitals over a period five months. An emergency response was implemented in each hospital in order to connect clinical pharmacists with COVID‐19 patients living in rural areas. Pharmacists used phone calls and social media applications, such as WhatsApp® to conduct two types of interventions; (a) Proactive interventions and (b) outcome‐based interventions. IBM SPSS V26 was used for data analysis.
Results
Of the 418 patients included, 351 (83.97%) recovered, 60 (14.35%) were hospitalised and 7 (1.67%) were deceased. Medication orders per patient, high‐alert medications per patient and prescribing errors per patient were 5.82, 1.45 and 0.74, respectively. Telepharmacy teams conducted 3318 phone calls, 2116 WhatsApp® chats and 1128 interventions, of which 812 (71.92%) were process‐based and 316 (27.98%) were outcome‐based. Among these interventions, four significant determinants of improvement in clinical outcomes were found: substitution of a prescribed drug (Adjusted odds ratio [AOR] = 4.03; 95% confidence interval [CI], 2.54‐5.87), adding a drug to the prescription (AOR = 3.15; 95% CI, 1.87‐4.76), advice the patient to stop smoking (AOR = 3.53; 95% CI, 1.98‐5.17) and cessation of drug therapy (AOR = 3.11; 95% CI, 1.25‐4.55). The most common medications involved in drug‐related interventions were Hydroxychloroquine, Azithromycin and Paracetamol.
Conclusion
Our findings demonstrate significant impact of the remote pharmacist interventions on both medicines use and clinical outcomes of COVID‐19 patients in rural areas. Pharmacists in developing countries should be supported to implement remote clinical services to provide patients in rural places with optimal care.
What’s known
COVID‐19 has severe negative consequences on patient safety, especially in rural areas.
The impact of COVID‐19 in rural areas in developing countries is disastrous and more serious compared with other regions.
Remote clinical services are implemented worldwide to reduce the risk for COVID‐19 transmission and increase access to healthcare.
What’s new
Using phone calls and WhatsApp® by clinical pharmacists can improve the clinical outcomes of COVID‐19 patients in rural areas.
Hospital telepharmacy tools have an important role providing high‐quality clinical care for rural communities during disasters.
Remote clinical services may reduce inappropriate medicine prescribing and use.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) has cost the global health and economy an enormous humanistic and monetary loss, and posed unprecedented challenges to healthcare systems. 1 Inadequate personal protective equipment, poor sanitation, overburdened hospitals and scarce resources in low‐ and middle‐income countries (LMICs) may accelerate the transmission of the pandemic and thwart any attempts for effective implementation of public health measures. 2 In addition, inequitable access to healthcare because of socio‐economic gaps put a further strain on healthcare systems in LMICs. 3 , 4
In this context, Rural healthcare systems are the most impacted since they suffer from insufficient staffing, lack of healthcare infrastructure, isolation rooms and communication tools. 4 , 5 COVID‐19 patients in rural areas also suffer from inadequate access to needed health services because of long travel time to healthcare facilities, lack of reliable public transportation, insurance coverage issues and violence. 5 In the USA for instance, rural areas has become more diverse racially and ethnically, and thus different health challenges and social vulnerability to the pandemic among these communities are expected. 6
Amongst the avalanche of studies concerning the devastating effects of COVID‐19, evidence have emerged demonstrating pharmacists as potential key players in emergency response, since they are the most accessible healthcare professionals and can reduce the burden on healthcare systems by working directly with the public, 7 , 8 providing care for patients with chronic health conditions, 9 , 10 , 11 and providing pharmaceutical care for COVID‐19 patients. 12 More specifically, pharmacists’ scope of practice during COVID‐19 included providing drug information for healthcare personnel, patient counselling, optimisation of drug therapy, support infection prevention and control practices, monitoring laboratory results and drug inventory management. 12 , 13
The use of information technology to exchange medical data between two sites is called telemedicine and it has gained much more attention during the ongoing crisis. 14 Applying this concept to pharmacy practice produces telepharmacy, which strategy allows pharmacists to provide their services without direct physical contact with costumers. 15 Before this pandemic, telepharmacies applied in the United States (US) hospitals improved patient access to pharmaceutical care and contributed to engage hospital pharmacists more in patient‐centred care. 16 In Europe, hospital telepharmacy was a useful tool in remote outpatient consultation, home delivery of medications and coordination between healthcare personnel. 17 However, the vast majority of published articles on this topic are descriptive in nature and did not provide compelling evidence relating to usefulness and benefits gained from implementation of telepharmacy as an emergency response plan.
Therefore, a strategy was needed with the focus on improving access of underserved population to proper care, while reducing the risk for COVID‐19 transmission. A multidisciplinary expert team, comprising a group of clinical pharmacists, infectious disease specialists and nurses developed a response plan to standardise patient care in six Egyptian hospitals. The purpose of this strategy was to connect pharmacists with both physicians and self‐isolated COVID‐19 patients in rural areas using information technology tools. However, there were limited resources to create full telepharmacy model. Therefore, pharmacists mainly used phone calls and social media applications to initiate their response plan. The current study provides evidence on the outcomes of remote pharmacist interventions carried out to manage COVID‐19 patients in rural areas.
1.1. Aims of the study
To describe the experience of six hospitals in the management of COVID‐19 patients in rural areas through an assessment of frequency, nature and clinical outcomes of remote clinical interventions
2. METHODS
2.1. Study design
This cross‐sectional study used prospective data from six hospital‐based telepharmacies in Egypt over 5 months (from June to November 2020). Clinical pharmacists reported their interventions upon drug therapy of COVID‐19 patients living in rural areas and clinical outcomes of those patients. Participants included were informed about the purposes of the study and verbal consents were obtained. COVID‐19 patients who met the confirmatory laboratory evidence issued by the Ministry of Health in Egypt, and lived in rural areas were included in the study. Those who had no access to phone calls, moved into the urbans during the study, or not willing to be involved were excluded.
2.2. Characteristics of telepharmacy model
The model was simple, clinical pharmacists who had full access to patient records communicated virtually with physicians and patients (Figure 1). In this model, physicians prescribed medication orders for each patient using handwriting. Pharmacists reviewed the prescribed medications against the clinical data available from patient records. Then, medications were dispensed to patients’ representatives. Pharmacists followed‐up with patients on a daily basis using phone calls, social media applications such as WhatsApp®. Secure network connection, electronic prescribing system, electronic patient records, automated drug dispensing cabinets, cloud services and home delivery services were not available. Thus, we asked each pharmacist to record his/her interactions with patients and physicians on an excel sheet designed by the principle investigator.
FIGURE 1.
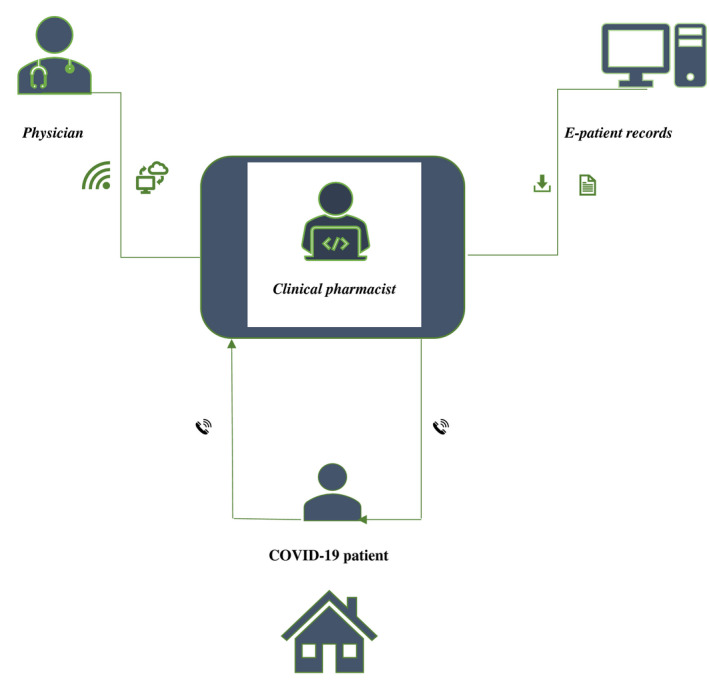
The structure of telepharmacy model
2.3. Definitions
To describe virtual interactions carried out between health providers and patients, and categorise remote pharmacist interventions, several operational definitions were adopted, tailored to the study aims, and constantly updated based on the interim guidance issued by governmental entities and international pharmaceutical organisations, and based on published articles. Some of these definitions are listed below:
Rural areas: there is no global standard definition for rural areas. In Egypt, they are defined as “very distant places where public transportation and services are lacking.” 18
Recovered COVID‐19 patients: Clinical pharmacists considered patients as recovered from the infection when fever disappeared for more than 72 hours, other symptoms including but not limited to: cough, chest pain, sore throat and difficulty in breathing disappeared or significantly improved, and the results of a minimum of two consecutive Polymerase Chain Reaction (PCR) tests conducted at least 24 hours apart were negative.
2.3.1. Data collection and validation
At the beginning of the research project, five online meetings were conducted, at which the principal researcher explained to telepharmacy teams the main purposes of the project, the nature of data collection and expected outcomes. The followings are the key points of the meetings:
The nature of the task: The data collection should be prospective and all eligible patients should be included. Any patients who withdraw while the study is ongoing should be excluded and their data should be erased. It was decided that pharmacists should follow‐up with patients until they are cured, hospitalised or deceased. When a patient entre the hospital, pharmacists were asked to record the final outcome (discharged or deceased).
The expected outcomes: First, pharmacists were asked to record the baseline characteristics of patients enrolled in the study. These included gender, age, weight, height, comorbidity, smoking status, level of education, marital status, symptoms and quarantine conditions, such as private tools, bathroom, utilities, proper disposal of wastes, proper washing of clothes and healthy foods. Second, pharmacists were asked to report their virtual interactions with patients or physicians. These interactions were divided based on the point of intervention into: (a) process‐based clinical interventions (proactive interventions), which were defined as any interventions performed on prescriptions or on any patient behaviours that may worsen the case, but without any signs of deterioration. (b) outcome‐based clinical interventions, which were defined as any interventions performed after appearance of any adverse effects or any signs of deterioration for the patient. Third, pharmacists were asked to report the clinical outcomes of their interventions.
Validation and conflict of interests: Three research associates were asked to supervise the data collection. They were assigned to perform double check for reported data, exclude any ambiguous information, try to get the missing information without contacting patients, update clinical pharmacists with the latest guidelines and protocols and ensure telepharmacy tools are fully functional. Clinical pharmacists participated in the study were asked to declare if any of the patients enrolled in the study is a close relative, and in this case, data related to this patient was excluded from the final dataset.
Data management: Research associates were responsible for collecting data sheets and notes from the six telepharmacies. The Principal investigator and the research associate built the final database and their work was validated by two independent assessors, who compared the database with data sheets and notes written by the clinical pharmacists. IBM SPSS (IBM Corp.) v26 and Microsoft Excel (Microsoft) were the softwares whereby data were organised and analysed.
2.3.2. Statistical analysis
Quantitative data are presented as mean ± standard deviation (SD) and qualitative variables as proportions (%). To compare the means of patient groups, ANOVA test was used. To measure differences in distribution of categorical variables between recovered, hospitalised and deceased patients, A Bonferroni test was applied. To assess predictors of improved clinical outcomes, a multivariable logistic regression model was created, in which the clinical outcome status (improved or worsened) was considered dependent variable and pharmacist interventions (process‐based and outcome‐based) were considered independent variables.
3. RESULTS
Overall, 489 patients gave their consent for participation, of which 71 were excluded because of lost connection, their data were incomplete, or the patient refused to complete the study. Of the 418 patients included, 257 (61.48%) were females, 84 (20.09%) had anaemia, 164 (39.23%) had polymorbidity, 188 (44.97%) were smoking every day and had smoked more than 100 cigarettes throughout their lives (Table 1). The mean age was 56.47 (±7.24) years and the mean of symptom appearance after exposure was 3.63 (±1.84) days. Hospitalisation and death rates were 14.35% and 1.67%, respectively.
TABLE 1.
Characteristics of patients enrolled in the study* β (n = 418)
| Baseline parameters | Total | Recovered patients | Hospitalised patients | Deceased patients |
|---|---|---|---|---|
| No. of patients | 418 (100) | 351 (83.97) | 60 (14.35) | 7 (1.67) |
| Age, (y), mean (SD) | 56.47 (±7.24) | 39.52 (±8.24) | 62.36 (±13.86) | 67.53 (±11.87) |
| Gender, female | 257 (61.48) | 211 (60.11) | 42 (70.00) | 4 (57.14) |
| Marital status, married | 357 (85.40) | 315 (89.74) | 38 (63.33) | 4 (57.14) |
| Educational level, below college | 178 (42.58) | 150 (42.73) | 26 (43.33) | 2 (28.57) |
| Smoking status, smokers | 188 (44.97) | 130 (37.03) | 53 (88.33) | 5 (71.42) |
| Anaemia, yes | 84 (20.09) | 49 (13.96) | 32 (53.33) | 3 (42.85) |
| Lymphocyte count, low | 225 (53.52) | 160 (45.58) | 59 (98.33) | 6 (85.71) |
| Chest CT, GGO | 251 (60.04) | 189 (53.84) | 54 (90.00) | 6 (85.71) |
| Symptoms appearance after exposure, mean (SD) | 3.63 (±1.84) | 4.81 (±2.84) | 2.13 (±0.82) | 1.98 (±0.98) |
| Polymorbidity, yes | 164 (39.23) | 99 (28.20) | 59 (98.33) | 6 (85.71) |
| Comorbidities | ||||
| COPD | 38 (9.09) | 7 (1.99) | 28 (46.66) | 3 (42.85) |
| Diabetes | 123 (29.42) | 84 (23.93) | 37 (61.66) | 2 (28.57) |
| Atrial fibrillation | 7 (1.67) | 2 (0.56) | 3 (5.00) | 2 (28.57) |
| Hypertension | 156 (37.32) | 98 (27.92) | 53 (83.33) | 5 (71.42) |
| Coronary artery disease | 12 (2.87) | 7 (1.99) | 3 (5.00) | 2 (28.57) |
| Hyperlipidemia | 148 (35.40) | 96 (27.35) | 50 (83.33) | 2 (28.57) |
| Heart failure | 35 (8.37) | 11 (3.13) | 22 (36.66) | 2 (28.57) |
| Liver disease | 16 (3.82) | 6 (1.70) | 9 (15.00) | 1 (14.28) |
| Renal disease | 34 (8.13) | 8 (2.27) | 22 (36.66) | 2 (28.57) |
| Cancer | 2 (0.47) | 0 (0.00) | 2 (3.33) | 0 (0.00) |
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computerised tomography; GGO, ground glass opacity; SD, standard deviation; β, no significant difference in baseline characteristics (all P > .05).
Items listed as numbers bed as proportions [n (%)] unless stated otherwise.
Physicians wrote 2431 medication orders, of which 609 were high‐alert medications. Telepharmacy teams identified 312 prescribing errors (PEs), of which 287 were corrected. There were no significant differences in the incidence of PEs between cured, hospitalised and deceased patients (Table 2). Telepharmacy teams conducted 3318 phone calls, of which 377 were dropped. The mean duration of phone calls with patients was 11.87 (±5.54) minutes. The total number of photos and audios shared via WhatsApp® during the 2116 chats carried out were 190 and 123, respectively.
TABLE 2.
Outcomes related to medication errors, pharmacist interventions and communication with patients *
| Parameters | Total | Recovered patients | Hospitalised patients | Deceased patients |
|---|---|---|---|---|
| Total number of medication orders | 2431 | 1673 | 717 | 41 |
| Medication orders per patient | 5.82 | 4.77 | 11.95 | 5.85 |
| High‐alert medications | 609 | 210 | 375 | 24 |
| High alert medications per patient | 1.45 | 0.59 | 6.25 | 3.42 |
| Total PEs | 312 | 191 | 103 | 18 |
| PEs per patient | 0.74 | 0.54 | 1.71 | 2.57 |
| PEs incidence (incidence = total PEs/total No. of medication orders × 100) | 12.83% | 11.41% | 14.36% | 43.90% |
| Corrected PEs | 287 | 176 | 96 | 15 |
| Pharmacist interventions | ||||
| Total number of pharmacist interventions | 1128 | 567 | 503 | 58 |
| Process‐based pharmacist intervention, n (%) a | 812 (71.98) | 487 (85.89) | 298 (59.24) | 27 (45.76) |
| Outcome‐based pharmacist interventions, n (%) a | 316 (28.01) | 79 (14.03) | 205 (40.75) | 32 (54.23) |
| Communication with patients | ||||
| Number of phone calls | 3318 | 2621 | 626 | 71 |
| Phone call duration (min), mean (SD) | 11.87 (±5.54) | 14.29 (±7.58) | 9.86 (±4.77) | 11.47 (±3.86) |
| Dropped calls | 377 | 256 | 98 | 23 |
| Number of WhatsApp® chats | 2116 | 1184 | 863 | 69 |
| Photos shared via WhatsApp® | 190 | 78 | 90 | 22 |
| Audios shared via WhatsApp® | 123 | 63 | 46 | 14 |
| Losing connection while texting on WhatsApp® | 416 | 296 | 102 | 18 |
Abbreviations: PEs, prescribing errors; SD, standard deviation.
Differences among recovered, hospitalise, and deceased patients are significant (P ≤ .05).
Items listed as numbers (n) unless stated otherwise.
The total number of pharmacist interventions was 1128, of which 812 (71.98%) were process‐based and 316 (28.01%) were outcome‐based. The top five process‐based interventions were: (a) substitution of a prescribed drug 107 (13.17%), (b) patient education about medication use 106 (13.05%), (c) advice the patient to stop smoking 76 (9.35%), (d) advice the patient to eat health food 74 (9.11%), and (e) adjusting the duration of a prescribed drug 62 (7.63%). While cessation of drug therapy 93 (29.43%) and initiation of a new drug 83 (26.26%) were the most common outcome‐based interventions (Table 3). The most common signs of deterioration called for telepharmacy interventions were: Severe dyspnoea 24.71%, breathing difficulties 16.86%, persistent high‐grade fever 12.94% and bloody cough 11.37% (Figure 2). The logistic regression analysis suggested four significant determinants of improvement in clinical outcomes: substitution of a prescribed drug (AOR = 4.03; 95% CI, 2.54‐5.87), adding a drug to the prescription (AOR = 3.15; 95% CI, 1.87‐4.76), advise the patient to stop smoking (AOR = 3.53; 95% CI, 1.98‐5.17) and cessation of drug therapy (AOR = 3.11; 95% CI, 1.25‐4.55). Examples of pharmacist interventions and clinical outcomes are summarised in Table 4.
TABLE 3.
Association of process‐based (N = 812) and outcome‐based (N = 316) pharmacist interventions with clinical outcome status (improved vs worsened)
| Parameters | Total | Improvement in clinical outcomes | Worsening in clinical outcomes | Predicting improvement in clinical outcomes, AOR (95% CI) |
|---|---|---|---|---|
| Process‐based pharmacist intervention | ||||
| Adjusting the dose of a prescribed drug (Ref) | 57 (7.01) | 25 (5.13) | 32 (9.84) | 1.00 |
| Substitution of a prescribed drug | 107 (13.17) | 82 (16.83) | 25 (7.69) | 4.03 (2.54‐5.87) |
| Adjusting the duration of a prescribed drug | 62 (7.63) | 34 (6.98) | 29 (8.92) | 1.42 (0.54‐1.96) |
| Adding a drug to the prescription | 51 (6.28) | 37 (7.59) | 14 (4.30) | 3.15 (1.87‐4.76) |
| Removing a drug from the prescription | 35 (4.31) | 9 (1.84) | 26 (8.00) | 0.43 (0.28‐1.38) |
| Advice the patient to use private toilet | 49 (6.03) | 32 (6.57) | 16 (4.92) | 2.44 (0.98‐4.55) |
| Advice the patient to use private tools and utilities | 21 (2.58) | 11 (2.25) | 10 (3.07) | 1.38 (0.74‐2.32) |
| Recommend proper disposal of waste | 37 (4.55) | 18 (3.69) | 19 (5.84) | 1.16 (0.68‐1.84) |
| Recommend proper washing of clothes | 33 (4.06) | 27 (5.54) | 6 (1.84) | 5.25 (0.56‐9.33) |
| Recommend one room staying | 45 (5.54) | 31 (6.36) | 14 (4.30) | 2.70 (0.65‐5.44) |
| Psychological support | 59 (7.26) | 35 (7.18) | 24 (7.38) | 1.87 (0.48‐5.28) |
| Patient education about medication use | 106 (13.05) | 54 (11.08) | 52 (16.00) | 1.15 (0.96‐4.33) |
| Advice the patient to eat health food | 74 (9.11) | 40 (8.21) | 34 (10.46) | 1.41 (0.85‐3.54) |
| Advice the patient to stop smoking | 76 (9.35) | 52 (10.78) | 24 (7.38) | 3.53 (1.98‐5.17) |
| Outcome‐based pharmacist interventions | ||||
| Cessation of drug therapy (Ref) | 93 (29.43) | 22 (27.84) | 71 (29.95) | 1.00 |
| Initiation of a new drug | 83 (26.26) | 41 (51.89) | 42 (17.72) | 3.11 (1.25‐4.55) |
| Adjusting the dose of a dispensed drug | 50 (15.82) | 7 (8.86) | 43 (18.14) | 0.49 (0.33‐1.91) |
| Recommending a laboratory test | 28 (8.86) | 3 (3.79) | 25 (10.54) | 0.37 (0.19‐1.18) |
| Referral to physician | 62 (19.62) | 6 (7.59) | 56 (23.62) | 0.30 (0.21‐1.47) |
Bold adjusted odds ratio indicates significant findings, parameters described as proportions [n (%)] unless stated otherwise.
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; Ref, reference.
FIGURE 2.
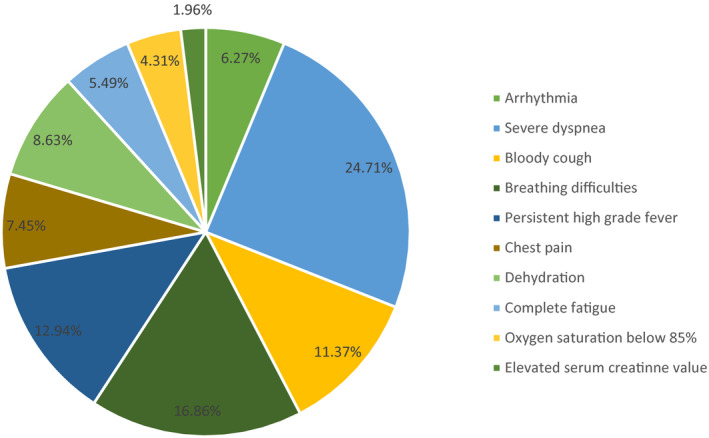
Signs of deterioration that pharmacists intervened upon during the study
TABLE 4.
Examples of pharmacist process‐based and outcome‐based interventions
| Clinical scenarios of pharmacist interventions | Type of intervention | Clinical outcome |
|---|---|---|
| A 62‐year old male patient with COVID‐19 suffered uncontrolled rise in body temperature. He was on Paracetamol 500 mg/8 h and Hydroxychloroquine. The pharmacist increased the dose of Paracetamol to 1000 mg tablet to be taken every 6 h | Outcome‐based intervention/adjusting the dose of a dispensed drug | The body temperature was stabilised and the patient was recovered on day 15 |
| A 44‐year old female patient with COVID‐19 and with history of liver disease. The prescription contained Paracetamol and Oseltamivir. The pharmacist substituted Paracetamol with the combination of Paracetamol and Methionine | Process‐based intervention/substitution of a prescribed drug | The patient was recovered on day 13 |
| A 43‐year old male patient with COVID‐19 and with history of Glucose‐6‐phosphate dehydrogenase (G6PD) deficiency WHO class II. The prescription contained Hydroxychloroquine. The pharmacist excluded Processed Hydroxychloroquine from the patient’s treatment to prevent blood haemolysis | Process‐based intervention/removing a drug from the prescription | The patient was recovered on day 19 |
| A 32‐year old female patient took Nifuroxazide tablets to control diarrhoea, but it was failed and even worsened. The pharmacist stopped the Nifuroxazide and dispensed a combination therapy of Ciprofloxacin and metronidazole | Outcome‐based intervention/cessation of drug therapy/initiation of new drug therapy | The diarrhoea was controlled after two days of starting the new therapy and the patient was recovered on day 15 |
| A 64‐year old female patient with COVID‐19 and with history of diabetes mellitus type II and hypertension. The patient had high D‐dimer value and suffered from hypoxia during her daily life activity. She was on Azithromycin, vitamin C, zinc supplement, actoferrin and oral prednisone. The telepharmacy team noticed a spike in blood glucose level after seven days follow‐up. The processed intervention was stopping prednisone and further follow‐up for glucose level and oxygen saturation | Outcome‐based intervention/cessation of drug therapy | The blood glucose level got back to normal after 7 days and the patient was recovered on day 18 |
Of the 1128 interventions conducted by telepharmacy teams, 743 (65.86%) were drug‐related. The top five drugs affected by pharmacist interventions were Hydroxychloroquine (14.93%), Azithromycin (13.99%), Paracetamol 12.51%, Corticosteroid inhalers 9.95%, and long‐acting beta agonist (6.86%) (Figure 3).
FIGURE 3.
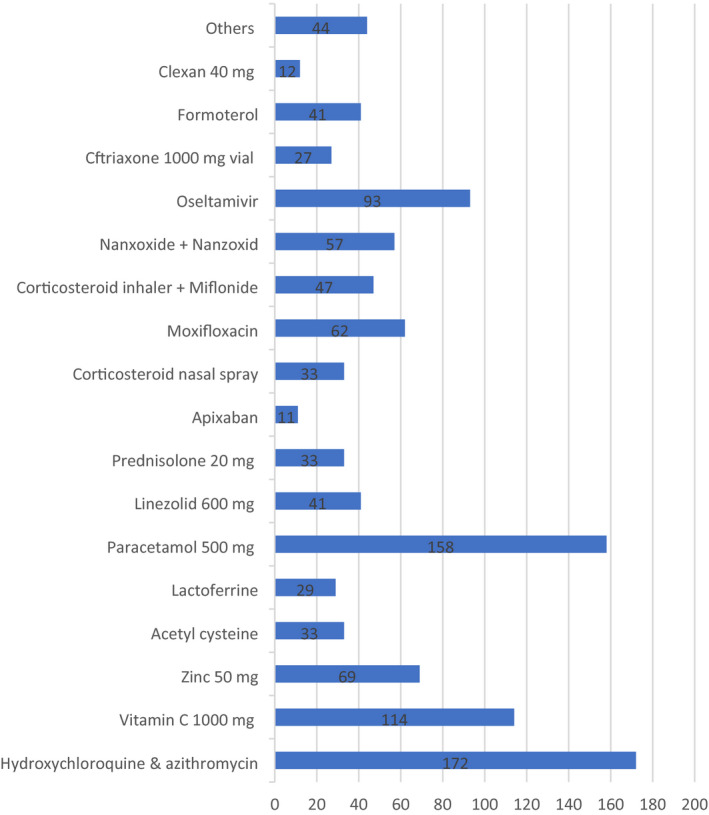
Drugs that pharmacists intervened upon during the study
4. DISCUSSION
Rural communities in developing countries need urgent healthcare plans because of considerable health disparities. 19 To our knowledge, this is one of the first multicentric studies on hospital‐based remote pharmaceutical services, and associated interventions, during the current pandemic. Six telepharmacy teams, comprising nine clinical pharmacists provided patient‐centred care to COVID‐19 patients in rural areas. This study adds new value into hospital pharmacies’ preparedness plans and underscores the importance of telepharmacy tools in providing high‐quality care for rural communities during disasters.
Because patients in rural communities in developing countries are geographically separated and access to care can be time and cost‐prohibitive, development of hospital telepharmacies that link patients isolated in rural places directly with healthcare providers and help pharmacists to perform patient counselling and monitoring could increase access to pharmaceutical care, improve the clinical outcomes of patients and reduce the burden on healthcare systems. Furthermore, because a lot of patients living rural areas are hard‐to‐reach and do not have access to stable phone lines or consistent internet connection, it was expected to encounter many dropped calls, disconnected WhatsApp® chats, and complaints about drug delivery services. Nevertheless, telepharmacy teams managed to partially overcome these drawbacks by using different means in the attempt to reach patients, such as calling patients’ representatives, using WhatsApp® and short messages services (SMS).
The findings of this study indicate that telepharmacy teams reduced potential adverse drug events (ADEs) by identifying and correcting prescribing errors (PEs) before reach patients. In addition, most of telepharmacy interventions were process‐based. A recent study indicates a growing need for a proactive approach to minimise inappropriate medicines use. 20 Telepharmacy tools enabled clinical pharmacists to be in a good position to perform proactive interventions on prescriptions and patient negative health behaviours to prevent patient harm. Patient education and advice including eating healthy food and quitting cigarettes accounted for a significant proportion of telepharmacy interventions. In this context, Koster et al 21 emphasised the urgent need for extended implementation of remote pharmaceutical care to support patient counselling and organisational procedures, and thus ensure high quality pharmacotherapy.
The findings of our interventional analysis demonstrate significant correlation between telepharmacy interventions and improved clinical outcomes. Several factors might have contributed to this outcome. First, although a clear agreement was not applied, a high level of collaboration between telepharmacy teams and prescribers was found. Second, though the structure of telepharmacy model was simple, it facilitated communication and prompted pharmacists to demonstrate their clinical skills. Third, patient engagement in telepharmacy is considered vital in this case. Unlike some patients in the urban areas who might be sceptical towards use of telepharmacy, 22 given health challenges they face, patients in rural areas were more convinced of remote healthcare importance.
Besides proactive clinical interventions that prevented serious patient harm, telepharmacy teams dealt with signs of deterioration and adverse effects of some cases and referred them to hospitals. This might explain the high rate of hospitalisation in our study. For example, some patients suffered bloody cough with breathing difficulty, and for patients with poor health literacy, a telepharmacy intervention was a lifesaver.
4.1. Limitations
This study has several limitations. First, using a convenience sample of six hospital telepharmacies in five regions limits generalisability; hospital pharmacies in other regions and countries may have responded differently. Second, absence of video communication, electronic prescribing system and electronic patient record in response plan affects the nature of patient‐provider interactions, and may induce doubts in the minds of patients. Third, because of scarce resources, it was impractical to perform two consecutive PCRs for all patients who considered recovered from the infection. Third, the privacy issue was beyond the scope of the study’s aims. Some patients refused to participate in the study for privacy reasons, and this could compromise the efforts of telepharmacy. Health authorities and professional bodies could solve this issue by drawing up regulatory procedures and guidelines that encourage patient engagement in telepharmacy.
5. CONCLUSION
Implementation of hospital telepharmacy services reduces irrational medicines use and improves clinical outcomes of COVID‐19 patients in rural areas. Pharmacists in developing countries should be supported to implement remote services to provide patients in rural places with optimal pharmaceutical care.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors have significantly contributed to the concept design, provision of data collection, statistical procedures, visualisation and drafting.
ETHICS APPROVAL
The Institutional Review Board of Damnhour University approved the research project (No. 221PP31).
ACKNOWLEDGEMENTS
We thank clinical pharmacists, health informatic specialists and physicians who participated in the study. Their efforts in data collection are much appreciated.
Al Meslamani AZ, Kassem AB, El‐Bassiouny NA, Ibrahim OM. An emergency plan for management of COVID‐19 patients in rural areas. Int J Clin Pract. 2021;75:e14563. 10.1111/ijcp.14563
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.
REFERENCES
- 1. Karim SA, Chen H‐F. Deaths from COVID‐19 in rural, micropolitan, and metropolitan areas: a county‐level comparison. J Rural Health. 2021;37(1):124–132. 10.1111/jrh.12533 [DOI] [PubMed] [Google Scholar]
- 2. Gaffney AW, Hawks L, White AC, et al. Health care disparities across the urban‐rural divide: a national study of individuals with COPD. J Rural Heal. 2020. 10.1111/jrh.12525 [DOI] [PubMed] [Google Scholar]
- 3. Kretchy IA, Asiedu‐Danso M, Kretchy J‐P. Medication management and adherence during the COVID‐19 pandemic: perspectives and experiences from low‐and middle‐income countries. Res Soc Adm Pharm. 2021;17(1):2023–2026. 10.1016/j.sapharm.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dandachi D, Reece R, Wang EW, Nelson T, Rojas‐Moreno C, Shoemaker DM. Treating COVID‐19 in rural America. J Rural Health. 2021;37(1):205–206. 10.1111/jrh.12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adunlin G, Murphy PZ, Manis M. COVID‐19: how can rural community pharmacies respond to the outbreak? J Rural Health. 2021;37(1):153–155. 10.1111/jrh.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention . Rural communities. 2020. https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/other‐at‐risk‐populations/rural‐communities.html. Accessed February 2, 2021.
- 7. Al‐Quteimat OM, Amer AM. SARS‐CoV‐2 outbreak: how can pharmacists help? Res Soc Adm Pharm. 2021;17(2):480–482. 10.1016/j.sapharm.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng S‐Q, Yang L, Zhou P‐X, Li H‐B, Liu F, Zhao R‐S. Recommendations and guidance for providing pharmaceutical care services during COVID‐19 pandemic: a China perspective. Res Social Adm Pharm. 2021;17(1):1819–1824. 10.1016/j.sapharm.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Basheti IA, Nassar R, Barakat M, et al. Pharmacists’ readiness to deal with the coronavirus pandemic: assessing awareness and perception of roles. Res Social Adm Pharm. 2021;17(3):514–522. 10.1016/j.sapharm.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ung COL. Community pharmacist in public health emergencies: quick to action against the coronavirus 2019‐nCoV outbreak. Res Social Adm Pharm. 2020;16(4):583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elbeddini A, Hooda N, Yang L. Role of Canadian pharmacists in managing drug shortage concerns amid the COVID‐19 pandemic. Can Pharm J. 2020;153(4):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Visacri MB, Figueiredo IV, de Mendonça Lima T. Role of pharmacist during the COVID‐19 pandemic: a scoping review. Res Soc Adm Pharm. 2021;17(1):1799–1806. 10.1016/j.sapharm.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arain S, Thalapparambath R, Al Ghamdi FH. COVID‐19 pandemic: Response plan by the Johns Hopkins Aramco Healthcare inpatient pharmacy department. Res Soc Adm Pharm. 2021;17(1):2009–2011. 10.1016/j.sapharm.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID‐19). J Telemed Telecare. 2020;26(5):309–313. 10.1177/1357633X20916567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le T, Toscani M, Colaizzi J. Telepharmacy: a new paradigm for our profession. J Pharm Pract. 2020;33(2):176–182. 10.1177/0897190018791060 [DOI] [PubMed] [Google Scholar]
- 16. Schneider PJ. Evaluating the impact of telepharmacy. Am J Health Pharm. 2013;70(23):2130–2135. 10.2146/ajhp130138 [DOI] [PubMed] [Google Scholar]
- 17. Margusino‐Framiñán L, Illarro‐Uranga A, Lorenzo‐Lorenzo K, et al. Pharmaceutical care to hospital outpatients during the COVID‐19 pandemic. Telepharmacy. Farm Hosp. 2020;44(7):61–65. 10.7399/fh.11498 [DOI] [PubMed] [Google Scholar]
- 18. Elmenofi GAG, El Bilali H, Berjan S. Governance of rural development in Egypt. Ann Agric Sci. 2014;59(2):285–296. [Google Scholar]
- 19. Carpenter DM, Hastings T, Westrick S, et al. Rural community pharmacies’ preparedness for and responses to COVID‐19. Res Soc Adm Pharm. 2021;17(7):1327–1331. 10.1016/j.sapharm.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes GA, Rowett D, Corlis M, Sluggett JK. Reducing harm from potentially inappropriate medicines use in long‐term care facilities: we must take a proactive approach. Res Social Adm Pharm. 2021;17(5):829–831. 10.1016/j.sapharm.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 21. Koster ES, Philbert D, Bouvy ML. Impact of the COVID‐19 epidemic on the provision of pharmaceutical care in community pharmacies. Res Soc Adm Pharm. 2021;17(1):2002–2004. 10.1016/j.sapharm.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poudel A, Nissen LM. Telepharmacy: a pharmacist’s perspective on the clinical benefits and challenges. Integr Pharm Res Pract. 2016;5:75–82. 10.2147/IPRP.S101685 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.


