Abstract
Background
Clinical symptoms of adults and paediatric inpatients with COVID‐19 disease are conflicting. This meta‐analysis was conducted to assess the effect of age of COVID‐19 inpatient on the severity of the disease.
Methods
A systematic literature search up to January 2021 was performed and 5 studies included 910 inpatients with COVID‐19 disease at the baseline of the study; 773 of them were adult inpatients, and 137 of them were paediatric inpatients. They reported a comparison between adults and children with COVID‐19 in the level of symptomatic severity, clinical features, computed tomography (CT) results and laboratory results. Odds ratio (OR) with 95% confidence intervals (CIs) were calculated assessing the effect of age of COVID‐19 inpatient on the severity of the disease using the dichotomous method with a random or fixed‐effect model.
Results
Adults with COVID‐19 disease had significantly lower number of mild cases (OR, 0.18; 95% CI, 0.04‐0.77, P = .02); higher number severe cases (OR, 4.90; 95% CI, 2.03‐11.83, P < .001); higher number of cases with fever (OR, 4.14; 95% CI, 2.31‐7.43, P < .001); and higher number of cases with CT positive COVID‐19 disease (OR, 2.04; 95% CI, 1.17‐3.55, P = .001) compared with children. However, no significant difference was found between adults and children in number of cases with shortness of breath (OR, 1.44; 95% CI, 0.41‐5.04, P = .57); dry cough (OR, 1.77; 95% CI, 0.64‐4.93, P = .27); leukopenia (OR, 0.89; 95% CI, 0.47‐1.66, P = .71); lymphopenia (OR, 0.96; 95% CI, 0.49‐1.88, P = .91); high platelets (OR, 0.41; 95% CI, 0.17‐1.02, P = .05); and high D‐dimer (OR, 0.82; 95% CI, 0.43‐1.56, P = .54).
Conclusions
Adults with COVID‐19 disease have a much higher level of symptomatic severity, fever and CT‐positive COVID‐19 disease than children. However, as shown in our results, the laboratory data were similar in both groups.
What’s known
Clinical symptoms of adults and paediatric inpatients with COVID‐19 disease are conflicting.
This meta‐analysis was conducted to assess the effect of age of COVID‐19 inpatient on the severity of the disease.
What’ new
Adults with COVID‐19 disease have a much higher level of symptomatic severity, fever and CT‐positive COVID‐19 disease than children.
However, as shown in our results, the laboratory data were similar in both groups.
1. INTRODUCTION
A new SARS‐CoV‐2 infection appeared in December 2019 in Wuhan and spread rapidly all over the world. 1 The World Health Organization has called it COVID‐19 disease and confirmed it as a pandemic. 2 Children are more prone to pneumonia infection than adults, which may cause deadly outcomes. 3 , 4 During the current COVID‐19 pandemic, it was reported that infants are less susceptible to such a violent virus. 5 , 6 So, the number of paediatric inpatients with COVID‐19 disease was much lower than the number of adult inpatients. 7 , 8 However, there is no proper explanation for this phenomenon. Numerous studies and meta‐analyses reported the demographics, clinical characters, laboratory indicators and computed tomography imaging characteristics of the adult with COVID‐19 disease. 9 , 10 But because of the inadequate number of children with COVID‐19 disease, a few meta‐analysis studies concentrated on children with COVID‐19 disease. 11 , 12 , 13 Though, they did not compare their children's results to adults because of the lack of comparative studies. Further compassion between adults and children on the clinical features of COVID‐19 disease is urgently needed to help in the clinical diagnosis and management of the subjects, of different ages, infected with SARS‐CoV‐2. This meta‐analysis aimed to assess the effect of age of COVID‐19 inpatient on the severity of the disease.
1.1. Methods
The study performed here followed the meta‐analysis of studies in the epidemiology statement, 14 which was conducted following an established protocol.
1.2. Study selection
Studies included were observation studies assessing the effect of age of COVID‐19 inpatient on the severity of the disease. Only human studies in any language were considered. Inclusion was not limited by study size. Publications excluded were review articles and commentary and studies that did not deliver a measure of an association. Figure 1 shows a schematic diagram of the study procedure. The articles were integrated into the meta‐analysis when the following inclusion criteria were met:
The study was observational.
The target population was subjects with COVID‐19 disease.
The intervention programme was based on the effect of age of COVID‐19 inpatient on the severity of the disease.
The study included a comparison between adults and children.
FIGURE 1.
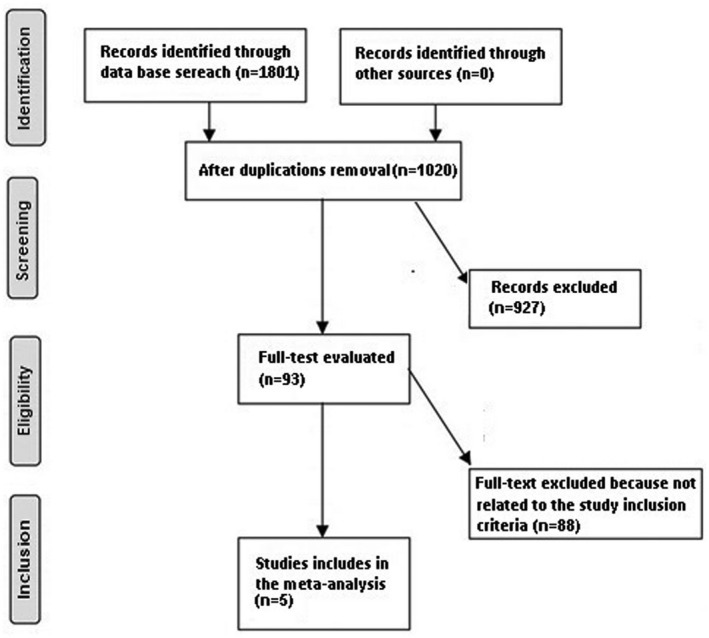
Schematic diagram of the study procedure
1.3. Identification
A protocol of search strategies was prepared according to the PICOS principle, 15 and we defined it as follow: P (population): subjects with COVID‐19 disease; I (intervention/exposure): effect of age of COVID‐19 inpatient on the severity of the disease; C (comparison): adults compared with children; O (outcome): level of symptomatic severity, clinical features, CT results and laboratory results; and S (study design): no restriction. 16
First, we conducted a systematic search of OVID, Embase, Cochrane Library, PubMed, Google Scholar databases till January 2021, using a blend of keywords and similar words for COVID‐19, SARS‐CoV‐2, adults, children, level of symptomatic severity, clinical features, computerised tomography and laboratory results, as shown in Table 1. All identified studies were pooled in an EndNote file, duplicates were omitted and the title and abstracts were reviewed to exclude studies that did not report an association of effect of age of COVID‐19 inpatient on the severity of the disease.
TABLE 1.
Search strategy for each database
| Database | Search strategy |
|---|---|
| Pubmed |
#1 "COVID‐19"[MeSH Terms] OR "SARS‐CoV‐2"[All Fields] OR "adults"[All Fields] OR "children"[All Fields] #2 "clinical features"[MeSH Terms] OR "COVID‐19"[All Fields] OR "computerized tomography"[All Fields] OR "laboratory results"[All Fields] #3 #1 AND #2 |
| Embase |
'COVID‐19'/exp OR 'SARS‐CoV‐2'/exp OR ' adults'/exp OR children #2 'clinical features'/exp OR 'ICBG'/exp OR 'computerized tomography'/exp OR laboratory results #3 #1 AND #2 |
| Cochrane library |
(COVID‐19):ti,ab,kw (SARS‐CoV‐2):ti,ab,kw OR (adults):ti,ab,kw (Word variations have been searched) #2 (children):ti,ab,kw OR (clinical features):ti,ab,kw OR (computerized tomography):ti,ab,kw OR (laboratory results):ti,ab,kw (Word variations have been searched) #3 #1 AND #2 |
1.4. Screening
Data were abridged on the following bases; study‐related and subject‐related characteristics onto a standardised form; last name of the primary author, period of study, year of publication, country, region of the studies and study design; population type, the total number of subjects, demographic data, clinical and treatment characteristics, categories, qualitative and quantitative method of evaluation, information source, outcome evaluation and statistical analysis. 17 When there were different data from one study based on the assessment of the effect of age of COVID‐19 inpatient on the severity of the disease, we extracted them independently. The risk of bias in these studies; individual studies were evaluated using the two authors independently assessed the methodological quality of the selected studies. The “risk of bias tool" from the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 was used to assess methodological quality. 18 In terms of the assessment criteria, each study was rated and assigned to one of the following three risks of bias: low: if all quality criteria were met, the study was considered to have a low risk of bias; unclear: if one or more of the quality criteria were partially met or unclear, the study was considered to have a moderate risk of bias; or high: if one or more of the criteria were not met, or not included, the study was considered to have a high risk of bias. Any inconsistencies were addressed by a re‐evaluation of the original article.
1.5. Eligibility
The main outcome focused on the assessment of the effect of age of COVID‐19 inpatient on the severity of the disease, we extracted them independently to form a summary.
1.6. Inclusion
Sensitivity analyses were limited only to studies reporting the level of symptomatic severity, clinical features, computerised tomography results and laboratory results of children with COVID‐19 disease compared with adults; we extracted them independently. For subcategory and sensitivity analysis, we used comparisons between adults and children.
1.7. Statistical analysis
The dichotomous method with a random‐effect model or fixed‐effect was used to calculate the odds ratio (OR) and 95% CI. The I 2 index was calculated; the I 2 index is between 0% and 100%. Values of about 0%, 25%, 50% and 75% indicate no, low, moderate and high heterogeneity, respectively. 19 When I 2 was higher than 50%, we chose the random effect model; when it was lower than 50%, we used the fixed‐effect model. A subcategory analysis was completed by stratifying the original evaluation per outcome categories as described before. In this analysis, a P‐value for differences between subcategories of <.05 was considered statistically significant. Publication bias was evaluated quantitatively using the Egger regression test (publication bias considered present if P ≥ .05), and qualitatively, by visual examination of funnel plots of the logarithm of ORs versus their standard errors (SE). 15 All P‐values were two tailed. All calculations and graphs were performed using reviewer manager version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
2. RESULTS
A total of 1801 unique studies were identified. Most of them were either showing data for adults only or children only. That made our studies much harder. Only 5 studies were found, conducted in 2020 and most of them were from China, comparing adults with children and fulfilled the inclusion criteria. 20 , 21 , 22 , 23 , 24 Details of the included studies are shown in Table 2.
TABLE 2.
Characteristics of the selected studies for the meta‐analysis
The 5 studies included 910 inpatients with COVID‐19 disease at the baseline of the study; 773 of them were adult inpatients, and 137 of them were paediatric inpatients.
The study size ranged from 32 to 625 subjects at the start of the study. 5 studies reported data stratified comparison between subjects’ age and level of symptomatic severity, 4 studies for clinical features, 4 studies for computerised tomography results and 4 studies for laboratory results.
The extent of the age outcome in subjects with COVID‐19 disease was higher in adults than that in children and this was statistically significant in all populations studied.
Adults with COVID‐19 disease had significantly lower number of mild cases (OR, 0.18; 95% CI, 0.04‐0.77, P = .02) with high heterogeneity (I 2 = 75%); higher number of severe cases (OR, 4.90; 95% CI, 2.03‐11.83, P < .001) with no heterogeneity (I 2 = 0%); higher number of cases with fever (OR, 4.14; 95% CI, 2.31‐7.43, P < .001) with no heterogeneity (I 2 = 0%); and higher number of cases with CT positive COVID‐19 disease (OR, 2.04; 95% CI, 1.17‐3.55, P = .001) with low heterogeneity (I2 = 49%) compared with children as shown in Figures 2, 3, 4. However, no significant difference between adults and children was observed in number of cases with shortness of breath (OR, 1.44; 95% CI, 0.41‐5.04, P = .57) with no heterogeneity (I 2 = 0%); dry cough (OR, 1.77; 95% CI, 0.64‐4.93, P = .27) with moderate heterogeneity (I 2 = 64%); leukopenia (OR, 0.89; 95% CI, 0.47‐1.66, P = .71) with low heterogeneity (I 2 = 31%); lymphopenia (OR, 0.96; 95% CI, 0.49‐1.88, P = .91) with low heterogeneity (I 2 = 25%); high platelets (OR, 0.41; 95% CI, 0.17‐1.02, P = .05) with no heterogeneity (I 2 = 0%); and high D‐dimer (OR, 0.82; 95% CI, 0.43‐1.56, P = .54) with no heterogeneity (I 2 = 0%) as shown in Figures 3 and 5.
FIGURE 2.
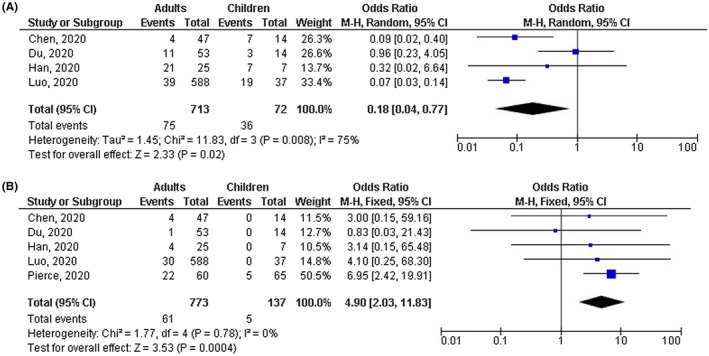
Forest plot of the effect of age on (A) number of mild cases, (B) number of severe cases
FIGURE 3.
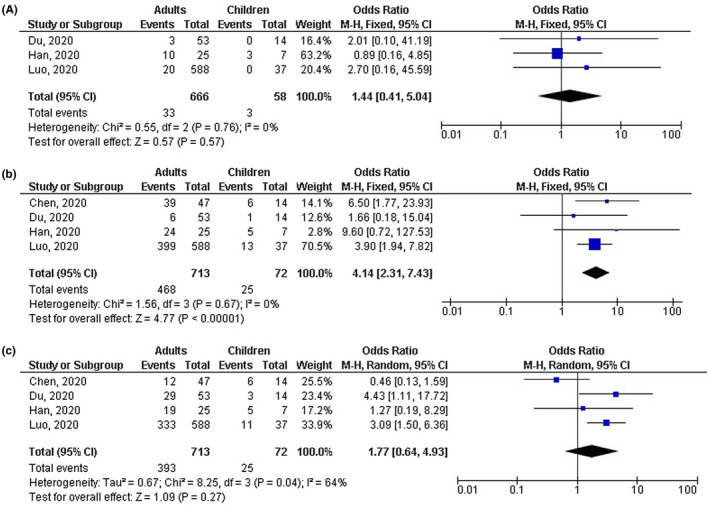
Forest plot of the effect of age on (A) number of cases with shortness of breath, (B) number of cases with fever, (C) number of cases with shortness of dry cough
FIGURE 4.
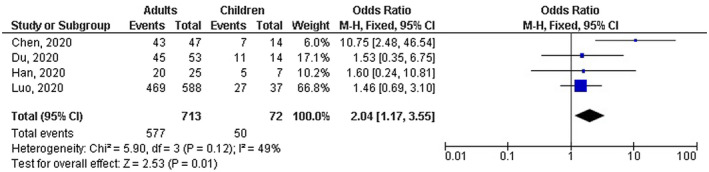
Forest plot of the effect of age on the number of cases with CT positive results
FIGURE 5.
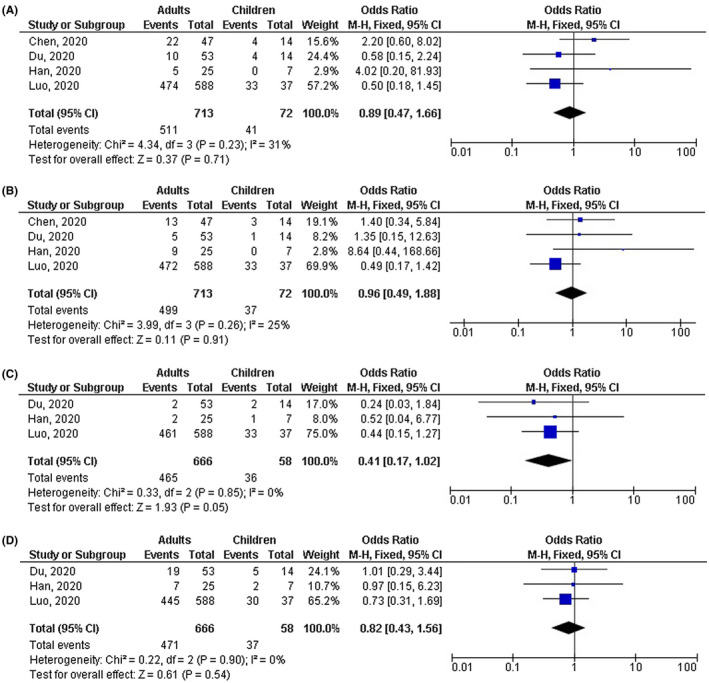
Forest plot of the effect of age on (A) number of cases with leukopenia, (B) number of cases with lymphopenia, (C) number of cases with shortness of high platelets, (D) number of cases with shortness of dry high D‐dimer
A stratified analysis of studies that did and did not adjust for the effect of ethnicity and gender on the results was not performed because no studies reported or adjusted for this factor.
Based on the visual inspection of the funnel plot as well as on quantitative measurement using the Egger regression test, there was no evidence of publication bias (P = .87). However, most of the included studies were assessed to be of a low methodological quality. All studies did not have selective reporting bias, and no articles had incomplete outcome data and selective reporting.
3. DISCUSSION
This meta‐analysis study based on 5 studies included 910 inpatients with COVID‐19 disease at the baseline of the study; 773 of them were adult inpatients, and 137 of them were paediatric inpatients. 20 , 21 , 22 , 23 , 24
The extent of poor outcome in the symptomatic severity, fever and CT positive in adults with COVID‐19 disease was higher than that in children and this was statistically significant in all populations studied. 20 , 21 , 22 , 23 , 24 However, no significant difference between adults and children was observed in shortness of breath, dry cough and all the laboratory data. Although, the low P‐values of the high platelet outcomes (P = .05) suggest that the insignificant difference found between adults and children could turn into significant if more studies comparing adults COVID‐19 cases to children. Though, the analysis of outcomes should be done with caution because of the small number of the study found (5 studies) and the low sample size in most of the selected studies (3 studies ≤100 subjects) in our meta‐analysis; suggesting the need for more studies to validate these findings or possibly to significantly influences confidence in the effect evaluation.
This finding suggests that adults with COVID‐19 disease have a much higher level of symptomatic severity, clinical features and CT results than children. Also, request more comparative studies rather than the hundreds of observational studies available that just describe the COVID‐19 patients’ status. The reasons for these findings are likely multi‐factorial. 20 , 21 , 22 , 23 , 24
To our knowledge, this is the first meta‐analysis comparing adults and children with COVID‐19. Though, the analysis of outcomes should be with caution because of the low number of studies in our meta‐analysis, the small sample size of the selected studies, and the potential risk of bias.
Children are much easier infected at school and playgrounds, 25 much less aware of the proper protection measures about SARS‐CoV‐2 infection spread 26 and much less aware of the proper way to handle COVID‐19 disease patients at home. 27 Hence, children can serve as a great source of infection to their families since mostly they have asymptomatic SARS‐CoV‐2 infection. 25 , 26 Several studies and consensus have been published describing the proper diagnosis, management and prevention of COVID‐19 disease. 25 , 28 , 29 However, COVID‐19 disease still infecting and taking the life of millions of people. 30 This disease required further description of the effect on different ages, gender and ethnicity. It has been shown previously and validated in our meta‐analysis that adults are more susceptible to SARS‐CoV‐2 infection than children. However, as shown in our results, the laboratory data were similar in both groups. These results are confusing and require a proper explanation of why symptoms and severity are high in adults and laboratory data are similar in both groups since most of the present explanations are suggestions. The reasons why paediatric inpatients are milder than adult inpatients are still unidentified. However, one of the suggested reasons is the existence of cross‐reaction between SARS‐CoV‐2 and any of childhood vaccines. 30 That could be valid since some relations were made between the Bacillus Calmette‐Guérin vaccine, one of the childhood vaccinations in most of the countries in the world, and the lower severity of the COVID‐19 disease. 30 Another explanation was based on the risk factors and the co‐morbid conditions that mostly occur in adults compared with children. A meta‐analysis study has shown that smoking, which mostly occurs in adults, could stimulate the development of COVID‐19 disease making the smoking subjects suffer more from COVID‐19 disease. 31 Also, the co‐morbid diseases which occur more in adults than in children could add some reasons why adults are much more susceptible to COVID‐19 disease than children. 32 , 33 However, still all of these explanations are suggestions. This meta‐analysis showed the effect of age of COVID‐19 inpatient on the severity of the disease. Yet, further studies are needed to validate these potential relationships and explain the mechanism of the effect of age of COVID‐19 inpatient on the severity of the disease. These studies must comprise larger with more homogeneous samples. Well‐conducted studies are needed to assess these factors and the combination of different childhood vaccination, gender, ethnicity and other variants of subjects; since our meta‐analysis study could not answer whether different ethnicity and gender are associated with the results. 34 , 35 , 36 , 37 , 38 , 39
In summary, the data suggest that adults with COVID‐19 disease may be at higher risk of the poor level of symptomatic severity, clinical features and computerised tomography results than children. However, the laboratory data were similar in both groups. From the study presented here, we recommend the use of further comparative studies to validate these findings.
3.1. Limitations
There may be selection bias in this study since so many of the studies found were excluded from the meta‐analysis. However, the studies excluded did not satisfy the inclusion criteria of our meta‐analysis. Also, we could not answer whether the results are associated with ethnicity and gender or not. The study designed to assess the effect of age of COVID‐19 inpatient on the severity of the disease was based on data from previous observational studies, which might cause bias induced by incomplete details. The meta‐analysis was based on 5 observational studies; 3 of them had a small sample size (n < 100). Variables including ethnicity, sex, nutritional status and comorbidity, for example diabetes mellitus, hypertension or obesity of subjects were also the possible bias‐inducing factors. Some unpublished articles and missing data might lead to a bias in the pooled effect. Also, some important paediatric cohorts were not included because of the low number of studies found in this meta‐analysis, given that only studies comparing children to adults were included. The sample sizes varied considerably across studies and four of the five included studies were conducted in China; non‐coverage of different geographical areas.
4. CONCLUSIONS
Adults with COVID‐19 disease have a much higher level of symptomatic severity, fever and CT‐positive COVID‐19 disease than children. However, as shown in our results, the laboratory data were similar in both groups. From the study presented here, we request further comparative studies between adults and children with COVID‐19 to validate these findings and find any possible explanation for the insignificant difference in laboratory results. Though, the analysis of outcomes should be done with caution because of the small number of the study found (5 studies) and the low sample size in most of the selected studies (3 studies ≤100 subjects) in our meta‐analysis; suggesting the need for more studies to validate these findings or possibly to significantly influences confidence in the effect evaluation.
5. DATA AVAILABILITY STATEMENT
The datasets analysed during this study are available from the corresponding author on reasonable request.
DISCLOSURE
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Conception and design: Xiaoxiao Xu, Mohamed EA Abdelrahim. Administrative support: All authors. Provision of study materials or subjects: All authors. Collection and assembly of data: Shaoqing Chai, Yan Li, Xuemei Li, Jie Tan. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors. All authors have read and approved the manuscript.
IRB APPROVAL
Not required for this study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Not applicable.
Chai S, Li Y, Li X, Tan J, Abdelrahim MEA, Xu X. Effect of age of COVID‐19 inpatient on the severity of the disease: A meta‐analysis. Int J Clin Pract. 2021;75:e14640. 10.1111/ijcp.14640
Shaoqing Chai and Yan Li both are the first authors, they contributed equally.
REFERENCES
- 1. Malik YS, Sircar S, Bhat S, et al. Emerging novel coronavirus (2019‐nCoV)—current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;40:68‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W‐J, Ni Z‐yi, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. JAMA Internal Med. 2020;180:1345‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo FR. Smoking links to the severity of COVID‐19: an update of a meta‐analysis. J Med Virol. 2020;92:2304‐2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan KW, Wong VT, Tang SCW. COVID‐19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese‐Western medicine for the management of 2019 novel coronavirus disease. Am J Chinese Med. 2020;48:737‐762. [DOI] [PubMed] [Google Scholar]
- 8. Li A, Ng P. Severe acute respiratory syndrome (SARS) in neonates and children. Arch Dis Childhood‐Fetal Neonatal Ed. 2005;90:F461‐F465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2: a systematic review and meta‐analysis. J Med Virol. 2020;92:1449‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2. Eur J Nucl Med Mol Imaging. 2020;47:1275‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma X, Liu S, Chen L, Zhuang L, Zhang J, Xin Y. The clinical characteristics of pediatric inpatients with SARS‐CoV‐2 infection: a meta‐analysis and systematic review. J Med Virol. 2021;93:234‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang T‐H, Wu J‐L, Chang L‐Y. Clinical characteristics and diagnostic challenges of pediatric COVID‐19: a systematic review and meta‐analysis. J Formos Med Assoc. 2020;119:982‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008‐2012. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1‐e34. [DOI] [PubMed] [Google Scholar]
- 17. Gupta A, Das A, Majumder K, et al. Obesity is independently associated with increased risk of hepatocellular cancer–related mortality. Am J Clin Oncol. 2018;41:874‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheikhbahaei S, Trahan Tyler J, Xiao J, et al. FDG‐PET/CT and MRI for evaluation of pathologic response to neoadjuvant chemotherapy in patients with breast cancer: a meta‐analysis of diagnostic accuracy studies. Oncologist. 2016;21:931‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pierce CA, Preston‐Hurlburt P, Dai Y, et al. Immune responses to SARS‐CoV‐2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12:eabd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen A, Huang J‐X, Liao Y, et al. Differences in clinical and imaging presentation of pediatric patients with COVID‐19 in comparison with adults. Radiol: Cardiothorac Imaging. 2020;2:e200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Du W, Yu J, Wang H, et al. Clinical characteristics of COVID‐19 in children compared with adults in Shandong Province. China. Infection. 2020;48:445‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han Y‐N, Feng Z‐W, Sun L‐N, et al. A comparative‐descriptive analysis of clinical characteristics in 2019‐coronavirus‐infected children and adults. J Med Virol. 2020;92:1596‐1602. [DOI] [PubMed] [Google Scholar]
- 24. Luo H, Liu S, Wang Y, et al. Age differences in clinical features and outcomes in patients with COVID‐19, Jiangsu, China: a retrospective, multicentre cohort study. BMJ open. 2020;10:e039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saeed H, Osama H, Madney YM, et al. COVID‐19; current situation and recommended interventions. Int J Clin Pract. 2021;75:e13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elgendy MO, El‐Gendy AO, Abdelrahim MEA. Public awareness in Egypt about COVID‐19 spread in the early phase of the pandemic. Patient Educ Couns. 2020;103:2598‐2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elgendy MO, Abd Elmawla MN, Abdel Hamied AM, El Gendy SO, Abdelrahim MEA. COVID‐19 patients and contacted person awareness about home quarantine instructions. Int J Clin Pract. 2021;75:e13810. [DOI] [PubMed] [Google Scholar]
- 28. Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID‐19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the global congress of hysteroscopy scientific committee. J Minimally Invasive Gynecol. 2020;27:988‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sayed AM, Khalaf AM, Abdelrahim MEA, Elgendy MO. Repurposing of some anti‐infective drugs for COVID‐19 treatment: a surveillance study supported by an in silico investigation. Int J Clin Pract. 2021;75:e13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Osama El‐Gendy A, Saeed H, Ali AMA, et al. Bacillus Calmette‐Guérin vaccine, antimalarial, age and gender relation to COVID‐19 spread and mortality. Vaccine. 2020;38:5564‐5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its Impact on Patients with COVID‐19. SN Compr Clin Med. 2020;1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Fang X, Cai Z, et al. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID‐19 patients: a systemic review and meta‐analysis. Research. 2020;2020:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elgendy MO, Abdelrahim ME, Eldin RS. Potential benefit of repeated dry powder inhaler’s inhalation technique counseling on asthmatic patients. Pulmonary Therapy. 2015;1:91‐101. [Google Scholar]
- 35. Elgendy MO, Abdelrahim ME, Eldin RS. Potential benefit of repeated MDI inhalation technique counselling for patients with asthma. Eur J Hosp Pharm. 2015;22:318‐322. [Google Scholar]
- 36. Ali AMA, Abdelrahim MEA. Modeling and optimization of terbutaline emitted from a dry powder inhaler and influence on systemic bioavailability using data mining technology. J Pharm Innovation. 2014;9:38‐47. [Google Scholar]
- 37. Abdelrahim ME, Assi KH, Chrystyn H. Relative bioavailability of terbutaline to the lung following inhalation, using urinary excretion. Br J Clin Pharmacol. 2011;71:608‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moustafa IO, Ali MRA‐A, Al Hallag M, et al. Lung deposition and systemic bioavailability of different aerosol devices with and without humidification in mechanically ventilated patients. Heart Lung. 2017;46:464‐467. [DOI] [PubMed] [Google Scholar]
- 39. Moustafa IO, ElHansy MHE, Al Hallag M, et al. Clinical outcome associated with the use of different inhalation method with and without humidification in asthmatic mechanically ventilated patients. Pulm Pharmacol Ther. 2017;45:40‐46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during this study are available from the corresponding author on reasonable request.


