Abstract
Background
Internationally, the COVID‐19 pandemic severely curtailed access to hospital facilities for those awaiting elective/semi‐elective procedures. For allergic children in Ireland, already waiting up to 4 years for an elective oral food challenge (OFC), the restrictions signified indefinite delay. At the time of the initiative, there were approx 900 children on the Children's Health Ireland (CHI) waiting list. In July 2020, a project was facilitated by short‐term (6 weeks) access to an empty COVID stepdown facility built, in a hotel conference centre, commandeered by the Health Service Executive (HSE), Ireland. The aim of this study was to achieve the rapid roll‐out of an offsite OFC service, delivering high throughput of long waiting patients, while aligning with existing hospital policies and quality standards, international allergy guidelines and national social distancing standards.
Methods
The working group engaged key stakeholders to rapidly develop an offsite OFC facility. Consultant paediatric allergists, consultant paediatricians, trainees and allergy clinical nurse specialists were seconded from other duties. The facility was already equipped with hospital beds, bedside monitors (BP, pulse and oxygen saturation) and bedside oxygen. All medication and supplies had to be brought from the base hospital. Daily onsite consultant anaesthetic cover was resourced and a resuscitation room equipped. Standardized food challenge protocols were created. Access to the onsite hotel chef facilitated food preparation. A risk register was established.
Results
After 6 weeks of planning, the remote centre became operational on 7/9/2020, with the capacity of 27 OFC/day. 474 challenges were commenced: 465 (98%) were completed and 9 (2%) were inconclusive. 135 (29%) OFCs were positive, with 25 (5%) causing anaphylaxis. No child required advanced airway intervention. 8 children were transferred to the base hospital. The CHI allergy waiting list was reduced by almost 60% in only 24 days.
Conclusions
Oral food challenges remain a vital tool in the care of allergic children, with their cost saving and quality‐of‐life benefits negatively affected by a delay in their delivery. This project has shown it is possible to have huge impacts on a waiting list efficiently, effectively and safely with good planning and staff buy‐in—even in a pandemic. Adoption of new, flexible and efficient models of service delivery will be important for healthcare delivery in the post–COVID‐19 era.
Keywords: COVID‐19, food allergy, food challenge, healthcare delivery
Short abstract

Abbreviations
- CNS
clinical nurse specialist
- HSE
Health Service Executive
- NTPF
National Treatment Purchase Fund
- OFC
oral food challenge
- QoL
quality of life
- RTI
respiratory tract infection
- SOP
standard operating procedure
1. INTRODUCTION
Health services around the world have had to adapt to the massive additional demands of the SARS‐CoV‐2/COVID‐19 pandemic. They had to change working practices and cultures, adapt existing facilities, build new ones and develop new ways to meet the needs of patients with COVID‐19, while balancing support for other aspects of health care. Some changes have become embedded, but some developed contingencies were unused.
The oral food challenge (OFC), the bedrock of allergy diagnostics, is a resource‐intensive, lengthy procedure, necessitating, in every case, experienced staff and immediately available facilities to manage anaphylaxis. Despite evidence of quality‐of‐life (QoL) benefits 1 and cost savings to health services, 2 access to adequate, dedicated facilities is unusual in many countries. Most hospital‐based allergy departments compete with other disciplines for shared dayward facilities resulting in long waiting times. Office‐based allergists in the United States and Canada report similar limitations of space and staffing. 3 , 4 In 2020, these universal barriers were raised further due to the unique service demands imposed by the pandemic.
During the early stages of COVID‐19 preparedness planning, Ireland's Health Service Executive (HSE) commissioned the construction of a 350‐bed stepdown facility to cope with the expected surge of convalescent cases of COVID, to be used after admission to acute hospital. It was built in a convention facility more than 10 km from the base hospital services. It opened in May 2020, but the surge of cases did not materialize. In July 2020, HSE advertised for projects that might avail of the facility before its planned decommissioning in October 2020.
We report here the planning, rapid implementation and results of an initiative to use the fully commissioned but hitherto unused 350‐bed COVID stepdown facility/Nightingale hospital for the concentrated delivery of a high volume of OFCs to address a long waiting list. At the time of the initiative, there were approx 900 children on a waiting list for OFC. In the other allergy centre in Cork University Hospital, 250 km away from Dublin, 250 other children were awaiting OFC.
2. METHODS
2.1. Planning
A planning committee was established including operational management, paediatric allergists, general paediatricians, anaesthesiologists, nurses, pharmacists, biomedical engineering and clerical staff. This committee met weekly for 5 weeks, before the planned 6‐week initiative. Inputs were sought ad hoc from other groups, such as resuscitation training teams and infection control.
2.2. Funding
‘Waiting list initiative’ funding was obtained from Ireland's Health Service Executive's (HSE) National Treatment Purchase Fund (www.ntpf.ie), established to fund access to health care as determined by national priorities. NTPF usually facilitates access to private healthcare facilities to address waiting lists for uninsured patients awaiting procedures in Ireland's public hospitals.
2.3. Critical risk assessment
A key decision made in the first planning meeting was to fund and fully resource attendance of a consultant paediatric anaesthesiologist for each day of the activity. This was decided as the facility was not close to the base hospitals, so it was necessary to ensure onsite advanced airway support in case it was needed during the expected cases of anaphylaxis, despite anaphylaxis being a relatively unusual event during food challenge—3%–5% of OFCs. 5 A further key enabler was a decision made later in planning to reduce the initiative from 5 working days each week to 4 days to allow consultant staff to attend to other responsibilities on the 5th day and to avoid overworking other staff with 5 working days starting at 7am, often lasting 12 hours.
2.4. Simulation
A dry run of patient flow from registration to discharge was attended by most staff, led by the resuscitation team and lead anaesthesiologist, to include initial challenge assessment, besides treatment and stabilization.
2.5. Documentation
Electronic health records are not yet established in Ireland, so paper charts were securely transported from each of the 4 base hospitals, as needed. A standardized proforma was used for the clerking of patients and recording of outcomes irrespective of the hospital of origin. A prepopulated prescription chart was developed, which only needed patient‐specific, weight‐based doses to be individually charted. A COVID‐19 health checklist was developed with questions about the attending child and the attending parent (only 1 parent was allowed to attend), and each family was telephoned the day before OFC attendance to ensure there were no emerging concerns regarding COVID.
2.6. Staffing
Colleagues working in regional allergy departments were invited to take part, providing a supervisory team of 3 consultant paediatric allergists, and 4 consultant paediatricians with a special interest in allergy. Similarly, trainee paediatricians with previous allergy experience were offered the opportunity to join the initiative creating a pool of 9 trainees. Allergy clinical nurse specialists (CNS) already employed by the base hospitals were made available as needed for the duration of the initiative. A retired allergy CNS, 2 allergy research fellows and a family doctor with experience in OFC were paid per diem to participate. Consultant anaesthesiologists attended during their resting time from their ordinary shift system; no elective perioperative or other work was compromised or delayed. Staff and patients travelling from Cork to Dublin were given free accommodation in a nearby hotel the night before their scheduled attendance for OFC. For most of the time, 2–4 senior cycle medical students were available to support nursing and medical staff, although they were principally there to take clinical histories and practise clinical examinations, which were then assessed by the trainees and consultants. The convention centre's full‐time chef and kitchen staff received instruction from clinical staff on food preparation for OFCs, risks of cross‐contamination, appropriate foods and condiments to provide. A standard operating procedure (SOP) was developed for the preparation of each type of food challenge. A senior member of the medical team liaised with the kitchen staff daily.
2.7. Staff well‐being
All staff wore masks at all times, adhered to hospital hand sanitizing policy and wore gloves to handle food and during patient contacts. A huddle occurred at the start of each day to focus on staff well‐being (COVID‐19 health in particular) and to disseminate lessons or ‘pearls’ and feedback on issues that had occurred during the initiative. A flip chart was used for information and motivational messaging. Free coffee, snacks and lunch were provided, and staff were rigorously rostered for breaks in an adjacent suite of clinical rooms, where they could rest, eat and support each other.
2.8. Waiting list validation
Long waiters from 3 to 4 years were reviewed in additional clinics during the planning phase to validate their ongoing need for OFC. This might have changed due to the natural resolution of their allergy or due to repeated exposures/reactions in the field, which would have negated the need for supervised OFC.
2.9. Adaptation of OFC conduct to the facility
Open, not double‐blind, challenges were performed, following the standard PRACTALL guidelines of incremental dosing, separated by 20–30 minutes with predose clinical assessments by the general nurse and doctor as needed. 6 To shorten the duration of each challenge, the usual first dose of 10 mg of allergen protein to which around only approximately 5% of children react 7 was omitted. Each challenge meal was prepared by the convention centre's full‐time chef and kitchstaff and was visually screened, signed and labelled by at least one doctor and nurse before being used at the bedside. The allergy CNS or doctor in each pod supervised and signed for each administered dose. Families arrived at the facility at 7.30 am with all challenges commenced by 8.30 am. Children were allocated to a pod randomly as they arrived; they were not pre‐allocated or grouped according to age or food type. Children had to stay on their bed, and no toys/media were provided, to prevent sharing. All children were brought to the remote bathroom in wheelchairs by staff/students, to prevent potential augmentation of emerging allergic reactions by exercise. Parents were not allowed circulate or socialize with each other but could singly access refreshments and the bathroom.
To perform multi‐person food challenges simultaneously, while maximizing human resource efficiency, an 18‐bed bay with piped oxygen available was divided into 3X6‐bed pods with movable screens placed between the pods to discourage ‘inter‐pod’ staff movement (Appendix 1). Each pod had a similar staff quota: 1 allergy CNS to oversee a total of 6 challenges, supported by 1 general paediatric nurse; 1 paediatric medical trainee, to clerk, examine each patient, take written parental consent and child assent and to prescribe emergency medication as appropriate; and 1–2 medical students. Staff were to stay in their pod, except when needed to support emergency care elsewhere, while the 2 supervising consultants moved between the 3 pods, assessing each of up to 18 simultaneous challenge's progress and evaluating emerging allergic reactions. Each pod was stocked and resupplied as needed with emergency medication sufficient for the needs of six patients. Medications were not pre‐prepared/drawn up separately for each patient.
Our local published experience was that up to 50% of OFCs are positive 5 and need medical attention for up to 2 hours after any reaction is complete. The other 50% negative OFCs need no treatment after the final dose. Therefore, a further 9 children were booked to arrive at 11.30 am in a further 2 pods. These were staffed by moving staff away from children with negative challenges, whose lower level of care was delegated to other staff for continuous observation. A total of 27 OFCs would be completed each day if every bed was used as projected.
The first day of the programme scheduled only 10 patients to allow for a smooth launch and necessary troubleshooting. A few days had lower planned numbers of challenges than 18, for operational reasons that predated the initiative.
2.10. Timeline
The first meeting took place on 17 July 2020, the first patients attended on 6 September 2020, and the last patient was challenged on 15 October 2020.
2.11. Ethical permission
Ethical permission was not sought for this quality improvement initiative. All parents and patients as appropriate gave written consent for the food challenge procedure, using standard, approved HSE forms.
3. RESULTS
3.1. COVID‐19 safety
No parent or child presented to the site with symptoms suggestive of COVID. Eight children did arrive at site with mild respiratory symptoms, but none was febrile, all 8 were isolated, medically assessed and discharged without starting OFC. Increasing COVID levels across Dublin, and escalation of pandemic national controls to level 3 (of 5) 8 in Dublin in the 4th week, led to the cancellation of patients travelling from Cork. Cork staff also stopped travelling in the 6th final week. One staff member developed COVID‐19 infection through a community contact. No close contact action in the field hospital was deemed necessary by occupational health.
3.2. Challenge outcomes
482 patients were admitted for OFC (Figure 1). Their age range was 2–18 with a median age of 8yr. Six patients were admitted for two separate challenges on different days. 474 OFCs were performed over 24 days. 396 (84%) of the OFC were to peanut, single tree nuts or sesame seed. The other 78 OFC included challenges to wheat, baked and whole egg and milk, fish, shellfish, kiwi and pulses.
FIGURE 1.
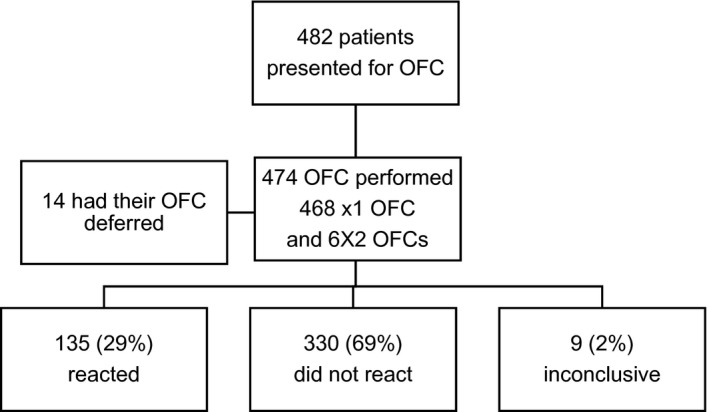
Flow diagram of oral food challenge outcomes
3.3. Reactions
135 (28.5%) of 474 children reacted during OFC (Table 1). The majority were advised to continue to avoid the food used in OFC, but 14 were discharged on graded introduction protocols. 9 9 (2%) OFCs were inconclusive, and those patients were advised to continue to avoid their index food. One child reacted mildly to the first dose. Reactions were witnessed to all allergens except white fish. The highest rate of reactions was seen with sesame, wheat and walnut. 25 (5%) patients were treated as per anaphylaxis protocol with 7 of these (1.5% of total OFCs and 28% of those who got any adrenaline) receiving a second dose of adrenaline. No child received advanced airway interventions. The 7 children who received two doses of IM adrenaline and a further child, with sustained tachycardia after 1 dose of adrenaline, were transferred to a base hospital. All were stable before transfer and were discharged home the following day. These cases are being reported in detail separately. 61 (13%) of patients were discharged from any follow‐up on the day of the OFC.
TABLE 1.
Oral food challenges performed by food type and outcome
| No. of OFC performed (not completed) | Percentage of total OFC performed | No. of +ve OFC (% of completed OFC) | |
|---|---|---|---|
| Peanut | 161 (3) | 34% | 61 (39) |
| Tree nut | 196 (4) | 41.35% | 40 (21) |
| Sesame | 39 (1) | 8.23% | 21 (55) |
| Fish | 14 (1) | 3% | 0 (0) |
| Shellfish | 8 | 1.7% | 1 (12.5) |
| Kiwi | 4 | 0.8% | 1 (25) |
| Wheat | 5 | 1% | 4 (80) |
| Milk or baked milk | 12 | 2.5% | 2 (17) |
| Egg or baked egg | 27 | 5.7% | 3 (11) |
| Pulses | 4 | 0.8% | 1 (25) |
| Other | 4 | 0.8% | 1 (25) |
| Total | 474(9) | 135 (29) |
3.4. Delayed reactions
One patient returned to the facility with ocular swelling, 2 hours post‐discharge after a negative pistachio challenge. This was considered a delayed allergic reaction, and pistachio avoidance was advised. One child who had reacted to baked egg continued to experience abdominal symptoms after discharge, which settled by the next day without further medical intervention. Nine (2%) families contacted the base hospital's allergy department within 2 weeks of food challenge with concerns regarding tolerance of the introduced food. One child was experiencing diarrhoea associated with increased exposure to baked egg, and 1 reported perioral reaction associated with peanut ingestion. On subsequent rechallenge, the latter patient reacted on the 3rd dose of peanut. The other 7 children's reported symptoms were assessed as unrelated to the introduction of the allergen into their diet and were able to maintain it in their diet.
3.5. Productivity
The maximum daily number of OFCs performed was 25. Late cancellations due to COVID infection, close contact status, other illness and work commitments (these were not all formally recorded) prevented the full quota of 27 being achieved on any day. The average number of challenges performed was 20 per 8 h day under the supervision of 2 consultants. This is equivalent to 1.25 OFC/allergist work hour. This was compared with the traditional in‐hospital supervision of average 3 food challenges/consultant at one time (5 h) equivalent to 0.6 OFC/per allergist work hr. A review showed specific adaptations or enabling resources that had been made available could be retained in our allergy services and others could be discontinued (Table 2).
TABLE 2.
Organizational lessons learned from the food challenge initiative
| Things we need to ‘drop or stop’ | Things we need to ‘grow’ or pick up again | |
|---|---|---|
|
Things we started in the initiative |
|
|
|
Things we stopped in the initiative |
|
|
3.6. Impact on waiting list
60% of the overall challenges performed came from the major centre (CHI). Clinical revalidation and 279 challenges reduced the total waiting list there from 502 to 172, a 57% reduction. 31% of CHI patients had been on the waiting list for over 2yr, 13% over 3yr and 0.6% over 4yr. At the end, only 14% were waiting over 2 years, 4% were waiting more than 3yr, and only 1 patient was waiting (for a drug challenge) more than 4yr. The ‘long waiters’ that remained had either been unable to attend or had medical or behavioural requirements considered unsuitable to attend the field hospital environment.
3.7. Staff satisfaction
Feedback from the clinical staff involved was overwhelmingly positive about the episode, mentioning team building, interdisciplinary working and the unique setting for immersive training of junior doctors, with the constant consultant presence. Consultant paediatricians commented on the high volume of exposure to OFC. Feedback from other sectors was not only largely positive about the concept but also commented on lack of time and poor consultation, being up against an imposed, not agreed, deadline (Appendix 2). The initiative was reported to have created a strong and visible identity for participating paediatric allergy units and nationally; it was a ‘good news story during COVID’.
3.8. Family satisfaction
A single family opted not to avail of the offsite OFC, requesting a future, hospital‐based option. 2 patients of CHI were reluctant to attend due to COVID concerns. 178 carers completed satisfaction surveys before discharge. Patient experience was scored as ‘excellent’ by 83% of respondents with a further 12% reporting it as above average. 81% were highly satisfied with the ease of use of a non‐hospital facility. 81% reported that the site was ‘child‐friendly’. Communication was effective with 89% reporting good understanding of the results of the OFC. 95% stated that their questions were answered adequately by the allergy team.
4. DISCUSSION
The extra demands and limitations on existing capacity imposed by the COVID pandemic have challenged all clinical services to critically examine service delivery and to rapidly introduce new models of care. Allergy services have redesigned approaches to numerous facets of care including allergy prevention, 10 tolerance promotion, 10 clinic models and training. 10 , 11 , 12 , 13 All of these changes in practice were born out of necessity. However, as the world embarks on the COVID vaccination phase, it is important to reflect on how these novel approaches inform future thinking on service planning.
This report describes the rapid deployment of a time‐limited mass OFC initiative achieved through the mobilization of trained paediatric allergy staff from across an entire country. The motivation was the loss of access to daybeds due to COVID‐19. The opportunity was a stepdown COVID‐19 facility lying empty. However, despite the apparent uniqueness of the situation, there are clear take‐home messages for all involved in the provision of ambulatory allergy services.
This project presents as the successful bringing together of variable clinic processes to rapidly create a unified model with long‐lasting learning across all involved units. Specific adaptations or enabling resources made available could be retained in our allergy services, and others could be discontinued (Table 2). The deployment of general nurses and the decreased ratio of patients per consultant and per allergy clinical nurse specialist were safe adaptations, with no related adverse outcomes.
In the hospital setting, allergy departments run with minimal day‐to‐day interaction with services such as infection control, clinical engineering, anaesthesiology/resuscitation and clinical risk. However, in this offsite venture, safety was ensured by the early engagement with these key stakeholders.
This model could act as a blueprint for transforming the limitations of multiple small independent allergy services into a highly effective collective. The initiative delivered 474 OFCs in 6 weeks by combining staff from 4 separate allergy units. Collectively, these 4 units would normally only deliver 900 challenges across 46 weeks, using a 1:1 nurse‐to‐patient ratio. Increasingly, an efficient use of resources is going to be essential for allergy services across the globe as they deal with the anticipated, exponential increase in pre‐desensitization OFCs. The increased efficiency we demonstrated required a degree of short‐term sacrifice/suspension of other aspects of clinical practice by clinical leads. Thus, this initiative challenges the more traditional model of allergy practice, where OFCs are factored into/limited by complex schedules and shared facilities.
The oral food challenge is a safe and reliable diagnostic tool when used by an experienced and trained physician. It is essential that trainees are provided with hands‐on experience of all aspects of OFC management including anaphylaxis. A recent publication by AAAAI reports that 56% of fellows reported performing 10 or less OFCs during their fellowship, and 29% had done none at all. 4 This high‐volume model has the added advantage of giving doctors in training an intensive fully supervised experience with almost quarantined exposure to anaphylaxis.
Lack of space is a universal limiting factor to the delivery of OFC. Allergists in the United States and Canada, surveyed in separate studies, reported it as a major barrier. 3 , 4 54.8% of Canadian allergists were in favour of creating dedicated OFC centres. This initiative establishes that OFC delivery is extremely portable, requiring very little in terms of specialist equipment. Even pumped oxygen is not necessarily required; although available at the bedsides, the resuscitation room was supplied only with tanked oxygen. Thus, OFC is a procedure that lends itself to offsite spaces, not just COVID‐19 stepdown facilities. Agreeing for a child to partake in an OFC is stressful for parents. It could be anticipated that being asked to attend at an offsite venue, during a pandemic, would dissuade many candidate families. In contrast, uptake was almost 100%. Families were not surveyed regarding their enthusiasm specifically, but it likely reflects a trust in the clinicians and in the institutions behind the initiative. Impairment of good communication and supportive behaviours due to high throughput, rotating staff and social distancing had been a concern. However, the post‐OFC survey revealed extremely high rates of satisfaction with overall experience and communication. This contrasts reassuringly with published qualitative data reporting parents feeling overwhelmed and in need of psychology support pre‐ and post‐OFC. 4
It is important to note that not all patients’ clinical needs can be served by a mass OFC practice. Patients likely to require more individual care by nursing such as infants <2 years and those with extreme anxiety or those who may need extra support by play specialists, including those with autism spectrum disorder, were all offered alternate appointments in the hospital setting. Similarly, the model did not lend itself to the care of patients with known specific infection control needs such as MRSA.
Only 13% of subjects were able to be fully discharged from the allergy service, as the remainder had ongoing care needs relating to existing food allergies or still needed OFC with other foods. The use of combined food/multi‐nut challenges 14 could increase the discharge rate.
The allergy team has been available to advise other services that are similarly limited by access to high‐volume clinical areas, and some have followed them in using the COVID‐19 facility for their activities. Two COVID‐19 era mantras that the allergy team adopted were ‘Perfect is the enemy of good’ and ‘Good enough is the new perfect’. These are unprecedented times with widespread curtailment of non‐essential medical services worldwide.
Project success is a balance between over‐ and underpreparation. This project was delivered after 5 weeks of planning because the opportunity/availability of the facility was time‐sensitive. Post‐event feedback revealed that, although overall satisfaction was high, it was lower amongst non‐clinical stakeholders who advocated greater inclusion and time to plan. Medical supply chain economics to only receive supplies ‘just in time’, which might work for fresh food in supermarkets, was shown in the early stages of the pandemic to leave health care short of critical supplies such as PPE and even ventilators. While being cautious about using metaphors of war on COVID‐19, we propose that medical institutions need to establish the principle of ‘readiness’, a military tool that enables adequate planning and preparation in order to achieve rapid responses in the face of sudden opportunity/need. This report shows that using the vacant COVID‐19 stepdown facility changed the way elective allergy care can be delivered. The retention and use of facilities built to meet the challenges of COVID‐19 in 2020–2022 must be considered as they can be adapted to many services’ needs, while remaining available for any further public health emergencies.
One of the greatest demands on international health services in the post‐COVID era will be the delivery of semi‐elective services cancelled or deferred due to COVID restrictions. We have shown here how critical it is for those who advocate for these patients to aggressively chase any opportunity offered, as it is surely experienced clinicians and their partners in front‐line healthcare who are best suited to reinventing models of care.
CONFLICT OF INTEREST
None to declare.
AUTHOR CONTRIBUTIONS
Aideen Byrne: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Funding acquisition (equal); Investigation (equal); Methodology (equal); Resources (equal); Software (equal); Supervision (equal); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Juan Trujillo: Conceptualization (supporting); Formal analysis (supporting); Writing‐original draft (lead); Writing‐review & editing (supporting). John Fitzsimons: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Software (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Muhammad Tariq: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Software (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (lead); Writing‐review & editing (lead). Robert Ghent: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Software (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Cathryn O'Carroll: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (equal); Software (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). David Coghlan: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Jonathan Hourihane: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Software (equal); Supervision (lead); Validation (lead); Visualization (equal); Writing‐original draft (lead); Writing‐review & editing (lead).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13580.
Supporting information
Appendix 1
Appendix 2
ACKNOWLEDGEMENTS
The authors would like to thank patients and families, consultant anaesthesiologists and other colleagues in Children’s Health Ireland and Cork University Hospital (resuscitation officers, nursing, health care assistants, medical records, admissions and waiting list officers, and ICT, infection control, pharmacy, risk, clinical engineering, human resources and communications officers), Bumbulance Ambulance Service, Senior Cycle 1 medical students from Royal College of Surgeons in Ireland and staff of Citywest Hotel Dublin.
Byrne AM, Trujillo J, Fitzsimons J, et al; the Children's Health Ireland (CHI) Food Challenge Initiative Team . Mass food challenges in a vacant COVID‐19 stepdown facility: Exceptional opportunity provides a model for the future. Pediatr Allergy Immunol. 2021;32:1756–1763. 10.1111/pai.13580
Funding information
This study was supported by the National Treatment Purchase Fund.
Contributor Information
Jonathan O’B Hourihane, Email: Jonathanhourihane@rcsi.com.
the Children's Health Ireland (CHI) Food Challenge Initiative Team:
Ali Alsalemi, Aoife Cassidy, Eva Corbet, Rita Creighton, Yvonne d’Art, Linda Farren, Rachel Flanagan, Niamh Flynn, Ruth Franklin, Claire Gray, Paul Harding, Ciara Hendrick, Fionnuala Herraghty, Sadhbh Hurley, Valerie Kavanagh, Dhanis Lad, Karen Leddy, Sarah Lewis, Triona McGlynn, Danielle O’Connor, Phil O’Neill, Orla O’Shea, Ann O’Toole, Rachel Quinn, Aisling Reid, Alison Russell, Emma Ruth, Anne Rynne, P Bhusan Sanneerappa, Mairead Sheehan, Claire Thompson, Ciara Tobin, James Trayer, Alison Wallace, Nicola Walsh, and Fiona Wilson
DATA AVAILABILITY STATEMENT
All data are available for sharing on written contact with the corresponding author.
REFERENCES
- 1. Kansen HM, Le TM, Meijer Y, et al. The impact of oral food challenges for food allergy on quality of life: A systematic review. Pediatr Allergy Immunol. 2018;29(5):527‐537. [DOI] [PubMed] [Google Scholar]
- 2. Couch C, Franxman T, Greenhawt M. The economic effect and outcome of delaying oral food challenges. Ann Allergy Asthma Immunol. 2016;116(5):420‐424. [DOI] [PubMed] [Google Scholar]
- 3. Greiwe J, Oppenheimer J, Bird AJ, Fleischer DM, Pongracic JA, Greenhawt M. AAAAI work group report: trends in oral food challenge practices among allergists in the United States. J Allergy Clin Immunol Pract Nov‐Dec. 2020;8(10):3348‐3355. [DOI] [PubMed] [Google Scholar]
- 4. Hsu E, Soller L, Abrams EM, Protudjer JLP, Mill C, Chan ES. Oral food challenge implementation: the first mixed‐methods study exploring barriers and solutions. J Allergy Clin Immunol Pract. 2020;8(1):149‐156. [DOI] [PubMed] [Google Scholar]
- 5. Yanishevsky Y, Daly D, Cullinane C, O'B Hourihane J. Differences in treatment of food challenge–induced reactions reflect physicians’ protocols more than reaction severity. J Allergy Clin Immunol. 2010;126(1):182. 10.1016/j.jaci.2010.03.038 [DOI] [PubMed] [Google Scholar]
- 6. Sampson HA, Gerth van Wijk R, Bindslev‐Jensen C, et al. Standardizing double‐blind, placebo‐controlled oral food challenges: American Academy of Allergy, Asthma & Immunology‐European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J All Clin Immunol. 2012;130(6):1260‐1274. 10.1016/j.jaci.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 7. Remington B, Westerhout J, Dubois A, et al. Suitability of low‐dose, open food challenge data to supplement double‐blind, placebo‐controlled data in generation of food allergen threshold dose. Clin Exp Allergy. 2020. 10.1111/cea.13753 [DOI] [PubMed] [Google Scholar]
- 8. Resilience‐recovery 2020‐2021, plan for living with Covid. https://www.gov.ie/en/campaigns/resilience‐recovery‐2020‐2021‐plan‐for‐living‐with‐covid‐19. Accessed February 1st 2021.
- 9. Garvey AA, O'Sullivan D, Hourihane JO'B. Home‐based induction of sustained unresponsiveness in children with mild reactions to high doses of peanut. J Allergy Clin Immunol Pract. 2017;5(6):1757‐1759. 10.1016/j.jaip.2017.03.027 [DOI] [PubMed] [Google Scholar]
- 10. Mack DP, Hanna MA, Abrams EM, et al. Virtually supported home peanut introduction during COVID‐19 for at‐risk infants. J Allergy Clin Immunol Pract. 2020;8(8):2780‐2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacMahon J, Hourihane JO’B, Byrne A. A virtual management approach to infant egg allergy developed in response to pandemic‐imposed restrictions. Clin Exp Allergy. 2020;26:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Codispoti CD, Bandi S, Moy JN, Mahdavinia M. Running a virtual allergy division and training program in the time of COVID‐19 pandemic. J Allergy Clin Immunol. 2020;145(5):1357‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MacMahon J, Byrne A. New ways of working. J Allergy Clin Immunol Pract. 2020;S2213‐2198(20). [Google Scholar]
- 14. Van Erp FC, Knust AC, Kok IL, et al. Usefulness of open mixed nut challenge to exclude tree nut allergy in children. Clin Transl Allergy. 2015;5(19). 10.1186/s13601-015-0062-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1
Appendix 2
Data Availability Statement
All data are available for sharing on written contact with the corresponding author.


