Abstract
Background
Annexin A1 (AnxA1) is an important endogenous glucocoticoid protein that contributes to the suppression of inflammation by limiting the production of neutrophil and pro‐inflammatory cytokines. This study aims to determine the clinical predictivity value of blood AnxA1 levels in patients with mild and severe–critical pneumonia induced by COVID‐19.
Methods
This study employed a prospective, case–control study design and was conducted at Ankara Training and Research hospital between 10 February 2021 and 15 March 2021. A total of 74 patients (42 of whom had moderate and 32 of whom had severe/critical cases of COVID‐19 disease according to World Health Organization guidelines) and 50 nonsymptomatic healthy volunteers participated in the study. Blood samples were taken from patients at the time of hospital admission, after which serum was isolated. Following the isolation of serum, AnxA1 levels were evaluated using the enzyme‐linked immunosorbent assay method.
Results
The serum AnxA1 levels were measured as 25.5 (18.6‐38.6) ng/ml in the control group, 21.2 (14.7‐32) ng/ml in the moderate disease group, and 14.8 (9.7‐26.8) ng/ml in the severe/critical disease group. Serum AnxA1 levels were significantly lower in the severe/critical disease group compared with the control and moderate disease groups (P = .01 and P = .0001, respectively). Using receiver operating characteristic analysis, a larger area under the curve (AUC) for the serum AnxA1 levels of the control group (AUC = 0.715, 95% CI = 0.626‐0.803; P = .0001) was calculated compared with the COVID‐19 patient group for the diagnosis of COVID‐19 disease. The AnxA1 level was found to be 80% sensitive and 54.1% specific at a cut‐off level of 18.5 ng/ml for the diagnosis of COVID‐19 disease. Moreover, the AnxA1 level was found to be 69.8% sensitive and 58.1% specific at a cut‐off level of 17.2 ng/ml in predicting the need for intensive care unit (ICU) treatment.
Conclusion
AnxA1 levels may be a beneficial biomarker in the diagnosis of COVID‐19 pneumonia and in predicting the need for ICU treatment in patients with COVID‐19 pneumonia at the time of admission to the emergency department.
What’s known
Endogenous corticosteroids contribute to the suppression of inflammation in a variety of ways.
AnxA1 limits the production of neutrophil and pro‐inflammatory cytokines.
The use of exogenous corticosteroids (especially dexamethasone) in severe/critical Coronavirus disease (COVID‐19) infections has also been recommended by World Health Organization guidelines for treating COVID‐19 and has been put into clinical practice.
What’s new
Serum Annexin A1 (AnxA1) levels were found to decrease in patients who had severe/critical cases of COVID‐19 disease.
AnxA1 levels may be a beneficial biomarker that can be used together with other known markers in the diagnosis of COVID‐19 pneumonia and in predicting the need for ICU treatment for patients with COVID‐19 pneumonia at the time of admission to the emergency department.
The present study suggests that one of the response mechanisms to glucocorticoid therapy in patients with severe COVID‐19 pneumonia using exogenous steroids may be through AnxA1.
1. INTRODUCTION
Coronavirus disease (COVID‐19) caused by the severe acute respiratory syndrome brought on by coronavirus 2 (SARS‐CoV‐2) was declared a global pandemic on 11 March 2020, and 157.2 million people had been infected and 3.2 million people had died worldwide by 6 May 2021. In the early days of May 2021, 800,000 new daily cases were confirmed, and 90,000 deaths were reported throughout the world. 1 COVID‐19 infection can cause a wide clinical spectrum ranging from mild upper respiratory tract infections to sepsis and acute respiratory distress syndrome (ARDS). 2
Cytokine storm, which was firstly defined by Cron and Behrens.It occurs when excessive and disregulated cytokine production due to an uncontrolled immune response to different initiators (such as infections, malignancies, and rheumatologic diseases). COVID‐19 can cause the production of cytokine storm inducing hyperinflammation, hyperactivation of the immune response, and multiple organ failure. 3 , 4 , 5 , 6
Annexin A1 (AnxA1) is an important endogenous glucocoticoid protein that contributes to the suppression of inflammation in a variety of ways. Also known as lipocortin‐1, it is one of the endogenous modulators of inflammation. AnxA1 is stored in high concentrations in the cytoplasm of neutrophils, macrophages, and monocytes in humans in states of no infection. 7 AnxA1 limits the production of neutrophil and pro‐inflammatory cytokines. Moreover, AnxA1 induces neutrophil apoptosis, mediates monocyte recruitment, and augments the scavenging of apoptotic cells by macrophages. Recent research has revealed that AnxA1 also induces macrophage reprogramming for providing optimal homeostasis. 7 AnxA1 levels are induced both by inflammatory reponses and by exogenously ingested glucocorticoids. Exogenous glucocorticoids induce AnxA1 expression in monocytes and neutrophils, which play a crucial role in the anti‐inflammatory responses. 8 Some studies on AnxA1 levels in sepsis patients have produced various results, such as in a study on patients with chronic obstructive pulmonary disease (COPD). AnxA1 levels were reported to decrease in sepsis patients, whereas another study found increased AnxA1 levels. 9 , 10 Serum AnxA1 levels were found to be higher in Stages 3 and 4 patients of Global Initiative for chronic obstructive lung disease (GOLD) COPD than patients with mild symptoms. 11 Another study found lower viral burden, higher mortality, and morbidity in ANXA1−/− rats infected with influenza compared with wild‐type rats. 12 In another study by Santana et al, low serum AnxA1 levels were found in patients infected with human T‐lymphotropic virus (HTLV‐1), and it was suggested that AnxA1 is an important marker on diagnosis and prognosis of HTLV‐1‐associated myelopathy/tropical spastic paraparesis. 13
As suggested by another study, the Ac2‐26 mimetic peptide of AnxA1 could be an important treatment agent in severe COVID‐19 disease. 14 However, to the authors' knowledge, there is no study based on the alterations of serum AnxA1 levels in patients with COVID‐19 infection or value of AnxA1 for clinical prediction.
We consider that blood AnxA1 levels may vary with clinical severity due to the significant increase in the inflammation cascade in patients with COVID‐19 pneumonia. The present study aims to determine the clinical predictivity value of blood AnxA1 levels in patients with mild and severe/critical pneumonia induced by COVID‐19 and to reveal the alterations of blood AnxA1 levels in patients with pneumonia compared with the control group.
2. METHODS
2.1. Study type
The present study is a prospective case–control study, and the required ethics approval was obtained from the Ethics Committee of Pamukkale University (Numbered: E‐60116787‐020‐15062). The study was conducted at Ankara Training and Research Hospital between 10 February 2021 and 15 March 2021. All procedures carried out on patients were in compliance with the Helsinki Declaration.
2.2. Study population
The patient groups and the healthy control group were informed in detail about the study, and they were requested to complete the written consent forms before participating in the study.
Study groups were established according to the inclusion and exclusion criteria. Patients whose diagnoses were clinically confirmed as COVID‐19 infection according to World Health Organization (WHO) guidelines using a positive reverse transcriptase polymerase chain reaction (RT‐PCR) test were included in the study. 15 Individuals were grouped in the moderate COVID‐19 disease group (N = 42), severe/critical COVID‐19 disease group (N = 32), and the healthy control group (N = 50).
The healthy control group included healthy volunteers with no history or diagnosis of any acute or chronic disease and infection and no known drug use.
2.3. Inclusion criteria
2.3.1. Patient groups
Patients whose diagnoses of COVID‐19 infection were confirmed by positive RT‐PCR in emergency department (ED) according to WHO guidelines and who gave their written consent were included in the study. 15
2.3.2. Control group
Subjects with no history of a known disease, no infectious symptoms, no drug use, and who provided written consent were included in the study.
2.4. Exclusion criteria
Patients who were diagnosed with heart, kidney, or liver failure, who had a history of acute pulmonary embolism, deep venous thrombosis, or chronic inflammatory disease, and who were pregnant were exclued from the study.
2.5. Clinical evaluation
The subjects included in the present study were clinically evaluated using WHO diagnosis and treatment guidelines for COVID‐19. 15 The patient management algorithms were administered due to the updates of these guidelines. The patient groups were categorised as moderate disease and severe/critical disease according to WHO guidelines. The severe/critical disease group consisted of intensive care unit (ICU) patients in line with WHO guidelines. 15 To evaluate the clinical severity, CURB‐65 scores of patients were calculated as indicated in the literature. The CURB‐65 score is a scoring system used in the evaluation of pneumonia. 16
2.6. Healthy group (control group)
This group included subjects who had no history or diagnosis of any disease, no infection history within the last 2 weeks, no history of any particular medication, who were admitted to ED with complaints other than infectious issues, and who gave their written consent to participate in the study.
2.7. Data collection
Demographic information and vital findings of the subjects and their laboratory findings (haemogram, C‐reactive protein [CRP], liver function tests [aspartat aminotransferaz and alanin aminotransferaz], creatinine, blood urea nitrogen [BUN], D‐dimer, creatinine kinase‐MB [CK‐MB], high sensitive troponin T [hsTnT], blood gas analysis parameters, and hospitalisation location [ICU or not]) were recorded in the data set.
2.8. AnxA1 level measurement
Venous blood samples that were taken when the patients were admitted to ED were withdrawn into a dry test tube that did not contain anticoagulant and were then centrifugated for 10 minutes at 4000 rpm. Serum samples obtained from centrifugation were collected for laboratory analysis. Serum AnxA1 levels were analysed using a commercially available AnxA1 enzyme‐linked immunosorbent assay (ELISA) Kit (Elabscience, E‐EL‐H5512, USA), per the manufacturer's protocol.
2.9. Statistical analysis
Given that a similarly organised reference study did not exist, a power analysis was performed in line with the presumptions. The results revealed that at least 92 people (min. 46 for each cohort) were needed to achieve 95% power at a 90% confidence interval, assuming that the projected effect size would be high (f = 0.7). The SPSS package program was used for data analysis. The continuous variables were presented as median (interquartile range [IQR]) and mean ± standard deviation. A Kolmogorov–Smirnov test was conducted to calculate the distribution type of the continuous variables. Mann–Whitney U or Kruskal–Wallis tests were used for analysing independent and nonparametric variables. Spearman correlation analysis was used to investigate correlation relationships between continous nonparametric variables. Receiver operating characteristic (ROC) curve analysis was used for the discriminant performance serum AnxA1 levels. The significance level was defined as P < .05 for all analyses.
3. RESULTS
Symptom duration time was statistically higher in the severe/critical disease group than in the moderate disease group (7.5 ± 1.7 and 5.9 ± 1.1 days, respectively) (P = .02).
As a result of the post hoc power analysis, the effect size of the AnxA1 concentrations for the differences between the two groups (patients and control) was moderate high (f = 0.66), and the power level observed for this effect size was 95%, and the reliability level was 93.96%.
Serum AnxA1 levels were measured as 25.5 (18.6‐38.6) ng/ml in the control group, 21.2 (14.7‐32) ng/ml in the moderate disease group, and 14.8 (9.7‐26.8) ng/ml in the severe/critical disease group. Serum AnxA1 levels were significantly lower in the severe/critical disease group compared with the control and moderate disease groups (P = .01 and P = .0001, respectively) (Table 1 and Figure 1).
TABLE 1.
Annexin A1 levels of the study groups
|
Controls (N = 50) |
Moderate disease group (N = 42) |
Severe/critical disease group (N = 32) |
P‐value | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD |
Median (IQR) |
Mean ± SD |
Median (IQR) |
Mean ± SD |
Median (IQR) |
||
| Annexin A1 | 33.46 ± 22 |
25.53 (18.7‐38.7) |
25.65 ± 14.81 |
21.21 (9.78‐19.71) |
16.06 ± 7.1 |
14.86 (9.78‐19.71) |
.0001* .044** .01*** .0001**** |
Abbreviations: IQR, interquartile range; SD, standard deviation.
P‐value derived from Kruskal–Wallis test and refers to the comparison between all the groups.
P‐value is derived from Mann–Whitney U test and refers to the comparison between control and moderate disease groups.
P‐value is derived from Mann–Whitney U test and refers to the comparison between control and severe/critical disease groups.
P‐value is derived from Mann–Whitney U test and refers to the comparison between moderate disease group and severe/critical disease groups.
FIGURE 1.
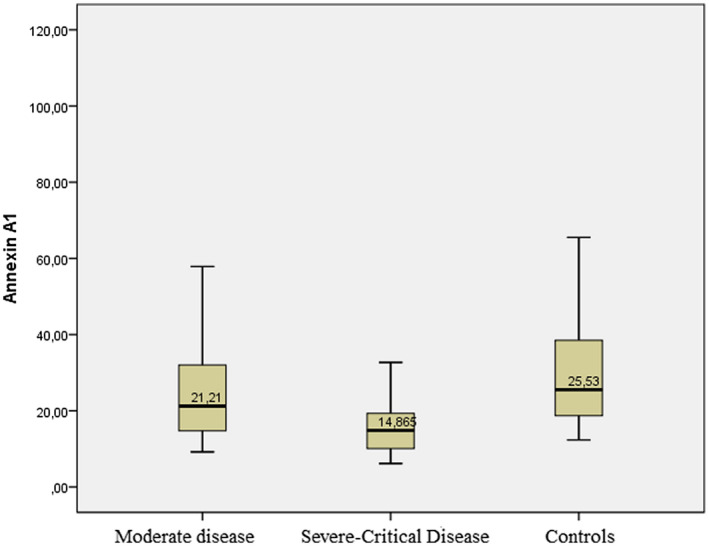
Serum annexin A1 levels of the groups
Subjects included in patient groups and healthy control group were matched by means of age and gender (P = .384 and P = .285, respectively) (Table 2).
TABLE 2.
Clinical data and comorbidity data of the groups
|
Controls (N = 50) |
Moderate disease group (N = 42) |
Severe/critical disease group (N = 32) |
P‐value | |
|---|---|---|---|---|
| Gender, N% | ||||
| Male | 24 | 22 | 11 | .285 * |
| Female | 26 | 20 | 21 | |
| Comorbidities, N (%) | ||||
| Diabetes mellitus | 19 (45.2%) | 15 (46.8%) | 1* | |
| Hypertension | 15 (35.7%) | 16 (50%) | .217* | |
| Coronary artery disease | 6 (14.2%) | 6 (18.7%) | .606* | |
|
Mean ± SD or median (IQR) | ||||
| Age (year) | 66.5 ± 16.4 | 66.8 ± 15.8 | 67.1 ± 12.8 | .384** |
| CURB‐65 score | 2 (1‐2) |
3 (2‐4) |
.0001 | |
| Body temperature (°C) | 37 (36.5‐37.3) |
36.6 (36.1‐37.4) |
.131 | |
| Heart rate (beat/min) |
91.5 (86‐101.25) |
94 (87‐116) | .32 | |
| Respiratory rate |
24 (20.75‐27.25) |
30 (23‐39) | .001 | |
| SpO2 |
92 (89.5‐96) |
80 (70‐87) | .0001 | |
| SBP (mm Hg) | 137.5 (120‐160) | 133 (116‐144) | .32 | |
| DBP (mm Hg) | 75.5 (68‐83.5) | 73 (63‐80) | .166 | |
Abbreviations: DBP, diastolic blood pressure; IQR, interquartile range; SBP, systolic blood pressure; SD, standard deviation.
P‐values are derived from Mann–Whitney U test.
P‐values are derived from chi square test
P‐values is derived from Student's t‐test.
Vital findings and clinical data for the study groups are given in Table 2, and Table 3 presents the laboratory parameters of the subjects.
TABLE 3.
Laboratory parameters of the patient groups
|
Moderate disease group (N = 32) |
Severe/critical disease group (N = 42) |
P‐value | |
|---|---|---|---|
| Mean ± SD or median (IQR) | Mean ± SD or median (IQR) | ||
| WBC (K/μL) | 5.57 (4.7‐6.73) |
9.19 (7.18‐15.16) |
.0001 |
| Haemoglobin (g/dL) |
13.19 ± 1.4 |
13.5 ± 1.49 | .351* |
| Platelet (K/μL) | 203.28 ± 59.7 | 256.53 ± 94.7 | .003* |
| NLR | 3.16 (1.88‐4.38) |
7.96 (4‐9.8) |
.0001 |
| ESR (mm/h) | 37 (21‐56) |
45 (23‐55) |
.272 |
|
CRP (mg/L) |
40.7 (13.98‐89.6) |
138.6 (77.8‐196.7) |
.0001 |
| BUN (mg/dL) | 34.5 (27.75‐48.5) |
49.6 (36.3‐70.3) |
.005 |
| Creatinine (mg/dL) | 0.87 (0.78‐1.13) | 1.12 (0.89‐1.42) | .011 |
| AST (U/L) | 27 (22‐33.5) |
44 (30‐79) |
.0001 |
| ALT (U/L) | 18 (12‐25) |
26 (22‐49) |
.001 |
| D‐dimer (ng/ml) | 580 (297.5‐1182.5) |
2000 (895‐4435) |
.0001 |
| hsTnT (μg/L) | 12.92 (6.97‐20.44) |
25.9 (18.7‐58.4) |
.0001 |
| CK‐MB (ng/ml) | 1.4 (0.91‐2.81) |
2.89 (1.85‐7.02) |
.0001 |
| Ph | 7.41 (7.37‐7.43) |
7.41 (7.35‐7.44) |
.71 |
| pCO2 (mm Hg) | 39.05 (35.12‐44.3) |
38.6 (33.2‐41.2) |
.43 |
| Lactate (mmol/L) | 1.8 (1.22‐2.1) |
2.8 (1.8‐4.6) |
.0001 |
| HCO3 (mEq/L) | 24 (22.45‐25.6) | 22.6 (20.6‐24.5) | .138 |
P‐values are derived from Kruskal–Wallis test.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; CK‐MB, creatinine kinase‐MB; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HCO3, bicarbonate; hsTnT, high sensitive troponin T; IQR, interquartile range; NLR, neutrophil leukocyte ratio; pCO2, partial carbon dioxide pressure; pH, power of hydrogen; SD, standard deviation; WBC, white blood cell.
P‐values are derived from Student's t‐test.
When the correlation of AnxA1 with clinical and laboratory findings was analysed, a negative mild–moderate correlation was found between serum AnxA1 and CURB‐65 score for the patients (ρ = −.381 and P = .001). Serum AnxA1 levels were mildly positively correlated with breath rate and oxygen saturation (SpO2) (ρ = .32 and P = .0001 and ρ = .202 and P = .025, respectively).
Using ROC analysis, a larger area under the curve (AUC) for the serum AnxA1 levels of the control group (AUC = 0.715, 95% CI = 0.626‐0.803; P = .0001) was calculated compared with the COVID‐19 patient group. The AnxA1 level was found to be 80% sensitive and 54.1% specific at a cut‐off level of 18.5 ng/ml for the diagnosis of COVID‐19 disease (Figure 2).
FIGURE 2.
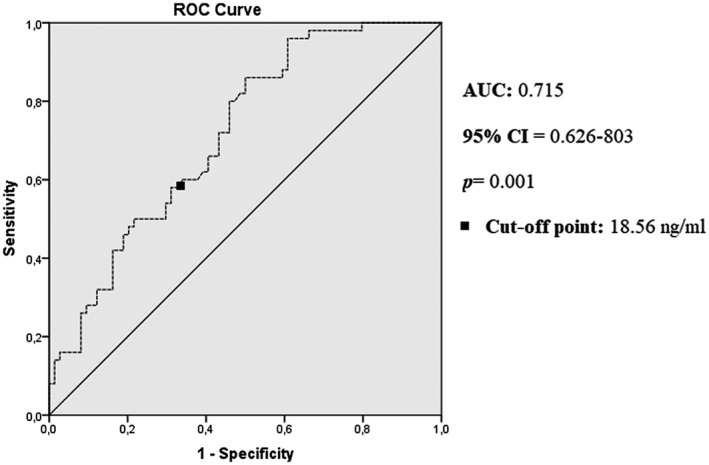
ROC curve analysis of the clinical diagnosis of the patients. AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic
Furthermore, a larger AUC for the serum AnxA1 levels of patients who needed ICU treatment (AUC = 0.701, 95% CI = 0.582‐0.819; P = .003) was calculated using ROC analysis. The AnxA1 level was found to be 69.8% sensitive and 58.1% specific at a cut‐off level of 17.2 ng/ml for predicting the need for ICU treatment (Figure 3).
FIGURE 3.
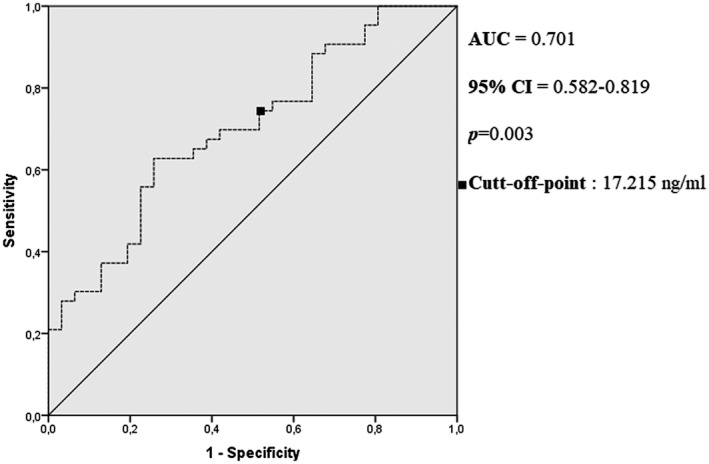
Receiver operating characteristic curve analysis of the clinical prognosis of the patients (non‐ICU needed and ICU needed). AUC, area under the curve; CI, confidence interval; ICU, intensive care unit
4. DISCUSSION
The present study evaluated the clinical significance of serum AnxA1 level in COVID‐19 pnemonia and concluded that the serum AnxA1 levels decreased as the clinical severity increased, and serum AnxA1 levels were 18.56 ng/ml in COVID‐19 pnemonia with a sensitivity of 80% and specifity of 54%. Moreover, serum AnxA1 level may be considered as an indicator for predicting the need for ICU treatment even with its lower sensitivity and specifity.
Many biomarkers, which are routine or nonroutine laboratory parameters, have been analysed both in diagnosing the disease and predicting the clinical prognosis throughout the COVID‐19 pandemic. Previous studies have investigated whether laboratory parameters such as monocyte/lymphocyte ratio, CRP, ferritin, lactate dehydrogenase (LDH), interleukin‐6 (IL‐6), D‐dimer, p selectin, calprotectin, and surfactant can be used as biomarkers for the clinical diagnosis and prediction of prognosis for COVID‐19. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 The sensitivity of the markers for COVID‐19 diagnosis mentioned in these studies ranged between 66% and 97.5%. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26
The serum AnxA1 levels in this study had a sensitivity of 80% and specifity of 54.1%, which might suggest that serum AnxA1 levels may be a useful marker in the diagnosis of COVID‐19. A Cochrane database review revealed the sensitivity of CRP, IL‐6, and LDH levels in clinical diagnosis as 66%, 73%, and 77%, respectively. Thus, AnxA1 might be considered as a more powerful biomarker compared with these markers. 17
AnxA1 is an endogenous glucocorticoid that plays a crucial role in inhibiting inflammation. AnxA1 regulates inflammation by mediating recruitment of neutrophils and macrophages. 28 Endogenous glucocorticoids are evaluated as the potential target in inflammation control. Notably, recombinant AnxA1 and N‐terminal peptides of AnxA1 have been found to have an anti‐inflammatory effect in pharmacologic and experimental models. 29 , 30
Although the mechanism of glucocorticoid‐induced leucine zipper (GILZ) protein, which is one of the proteins whose production is stimulated by exogenous glucocorticoids, has not been fully elucidated, it has been found to affect the functions of AnxA1. 8 AnxA1 mRNA expression is upregulated by glucocorticoids. 31 Studies have reported that inflammatory stimulation was much stronger and longer lasting in AnxA1 defective mice than wild‐type mice. 32 , 33 , 34 In a study conducted by Tsai et al, AnxA1 levels and associated lipoxin were found to diminish in patients with sepsis, although no significant differences were found between survivor and nonsurvivor sepsis patients in terms of these markers. In addition, the study also found that pro‐inflammatory cytokines increased and these markers decreased. 35 Among studies investigating the relationship between AnxA1 level and clinical prognosis in viral infections, a study by Santana et al is notable in that it found low serum AnxA1 levels in patients with HTLV‐1 infection. 13
When studies on the serum AnxA1 levels in COVID‐19 infection and other pulmonary pathologies were examined, serum AnxA1 levels were found to be higher in GOLD Stages 3 and 4 COPD patients compared with patients with mild symptoms. In addition to the present study, many mechanisms have been proposed as therapeutic targets in COVID‐19 infection. 36 In a hypothesis published by Bonavita, it was suggested that the Ac2‐26 mimetic protein of the AnxA1 protein could be a therapeutic target, especially in the treatment of severe COVID‐19 infection, and this peptide could be one of the main mediators of cytokine storm syndrome by lowering IL‐6 levels. 14 Additionally, the use of exogenous corticosteroids (especially dexamethasone) in severe/critical COVID‐19 infection has also been recommended by WHO guidelines for COVID‐19 treatment and has been put into clinical practice. 37 To the authors' knowledge, there exist no studies on the clinical and prognostic significance of AnxA1 level in COVID‐19 patients, and the present study is the first research in this regard. In the present study, lower serum AnxA1 level in severe/critical patients compared with mild patients and the control group suggested that hyperinflammation due to COVID‐19 and exacerbation of septic clinic might be attributed to the decrease in endogenous glucocorticoids. When the effect of exogenous corticoid therapy on endogenous corticoid levels and the role of glucocorticoids in the treatment of COVID‐19 are considered, the mechanism of response to glucocorticoid therapy may be through AnxA1. Studies on the effect of exogenous steroid administration on endogenous glucocorticoids in the treatment of severe COVID‐19 may further investigate the role of AnxA1 protein in COVID‐19 infection.
In addition, the findings regarding lower AnxA1 in severe/critical COVID‐19 patients have provided an idea about the clinical adaptability of the Ac2‐26 mimetic peptide of the AnxA1 protein, which was previously hypothesised and can be administered in the treatment of severe COVID‐19.
Moreover, this study found 69.8% sensitivity and 58.1% specificity at 17.2 ng/ml serum level of AnxA1 in order to predict the need for ICU treatment in patients admitted to ED, suggesting that serum AnxA1 level may be a useful biomarker in predicting the need for ICU treatment.
The present study has some limitations. Recurrent serum AnxA1 levels were not measured during the clinical visit, ICU hospitalisation, or treatment of the patients, and alterations of serum AnxA1 levels during the infection process were not understood.
5. CONCLUSIONS
The present study has found that serum AnxA1 levels may be a beneficial biomarker in the diagnosis of COVID‐19 pneumonia and in predicting the need for ICU treatment in patients with COVID‐19 pneumonia at the time of admission to the ED.
The lower serum AnxA1 level in severe COVID‐19 patients revealed the role of the AnxA1 protein in the clinical severity and the balance of anti‐inflammatory/inflammatory mechanisms.
Moreover, the present study has pointed out that one of the response mechanisms to glucocorticoid therapy in patients with severe COVID‐19 pneumonia using exogenous steroids may be through AnxA1.
ACKNOWLEDGEMENTS
There is no funding statement for this study.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interests.
DATA AVAILABILITY STATEMENT
All the data (other than patient names) are available to share.
Canacik O, Sabirli R, Altintas E, et al. Annexin A1 as a potential prognostic biomarker for COVID‐19 disease: Case–control study. Int J Clin Pract. 2021;75:e14606. 10.1111/ijcp.14606
REFERENCES
- 1. WHO Coronavirus (COVID‐19) Dashboard. Accessed at: https://covid19.who.int/. Accessed May 5, 2021.
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cron R, Behrens EM. Cytokine Storm Syndrome. 1st ed. Cham: Springer Nature Switzerland AG; Springer International Publishing; 2019. [Google Scholar]
- 4. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID‐19. J Infect. 2020;80:607‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu D, Yang XO. TH17 responses in cytokine storm of COVID‐19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gustine JN, Jones D. Immunopathology of hyperinflammation in COVID‐19. Am J Pathol. 2021;191:4‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Annexin A1 and the resolution of inflammation: modulation of neutrophil recruitment, apoptosis, and clearance. J Immunol Res. 2016;2016:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62‐70. [DOI] [PubMed] [Google Scholar]
- 9. Tsai WH, Shih CH, Yu YB, Hsu HC. Plasma levels in sepsis patients of annexin A1, lipoxin A4, macrophage inflammatory protein‐3a, and neutrophil gelatinase‐associated lipocalin. J Chin Med Assoc. 2013;76:486‐490. [DOI] [PubMed] [Google Scholar]
- 10. Tsai WH, Li IT, Yu YB, Hsu HC, Shih CH. Serial changes in plasma annexin A1 and cortisol levels in sepsis patients. Chin J Physiol. 2014;57:1‐7. [DOI] [PubMed] [Google Scholar]
- 11. Lai T, Li Y, Mai Z, et al. Annexin A1 is elevated in patients with COPD and affects lung fibroblast function. Int J Chron Obstruct Pulmon Dis. 2018;13:473‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ampomah PB, Kong WT, Zharkova O, Chua SCJH, Perumal Samy R, Lim LHK. Annexins in influenza virus replication and pathogenesis. Front Pharmacol. 2018;9:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santana BB, Queiroz MAF, Cerveira RA, et al. Low Annexin A1 level in HTLV‐1 infected patients is a potential biomarker for the clinical progression and diagnosis of HAM/TSP. BMC Infect Dis. 2021;21:219. 10.1186/s12879-021-05917-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonavita AG. Ac2‐26 mimetic peptide of annexin A1 to treat severe COVID‐19: a hypothesis. Med Hypotheses. 2020;145:110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization . Clinical management of COVID‐19: interim guidance (No. WHO/2019‐nCoV/clinical/2020.5). Accessed October 22, 2020.Available at: https://apps.who.int/iris/handle/10665/332196
- 16. Ioachimescu OC, Ioachimescu AG, Iannini PB. Severity scoring in community‐acquired pneumonia caused by Streptococcus pneumoniae: a 5‐year experience. Int J Antimicrob Agents. 2004;24:485‐490. [DOI] [PubMed] [Google Scholar]
- 17. Stegeman I, Ochodo EA, Guleid F, et al. Cochrane COVID‐19 diagnostic test accuracy group. Routine laboratory testing to determine if a patient has COVID‐19. Cochrane Database Syst Rev. 2020;11:CD013787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng J, Qi D, Yuan G, et al. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID‐19): A multicenter, cross‐sectional study. J Clin Lab Anal. 2020;34:e23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yilmaz A, Sabirli R, Seyit M, et al. Association between laboratory parameters and CT severity in patients infected with COVID‐19: a retrospective, observational study. Am J Emerg Med. 2021;42:110‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aceti A, Margarucci LM, Scaramucci E, et al. Serum S100B protein as a marker of severity in COVID‐19 patients. Sci Rep. 2020;10:18665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ozen M, Yilmaz A, Cakmak V, et al. D‐Dimer as a potential biomarker for disease severity in COVID‐19. Am J Emerg Med. 2021;40:55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seyit M, Avci E, Nar R, et al. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID‐19. Am J Emerg Med. 2021;40:110‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soraya GV, Ulhaq ZS. Crucial laboratory parameters in COVID‐19 diagnosis and prognosis: an updated meta‐analysis. Med Clin (Engl Ed). 2020;155:143‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. García‐Tardón N, Abbes AP, Gerrits A, Slingerland RJ, den Besten G. Laboratory parameters as predictors of mortality in COVID‐19 patients on hospital admission. J Lab Med. 2020;44:357‐359. [Google Scholar]
- 25. Kerget B, Kerget F, Koçak AO, et al. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID‐19? Lung. 2020;198:777‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karsli E, Sabirli R, Altintas E, et al. Soluble P‐selectin as a potential diagnostic and prognostic biomarker for COVID‐19 disease: a case‐control study. Life Sci. 2021;277:119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaya T, Yaylacı S, Nalbant A, et al. Serum calprotectin as a novel biomarker for severity of COVID‐19 disease. Ir J Med Sci. 2021;27:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosales C, Demaurex N, Lowell CA, Uribe‐Querol E. Neutrophils: their role in innate and adaptive immunity. J Immunol Res. 2016;2016:1469780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dalli J, Consalvo AP, Ray V, et al. Proresolving and tissue‐protective actions of annexin A1‐based cleavage‐resistant peptides are mediated by formyl peptide receptor 2/lipoxin A4 receptor. J Immunol. 2013;190:6478‐6487. [DOI] [PubMed] [Google Scholar]
- 30. Pederzoli‐Ribeil M, Maione F, Cooper D, et al. Design and characterization of a cleavage‐resistant Annexin A1 mutant to control inflammation in the microvasculature. Blood. 2010;116:4288‐4296. [DOI] [PubMed] [Google Scholar]
- 31. Solito E, Mulla A, Morris JF, Christian HC, Flower RJ, Buckingham JC. Dexamethasone induces rapid serine‐phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel nongenomic mechanism involving the glucocorticoid receptor, protein kinase C, phosphatidylinositol 3‐kinase, and mitogen‐activated protein kinase. Endocrinology. 2003;144:1164‐1174. [DOI] [PubMed] [Google Scholar]
- 32. Hannon R, Croxtall JD, Getting SJ, et al. Aberrant inflammation and resistance to glucocorticoids in annexin 1‐/‐ mouse. FASEB J. 2003;17:253‐255. [DOI] [PubMed] [Google Scholar]
- 33. Yang YH, Morand EF, Getting SJ, et al. Modulation of inflammation and response to dexamethasone by Annexin 1 in antigen‐induced arthritis. Arthritis Rheum. 2004;50:976‐984. [DOI] [PubMed] [Google Scholar]
- 34. Damazo AS, Yona S, Flower RJ, Perretti M, Oliani SM. Spatial and temporal profiles for anti‐inflammatory gene expression in leukocytes during a resolving model of peritonitis. J Immunol. 2006;176:4410‐4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsai WH, Shih CH, Yu YB, Hsu HC. Plasma levels in sepsis patients of annexin A1, lipoxin A4, macrophage inflammatory protein‐3a, and neutrophil gelatinase‐associated lipocalin. J Chin Med Assoc. 2013;76:486‐490. [DOI] [PubMed] [Google Scholar]
- 36. Krumm ZA, Lloyd GM, Francis CP, et al. Precision therapeutic targets for COVID‐19. Virol J. 2021;18:66. 10.1186/s12985-021-01526-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corticosteroids for COVID‐19. Living guidance. https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐Corticosteroids‐2020.1. Accessed May 27, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data (other than patient names) are available to share.


