Abstract
Problem
Limited data exists on the temporal trend of the Sars‐CoV‐2 immunologic response and duration of protection following natural infection. We sought to investigate the presence and duration of Sars‐CoV‐2 serum antibodies in obstetrical healthcare workers (HCW) on serial assessments over a 6‐month period, and to assess rates of vaccine acceptance and reported vaccine side effects among this cohort.
Method of study
A prospective cohort study of a convenience sample of obstetrical HCWs at a tertiary hospital. Serum Sars‐CoV‐2 antibodies for Immunoglobulin G (IgG) and Immunoglobulin M (IgM) were measured longitudinally at four intervals: baseline, 4 weeks, 12 weeks, and 6 months. Participants completed voluntary surveys on COVID19 testing, high‐risk exposures, vaccine acceptance, and vaccine side effects.
Results
One hundred twenty‐six of 150 (84%) HCWs who volunteered for participation completed all four blood draws. Prevalence of seropositive HCWs based on positive Sars‐CoV‐2 IgG antibodies increased from 2% at baseline to 31% at 12 weeks but declined to 21% by 6 months. Forty‐two percent (19/43) of the participants considered seropositive for Sars‐CoV‐2 IgG antibodies at any of the initial three blood draws converted to seronegative status at the 6‐month follow‐up. Eighty‐seven percent (72/83) of participants who responded to a follow‐up survey were willing to accept the COVID19 vaccine. Rates of acceptance did not differ by participant antibody status. Those that experienced symptoms with the first injection were more likely to have positive Sars‐CoV‐2 IgG antibodies (36.8% vs. 9.6%, p = .01).
Conclusion
Sars‐CoV‐2 IgG antibodies wane over time and may not provide prolonged and robust immune protection. This underscores the importance of vaccination and continued research in this area while the COVID19 pandemic continues.
Keywords: antibodies, COVID19, healthcare workers, Sars‐CoV‐2, vaccine hesitancy
1. INTRODUCTION
The rapid spread of the Sars‐CoV‐2 virus has led to the current COVID19 global pandemic. The social and economic consequences of this pandemic are vast, and until recently, the mainstay of mitigating this disease has been through health prevention measures such as social distancing, facial masking, and contact tracing. 1 With the rapid production, mass distribution, and robust vaccine acceptance of novel COVID19 vaccines, we now have an intervention that can end the pandemic. To date, 65% of the United States population has received at least a single dose of the Moderna, the Pfizer, or the Johnson and Johnson COVID19 vaccine; however, daily vaccination rates are decreasing. 2 This decline in vaccination is due to COVID19‐associated vaccine hesitancy, from factors such as perceived rushed production, and limited safety and long‐term data. 3 Still, others cite prior Covid19 infection and a perception of natural immunity, despite the lack of knowledge on the long‐term protection such an infection may provide. Even though data suggest reduced re‐infection rates in those that have had prior COVID19 infection, 4 , 5 we do not know the duration of this protection, especially against variant strains. Furthermore, vaccination in those with prior infection is recommended and produces significantly higher antibody titer levels than those without prior infection. 6
Current data show high rates of COVID19 seroconversion by presence of Sars‐CoV‐2 Immunoglobulin G (IgG) antibodies following acute infection. 7 , 8 However, recent data suggests a rapid decline in serum IgG antibody titers when followed serially for 60 days, 8 , 9 , 10 and a rate of re‐conversion to seronegative status ranging from 28% to 58% during this same study period. 9 , 10 Moreover, the half‐life of Sars‐CoV‐2 IgG antibodies is suggested to be as short as 36–85 days. 11 , 12 This is in stark contrast to antibodies to other known viral pathogens such as varicella, mumps, measles, and rubella all with calculated half‐lives greater than 50 years. 13 Still, the data are mixed, and other studies cite rates of persistence of Sars‐CoV‐2 IgG antibodies ranging from 92–94% at 3–6 month follow ups. 12 , 14
Information about the trend in Sars‐CoV‐2 IgG antibodies is of particular importance in obstetrical healthcare workers (HCW), as they are exposed to large volumes of asymptomatic patients with rapid turnover and have increased potential to spread COVID19 15 We sought to investigate the presence and duration of Sars‐CoV‐2 serum antibodies in obstetrical healthcare workers on serial assessments over a 6‐month period. We additionally assessed rates of vaccine acceptance and reported vaccine side effects among this population.
2. METHODS
This was a prospective cohort study that investigated the longitudinal presence of serum Sars‐CoV‐2 specific antibodies for both Immunoglobulin G (IgG) and Immunoglobulin M (IgM) in obstetrical HCWs at a tertiary hospital. This was a convenience sample of HCWs employed in the antepartum and triage units, labor and delivery, and outpatient facilities inclusive of clinic and ultrasound staff. Staff were recruited by invitation in each respective study site and volunteered to participate. Written informed consent was obtained, and an initial blood draw took place over a one‐week interval beginning March 25, 2020. Subsequent blood draws took place over a similar one‐week interval timed 4 weeks, 12 weeks, and 6 months later. Data regarding demographics, symptoms, prior Sars‐CoV‐2 nasopharyngeal PCR results, and timing of high‐risk exposures were collected through a voluntary survey conducted at the time of each blood draw. Follow‐up contact was made via email with the participants approximately 1 month after this cohort became eligible for COVID19 vaccination. The follow‐up survey included questions regarding plans to get the COVID19 vaccine and any adverse side effects experienced following administration. Side effects to the vaccine were considered positive if they included systemic symptoms such as fever, chills, myalgias, or fatigue. Localized symptoms such as arm pain or injection irritation were considered an expected reaction and classified as negative. Those that were no longer employed at our institution or did not present for follow‐up blood draws after a reminder email about collection dates were considered lost to follow up.
The concentrations of Sars‐CoV‐2 IgM and IgG antibodies were measured in serum from whole blood samples using a validated Sars‐CoV‐2 enzyme‐linked immunosorbent assay per manufacture's protocol (Novel Coronavirus COVID19 IgG ELISA kit; Epitope Diagnostics, San Diego, CA, USA). 16 The optical density (OD) ratio for positive IgM was > .201 (negative cut‐off value < .179) and positive IgG was > .439 (negative cut‐off value < .359). The minimal detectable concentration for IgM and IgG was 5 IU/mL. The inter‐ and intra‐assay coefficients of variation were < 15% and < 20%, respectively. The study was reviewed and approved by the hospital's biomedical institutional review board (IRB study ID#2020H0133).
Data are expressed by median [interquartile range] for continuous nonparametric variables, and number and percentage of subjects in each level of a categorical measurement. Relevant data were analyzed using Chi‐square. A p‐value < .05 was used to determine statistical significance.
3. RESULTS
150 female HCWs volunteered for participation. Median age of participants was 35 years old (IQR 29.5‐47.5), 63% (n = 95) were registered nurses, and 75% (n = 113) were primarily employed in the inpatient setting. Forty‐five percent (n = 68) of participants were tested for COVID19 by nasopharyngeal PCR antigen testing during the study period, of which 26% (n = 18/68) reported positive results (Table 1).
TABLE 1.
Characteristics of obstetrical healthcare workers
| Demographics/Outcomes | n = 150 |
|---|---|
| Median age, years [IQR] | 35 [29.5‐47.5] |
| Race/Ethnicity | |
| African American | 17 (11.3) |
| Caucasian | 125 (83.3) |
| Hispanic Other / Unknown | 8 (5.3) |
| Position | |
| Registered nurse | 95 (63.3) |
| Physician/CNM | 30 (20.0) |
| Administration | 8 (5.3) |
| Technician | 17 (11.3) |
| Primary location | |
| Inpatient | 113 (75.3) |
| Outpatient | 37 (24.7) |
| Tested for Sars‐CoV‐2 (PCR) | 68 (45.3) |
| Positive Sars‐CoV‐2 (PCR) | 18 (12.0) |
aData are reported as median [IQR] or n (%).
Three people did not complete any of the blood draws after volunteering, thus a total of 147 samples were available for analysis in the initial and 4‐week follow‐up blood draw. Six participants in the 12‐week blood draw, and 15 participants in the 6‐month blood draw were lost to follow up due to employment change or failure to present for collection. A total of 126 participants (84%) of those originally enrolled in the study had samples collected at all four time intervals (Figure 1). Sars‐CoV‐2 specific IgM antibodies were present in two participants (1.4%) and nine participants (6.1%) in the initial and 4‐week follow up assessments, respectively. No participants were positive for IGM antibodies in the 12‐week and 6‐month follow‐up assessments. All participants with positive IGM antibodies at any of the blood draws demonstrated presence of IGG antibodies in their subsequent blood draw. The proportion of HCWs with Sars‐CoV‐2 specific IgG antibodies increased sequentially from 2% (n = 3) at baseline to 31% (n = 44) at 12 weeks, before declining to 21% (n = 27) at the 6‐month follow‐up (Figure 2).
FIGURE 1.
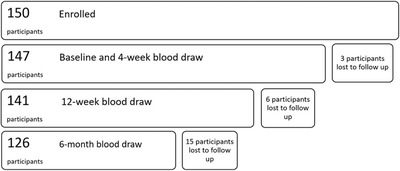
Flowchart of number of participants available for each blood draw period
FIGURE 2.
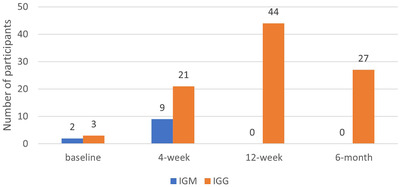
Frequency of Sars‐CoV‐2 specific IGM/IGG antibodies in serial assessments of HCW
Forty‐three participants were positive for Sars‐CoV‐2 specific IgG antibodies on at least one of the first three blood draws and were available for the final 6‐month follow up blood draw. Only 58% (25/43) remained positive at the final blood draw, which was 3–6 months later depending on the timing of initial seropositivity. This included 66% (2/3) of participants that were positive on the baseline assessment, 71% (12/17) of participants initially positive on the 4‐week blood draw, and 48% (11/23) of participants positive on the 12‐week blood draw.
Twenty‐six percent (18/68) of participants who were tested for COVID19 by nasopharyngeal PCR antigen testing for symptoms or exposure reported positive results during the study period. All of these had presence of IgG antibodies on a subsequent blood draw signifying seroconversion. Figure 3 depicts the prevalence of positive IgG antibodies over time with the date of positive PCR test being time point zero. We found in general highest titers were achieved within 1 month of acute infection and titers declined over time, with re‐conversion to seronegative status at the end of our collection period. In total, 27% (5/18) participants reconverted to negative status between 139 and 145 days after positive PCR test.
FIGURE 3.
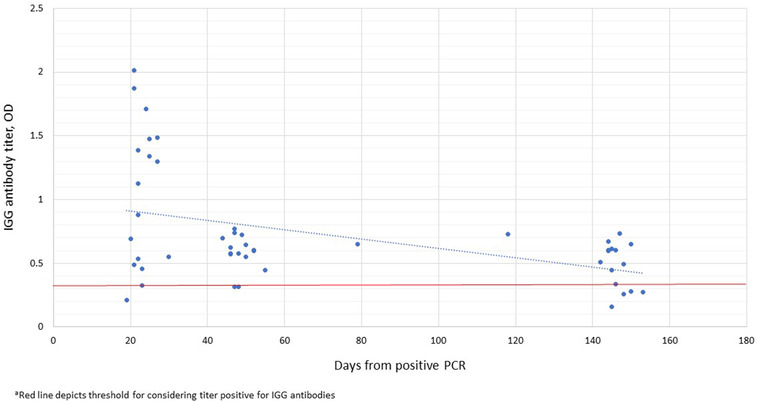
Persistence of Sars‐CoV‐2 specific antibodies from positive nasopharyngeal PCR antigen test
Finally, 55% (83/150) of participants responded to a follow‐up survey about vaccine uptake. Eighty‐six percent (71/83) received both doses of either the Moderna or Pfizer COVID19 vaccine and one participant was planning to but had not yet received the vaccine. Vaccine acceptance in this cohort was 87% (72/83) and did not differ by serum antibody status: whether IGG was present at any time point during the collection period. Of those that received the vaccine, 17% (12/71) experienced systemic side effects with the first injection, and 69% (49/71) with the second. Those that had Sars‐CoV‐2 specific IgG antibodies present prior to vaccination at any of the collection time points were more like to experience symptoms with the first injection (36.8% vs. 9.6%, p = .012), however there was no difference in the rates of vaccine side effects with the second injection regardless of antibody status (Table 2).
TABLE 2.
Comparison of vaccine uptake and side effects by antibody status
| Positive IgG (N = 24) | Negative IgG (N = 59) | p‐value | |
|---|---|---|---|
| Acceptance of vaccine (n = 83) | |||
| Yes (N = 72) | 79.1% (19/24) | 91.4% (53/59) | .07 |
| No (N = 11) | 20.8% (5/24) | 10.2% (6/59) | |
| Side effects with first injection (n = 71) | |||
| Yes (N = 12) | 36.8% (7/19) | 9.6% (5/52) | .01 |
| No (N = 59) | 63.1% (12/19) | 90.4% (47/52) | |
| Side effects with second injection (n = 71) | |||
| Yes (N = 49) | 73.7% (14/19) | 67.3% (35/52) | .60 |
| No (N = 22) | 26.3% (5/19) | 32.7% (17/52) | |
aConsideredpositive for Sars‐CoV‐2 specific IgG antibodies if present at any time point during collection period.
Side effectsto the vaccine were considered positive if they included systemic symptoms such as fever, chills, myalgias, or fatigue. Localized symptoms such as arm pain or injection irritation were considered an expected reaction and classified as negative.
cOne participant reported willingness to get the vaccine however had not yet received it at the time of this reporting
4. DISCUSSION
Our results support previous reports demonstrating a rapid decline of serum Sars‐CoV‐2 specific IGG antibodies over time, and high rates of reconversion to seronegative status despite previous seropositivity. 8 , 9 , 10 This finding is concerning particularly due to the high incidence of vaccine hesitancy and decreasing daily vaccination rates in the United States, 2 , 3 and further supports the recommendation to vaccinate those with prior COVID19 infection. Interestingly, our cohort demonstrates a high rate of vaccine acceptance regardless of antibody status; however, those with positive antibodies were more likely to experience systemic side effects to the first vaccine injection.
With the emergence of more deadly COVID19 variant strains, vaccination offers cross‐protection, which may not be present with natural immunity. 17 Long‐term immunologic data with SARS‐CoV‐2 are limited, however data have been collected on other viruses within the coronavirus family such as the SARS‐CoV virus responsible for the SARS epidemic in 2003. Follow up data from this epidemic are mixed, and ranges from persistence of specific IgG antibodies at 1 year, 18 to significant decline in antibody titers by 3 years. 19 More concerning is the high re‐infection rate in the familial seasonal coronaviruses after 12 months. 20 To this end, it is necessary to understand the robustness and longevity of the immune response from natural infection with COVID19 to improve vaccination uptake and aid in future COVID19 public health measures.
A major strength of our study is that it follows a cohort of HCWs longitudinally with serial titers over a 6‐month time period. There are few studies that have followed Sars‐CoV‐2 specific IgG antibodies in a single cohort for a duration up to 6 months. These data are immensely important to the ever‐evolving COVID19 health crisis and implementation of health policy. Additionally, our baseline blood draw coincided with the arrival of the Sars‐CoV‐2 virus in our region, and an outbreak on our obstetrical unit. Thus, our cohort contains a large proportion of individuals with positive COVID19 nasopharyngeal PCR results and subsequent seropositive IgG antibody results early in the study period.
Our study is limited by the fact that it is a convenience sample and thus made up entirely of female identifying participants. Demographic and Sars‐CoV‐2 testing, and exposure data were collected via voluntary surveys which is subject to bias. Additionally, our study demonstrates high rates of vaccine acceptance among HCW similar to others 21 ; however, those that responded to the follow‐up survey may have been more inclined to respond if they already received the vaccine.
In conclusion, Sars‐CoV‐2 specific IGG antibodies wane over time and may not provide prolonged and robust immune protection underscoring the importance of vaccination and continued research in this area while the COVID19 pandemic continues.
ACKNOWLEDGMENTS
M.M. Costantine is supported by a grant from The Eunice Kennedy Shriver National Institute of Child Health and Human Development (5 UG1 HD027915‐29) and the National Heart, Lung, and Blood Institute (1UG3HL140131‐01). This paper does not necessarily represent the official views of the NICHD, NHLBI, or the National Institute of Health.
Kiefer MK, Allen KD, Russo JR, et al. Decline in Sars‐CoV‐2 antibodies over 6 month follow up in Obstetrical Healthcare workers. Am J Reprod Immunol. 2021;86:e13490. 10.1111/aji.13490
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Centers for Disease Control and Prevention. COVID‐19: Prevent Getting Sick . https://www.cdc.gov/coronavirus/2019‐ncov/prevent‐getting‐sick/index.html Accessed June 21, 2021
- 2. Centers for Disease Control and Prevention . COVID Data Tracker: Trends in Number of COVID‐19 Vaccinations in the US. https://covid.cdc.gov/covid‐data‐tracker/#vaccination‐trends Accessed June 21, 2021
- 3. Meyer MN, Gjorgjieva T, Rosica D. Trends in health care worker intentions to receive a COVID‐19 vaccine and reasons for hesitancy. JAMA Netw Open. 2021;4(3):e215344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harvey RA, Rassen JA, Kabelac CA, et al. Association of Sars‐CoV‐2 seropositive antibody test with risk of future infection. JAMA Intern Med. 2021;181(5):672‐679. PMID: 33625463; PMCID: PMC7905701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lumley SF, O'Donnell D, Stoesser NE, et al, Oxford University Hospitals Staff Testing Group . Antibody status and incidence of Sars‐CoV‐2 infection in health care workers. N Engl J Med. 2021;384(6):533‐540. Epub 2020 Dec 23. PMID: 33369366; PMCID: PMC7781098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with Sars‐CoV‐2. JAMA. 2021;325(14):1467‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O Murchu E, Byrne P, Walsh KA, et al. Immune response following infection with Sars‐CoV‐2 and other coronaviruses: a rapid review. Rev Med Virol. 2021;31(2):e2162. Epub 2020 Sep 23. PMID: 32964627; PMCID: PMC7536965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following Sars‐CoV‐2 infection in humans. Nat Microbiol. 2020;5:1598‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Self WH, Tenforde MW, Stubblefield WB, et al. Decline in Sars‐CoV‐2 antibodies after mild infection among frontline health care personnel in a multistate hospital network – 12 states, April‐August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1762‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel MM, Thornburg NJ, Stubblefield WB, et al. Change in antibodies to Sars‐CoV‐2 over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020;324(17):1781‐1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid decay of anti‐Sars‐CoV‐2 antibodies in persons with mild Covid‐19. N Engl J Med. 2020;383(11):1085‐1087. Epub 2020 Jul 21. Erratum in: N Engl J Med. 2020 Jul 23;: PMID: 32706954; PMCID: PMC7397184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lumley SF, Wei J, O'Donnell D, et al, Oxford University Hospitals Staff Testing Group . The duration, dynamics and determinants of Sars‐CoV‐2 antibody responses in individual healthcare workers. Clin Infect Dis. 2021:ciab004. Epub ahead of print. PMID: 33400782; PMCID: PMC7929225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903‐1915. PMID: 17989383. [DOI] [PubMed] [Google Scholar]
- 14. Maine GN, Lao KM, Krishnan SM, et al. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID‐19 patients using the Abbott Architect. J Clin Virol. 2020;133:104663. Epub 2020 Oct 27. PMID: 33161369; PMCID: PMC7590643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kiefer MK, McKiever ME, Russo JR, et al. Exposure and seroconversion to severe acute respiratory syndrome coronavirus 2 among obstetrical healthcare providers following a contained outbreak. Am J Obstet Gynecol. 2020;223(4):601‐603.e2. Epub 2020 Jun 15. PMID: 32553914; PMCID: PMC7295482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Epitope Diagnostics, Inc . COVID‐19, Diagnostic immunoassay solutions for coronavirus detection. 2020. https://eaglebio.com/wp‐content/uploads/data‐pdf/EagleBio‐COVID‐19‐ELISA‐Assay‐04.15.20.pdf Accessed May 18, 2020
- 17. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with Sars‐CoV‐2 variants. N Engl J Med. 2021:NEJMoa2105000. Epub ahead of print. PMID: 33882219; PMCID: PMC8117968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang SC, Wang JT, Huang LM, et al. Longitudinal analysis of severe acute respiratory syndrome (SARS) coronavirus‐specific antibody in SARS patients. Clin Diagn Lab Immunol. 2005;12(12):1455‐1457. PMID: 16339072; PMCID: PMC1317065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH. Disappearance of antibodies to SARS‐associated coronavirus after recovery. N Engl J Med. 2007;357(11):1162‐1163. PMID: 17855683. [DOI] [PubMed] [Google Scholar]
- 20. Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short‐lasting. Nat Med. 2020;26:1691‐1693. [DOI] [PubMed] [Google Scholar]
- 21. Dzieciolowska S, Hamel D, Gadio S, et al. Covid‐19 vaccine acceptance, hesitancy, and refusal among Canadian healthcare workers: a multicenter survey. Am J Infect Control. 2021. S0196‐6553(21)00274‐1. 10.1016/j.ajic.2021.04.079. Epub ahead of print. PMID: 33930516; PMCID: PMC8079260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


