Abstract
Background
Vitamin D deficiency has been linked to the increased severity of numerous viral infections.
Objective
To assess whether vitamin D supplementation is safe and effective for the treatment of COVID‐19.
Methods
We searched MEDLINE, EMBASE, CENTRAL, LILACS and LOVE for randomised controlled trials (RCTs) published up to 2 March evaluating the effects of vitamin D for the treatment of coronavirus disease (COVID‐19). Two authors selected the studies and analysed the data evidence following Cochrane Recommendations.
Results
We included three RCTs with a total of 385 participants. We found low certainty evidence indicating that hospitalised patients under calcifediol plus standard care (SC) treatment seem to present a significantly lower risk of being admitted to ICU but no difference in mortality. We found low to very low certainty evidence that the improvement in fibrinogen levels is slightly greater in mildly symptomatic or asymptomatic patients with COVID‐19 that used cholecalciferol plus SC than in those treated with placebo plus SC (mean difference), and the patients who used cholecalciferol plus SC achieved more SARS‐CoV‐2 negativity, but not on d‐dimer, c‐reactive protein (CRP) or procalcitonin compared with the patients in the placebo plus SC group. We also found low to moderate certainty evidence that a single high dose of vitamin D does not seem to be effective for reducing mortality, length of hospital stay, ICU admissions and d‐dimer or CRP levels when used in patients with moderate to severe COVID‐19.
Conclusions
As a practical implication, the use of vitamin D associated with SC seems to provide some benefit to patients with COVID‐19. However, the evidence is currently insufficient to support the routine use of vitamin D for the management of COVID‐19, as its effectiveness seems to depend on the dosage, on the baseline vitamin D levels, and on the degree of COVID‐19 severity.
Review criteria
This is the first systematic review applying the principles of evidence‐based medicine to find out a possible benefit of the use of vitamin D in patients with COVID‐19.
The quality of evidence was evaluated following the GRADE approach.
Rapid systematic review methodology following the recommendations proposed by the Cochrane Handbook.
Message for the clinic
The limited evidence based on two randomised clinical trials shows that vitamin D may have a positive effect on fibrinogen levels, viral clearance and length of Intensive Care Unit stay in patients with COVID‐19. However, the evidence is currently insufficient to support the routine use of vitamin D for the management of COVID‐19.
1. INTRODUCTION
Coronavirus disease (COVID‐19) shook the social, individual, economic and health systems worldwide. Since its first confirmed case in December 2019, COVID continues to spread very quickly and become fatal. As of 27 September 2020, almost 33 million cases were confirmed and over 1 million deaths occurred worldwide. 1 Never before has a virus led to a crisis that demanded so much individual and collective contribution to be overcome and with such urgency.
In response to the current coronavirus disease (COVID‐19) pandemic, extraordinary efforts have been made by researchers on trying to find effective interventions. However, there is no effective treatment yet. In the meantime, the hypothesis on whether vitamin D deficiency could play a role in increasing the risk of dying from COVID‐19 have arisen. 2 , 3 , 4 In line with this hypothesis, a recent meta‐analysis using about 10 000 individual participants data from 25 randomised controlled trials (RCT), concluded that vitamin D supplementation reduced the risk of upper respiratory infections by about 19%. 5
Of note, evidence from non‐randomised studies on the treatment of COVID‐19 with vitamin D has recently gained traction. Simultaneously, many physicians have held onto any thread of hope—not least because the panorama scares. However, the evidence on the effectiveness of vitamin D in patients with COVID‐19 cannot rely on observational studies, as they are tempered by the risk of bias, especially arising from selection bias. 6 Relying on non‐randomised studies’ results may lead to spurious associations and to the introduction of potentially hazardous interventions into the clinical practise mainly by the introduction of confounding factors into the comparative groups. 6 For instance, Panarese and Shanini reported that living in Northern countries—where vitamin D deficiency is more prevalent—is associated with a higher hospitalisation rate and mortality rate when infected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) compared to other countries. 4 Similarly, Ilie et al have reported that mean levels of vitamin D are negatively correlated with the number of COVID‐19 cases. 7 Epidemiology studies have reported that mortality and severity of COVID‐19 are highly prevalent in older people, 8 in whom vitamin D deficiency is also more likely. 9 It is unknown whether other factors, such as age‐related comorbidities would be the real mediators of worsening the clinical course of patients with COVID‐19, or otherwise, vitamin D would actually influence outcomes of patients with COVID‐19. Therefore, analysing the results of randomised controlled trials (RCTs) and the differences in using vitamin D in patients with different degrees of severity is critical for providing useful information upon which to base clinical decision‐making processes.
2. METHODS
2.1. Study design
This present article describes a rapid systematic review that followed the recommendations proposed by the Cochrane Handbook. 10 This review was performed at the Medical School of a public university in São Paulo (SP), Brazil. We performed rapid systematic review methods by not independently conducting our screening of titles and abstracts. 10
2.2. Criteria for including studies for this review
2.2.1. Type of studies
We included RCTs of parallel design that have employed individual allocation. Cross‐over design studies were also considered for inclusion. No language restrictions were applied in our eligibility criteria. We excluded all other study designs.
2.2.2. Type of participants
Patients without age restriction are diagnosed with COVID‐19 by any laboratory test [eg, polymerase chain reaction (PCR) testing or serology assay to detect IgG, IgA and IgM].
2.2.3. Type of interventions
We included RCTs that evaluated vitamin D supplementation in patients with COVID‐19. We prioritised hard endpoints related to deleterious consequences of COVID‐19, such as respiratory derangement, inflammatory response, intensive care unit (ICU) admission, the need for invasive mechanical ventilation, among others. Vitamin D interventions or its analogues used alone or in combination with other interventions were considered, as long as the vitamin D effect could be estimated compared with other study groups. We did not restrict our criteria to any route, dosage, duration or timing of administration.
2.2.4. Outcomes
Primary outcomes were:
Duration of invasive mechanical ventilation
Mortality rate
Adverse events
Secondary outcomes were:
Symptoms (change in severity and duration of symptoms)
Length of ICU stay
ICU admission
Length of hospital stay
Inflammatory response
Viral clearance
2.3. Search strategy
We conducted a systematic search on the literature on 2 March 2020 in the following databases: Medline via PubMed, Embase via Elsevier, Cochrane Library—Cochrane Central Register of Controlled Trials (CENTRAL), Portal Regional BVS—LILACS and LOVE platform which comprises studies on COVID‐19 from 41 databases, including preprints databases such as medRxiv and bioRxiv. We searched the RCT registry database (www.clinicaltrials.gov) to find additional published trials. Studies published in any language since November 2019 were considered for inclusion. The search strategies are shown in Data S1.
2.4. Data analysis
We estimated the effects of vitamin D treatments in each of the available results for our predefined outcomes. Relative risks (RR) with their 95% confidence intervals (CI) were estimated using the Review Manager 5.4.1 software.
2.5. Assessment of risk of bias in included studies
We assessed the methodological quality of each included study using the risk of bias (RoB 2.0) table per the Cochrane Collaboration recommendations. 6 We evaluated the following domains: risk of bias arising from the randomisation process, risk of bias as a result of deviations from the intended interventions (effect of assignment to intervention), missing outcome data, risk of bias in the measurement of the outcome, risk of bias in selection of the reported result and overall risk of bias. Each study was evaluated on all six domains, assigning the classifications “low risk of bias,” “some concerns of risk of bias” or “high risk of bias” to each domain.
2.6. Quality of the body of the evidence
We used the GRADE approach to classify the strength of evidence as high, moderate, low or very low. 11 We evaluated the following criteria: risk of bias, inconsistency, imprecision and indirectness. We created the summary of findings table considering the primary outcomes from comparisons using the GRADEpro platform.
3. RESULTS
Our database search strategies yield 578 records. After the two‐stage screening process, we excluded duplicated reports and that were clearly irrelevant or not directly related to the review question. We assessed 31 full‐text studies for further scrutiny. Three of them fulfilled our eligibility criteria. The PRISMA flow diagram is shown in Figure 1.
FIGURE 1 .
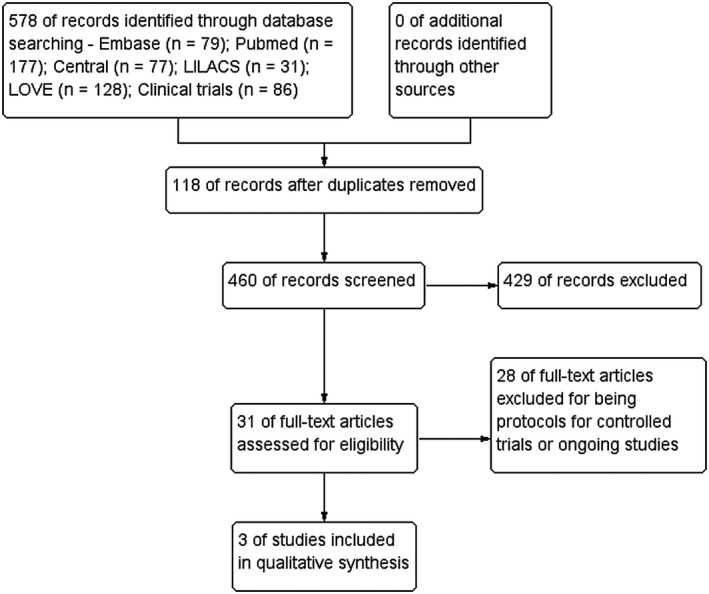
Flow diagram of study selection
3.1. Included studies
We included three parallel, randomised, double‐masked pilot clinical trials (Table 1). As the studies included very different degrees of COVID‐19 severity and pharmacological characteristics (combination with other treatments, dosage and schedules), pooling their results together was not possible. One study 12 evaluated the effect of calcifediol treatment on ICU admission and mortality rate among Spanish patients hospitalised for COVID‐19. All hospitalised patients received standard care (as per hospital protocol), including a combination of hydroxychloroquine (400 mg every 12 hours on the first day, and 200 mg every 12 hours for the following 5 days), azithromycin (500 mg orally for 5 days) and for patients with pneumonia and NEWS score ≥5, ceftriaxone 2 g intravenously every 24 hours for 5 days was added to hydroxychloroquine and azithromycin. The RCT by Rastogi et al 13 included participants asymptomatic or mildly symptomatic SARS‐CoV‐2 RNA positive vitamin D deficient (25(OH) D < 20 ng/mL) individuals. Patients requiring invasive ventilation or with significant comorbidities were excluded. All the participants received standard care for the SARS‐CoV‐2 infection and pre‐existing comorbidities. Murai et al 14 conducted a multicenter trial in Brazil and investigated the effect of a single high dose of vitamin D3 in hospitalised patients with moderate to severe COVID‐19. Participants were randomly assigned to receive a single oral dose of 200 000 IU of vitamin D3 or a placebo.
TABLE 1.
General characteristics of included studies
| Study | Castillo (2020) | Rastogi (2020) | Murai (2021) |
|---|---|---|---|
| Population | N: 76 | N: 69 | N: 240 |
| (I: 50/C: 26) | (I: 35/C: 34) | (I: 120/C: 120) | |
| Age: 53 ± 10 | Age: 48.8 ± 9.19 | Age: 56.2 ± 14.4 | |
| Intervention | 0.532 mg | 60 000 IU of cholecalciferol (oral nano‐liquid droplets) | 200 000 IU of vitamin D3 (dissolved in a 10‐mL peanut oil solution) |
| calcifediol (Day 1) | |||
| 0.266 mg calcifediol Days 3 and 7 and weekly (until discharge or ICU admission) | |||
| Control | Standard care | Placebo | Placebo |
| Outcomes | ICU admission; mortality | SARS‐CoV‐2 viral clearance inflammatory markers—fibrinogen, D‐dimer, procalcitonin and c‐reactive protein (CRP) | Hospital length of stay, mortality, number of patients admitted to the intensive care unit; the number of patients who needed mechanical ventilation; the duration of mechanical ventilation and serum levels of 25‐hydroxyvitamin D, total calcium, creatinine, C‐reactive protein (crp) |
| Follow up | Until discharge or ICU admission | 7 days | Single dose |
Abbreviations: C, control group; I, Intervention group; N, number of participants.
3.2. Excluded studies
We excluded 28 studies 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 as they were ongoing studies, with no results available.
3.3. Assessment of risk of bias in included studies
There was some concern for the overall risk of bias in the Entreans‐Castillo 2020 study 12 as it is not clearly described how the randomisation process was concealed and how interventionists were blind to the allocation of the interventions. The overall risk of bias in the Rastogi et al’s study 13 was judged as high as the placebo used in the study was not exactly matched with regards to the taste and consistency with the cholecalciferol nano‐formulation and possible bias arising from the randomisation process and from the blinding of outcome assessors. Murai et al 14 was the only study judged as having an overall low risk of bias. The risk of each study is presented in Figure 2.
FIGURE 2.
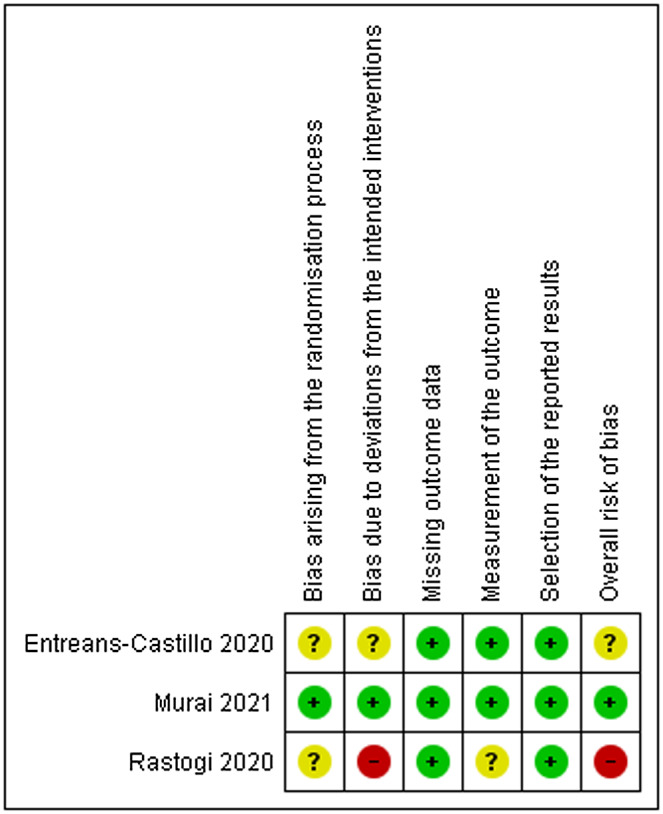
Risk of bias summary
3.4. Certainty of evidence
We rated the certainty of evidence using the GRADE approach. 11 We found low certainty of evidence for all the reported outcomes. We downgraded one level resulting from methodological limitation and one level resulting from the imprecision of the estimated effects, except for D‐dimer, which we downgraded one level resulting from methodological limitation and two levels resulting from the imprecision of the estimated effects.
3.5. Effects of intervention
To provide graphical visualisation of the results and to calculate mean differences between groups and risk ratios, we estimated the mean and SD of all outcomes by using median and interquartile ranges from primary studies as recommended by Wan et al 43 and Cochrane Handbook.
3.5.1. Mortality
We were not able to find any difference in mortality between using calcifediol plus standard care in patients with COVID‐19 compared with using standard care alone [risk ratio (RR) 0.11; 95% confidence interval (CI) 0.01 to 2.13]—low certainty of evidence (Figure 3). No difference was also found on mortality between using vitamin D compared with placebo [RR 1.49; 95% CI 0.55 to 4.05]—low certainty of evidence (Figure 4).
FIGURE 3.
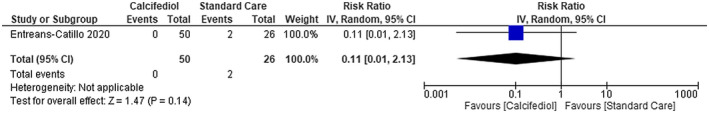
Mortality in COVID‐19 patients under calcifediol treatment and standard care
FIGURE 4.
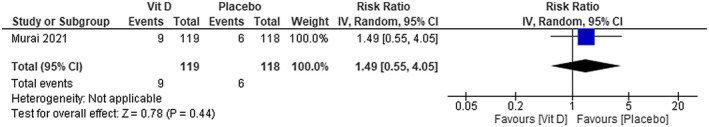
Mortality in COVID‐19 patients under vitamin D treatment and placebo
3.5.2. ICU admission
Patients with COVID‐19 that used calcifediol plus standard care presented a significantly lower risk of being admitted to ICU compared with those receiving standard care alone [RR 0.04; 95% CI 0.01 to 0.29]—low certainty of evidence (Figure 5). We were not able to find any difference on ICU admission between using vitamin D compared with placebo [RR 0.75; 95% CI 0.44 to 1.29]—moderate certainty of evidence (Figure 6).
FIGURE 5.
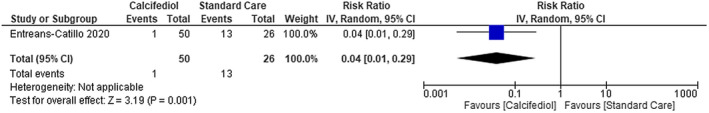
Intensive Care Unit admissions in patients with COVID‐19 under calcifediol treatment and standard care
FIGURE 6.
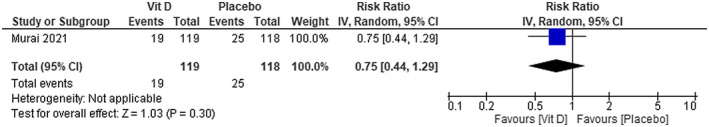
Intensive Care Unit admissions in patients with COVID‐19 under vitamin D treatment and placebo
3.5.3. Duration of invasive mechanical ventilation
Murai et al 14 reported only the mean difference (MD) between groups (MD 2.2 d, 95% CI –8.4 to 12.8)—low certainty of evidence. No difference was found between using vitamin D compared with using placebo on the duration of invasive mechanical ventilation.
3.5.4. Length of hospital stay
We were not able to find any difference between using vitamin D compared with placebo on the length of hospital stay (MD −1.30; 95% CI −2.63 to 0.03)—low certainty of evidence (Figure 7).
FIGURE 7.
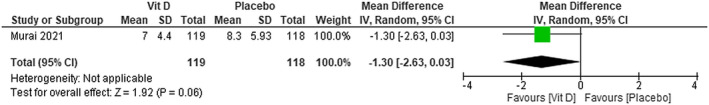
Length of hospital stay in patients with COVID‐19 under vitamin D treatment and placebo
3.5.5. Inflammatory markers
D‐dimer (g/L)
We were not able to find any difference in D‐dimer levels between using cholecalciferol plus standard care in patients with COVID‐19 compared with using placebo plus standard care (MD −47,20; 95% CI −124.87 to 30.47)—very low certainty of evidence (Figure 8).
FIGURE 8.
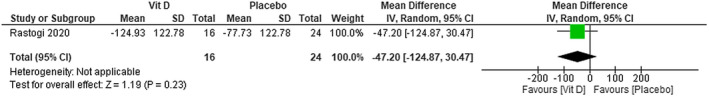
Change in D‐dimer (g/L) in patients with COVID‐19 under cholecalciferol treatment and placebo
In Murai et al’s study, 14 no difference was found between using vitamin D compared with using placebo on D‐dimer levels (MD 17.30; 95% CI −145.97 to 180.57)—moderate certainty of evidence (Figure 9).
FIGURE 9.
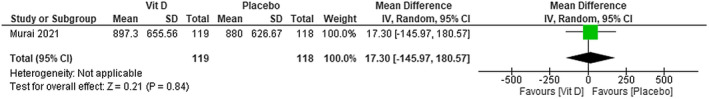
Change in D‐dimer (g/L) in patients with COVID‐19 under vitamin D treatment and placebo
The improvement in fibrinogen levels is slightly greater in patients with COVID‐19 that used cholecalciferol plus standard care than in those treated with placebo plus standard care. The MD is −0.95; 95% CI [−1.48 to −0.42]—low certainty of evidence (Figure 10).
FIGURE 10.
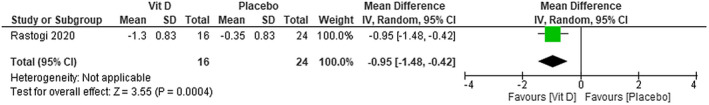
Change in fibrinogen (ng/mL) in patients with COVID‐19 under cholecalciferol treatment and placebo
We were not able to find any difference in CRP levels between using cholecalciferol plus standard care in patients with COVID‐19 compared with placebo plus standard care (MD −0.30; 95% CI −1.18 to 0.58)—low certainty of evidence (Figure 11). No difference was found between using vitamin D compared with using placebo on CRP levels (MD 0.13; 95% CI −2.99 to 3.25)—moderate certainty of evidence (Figure 12).
FIGURE 11.
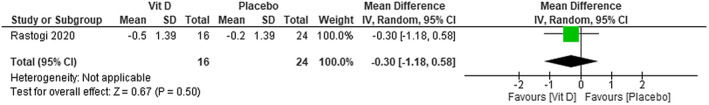
Change in CRP (ng/mL) in patients with COVID‐19 under cholecalciferol treatment and placebo
FIGURE 12.
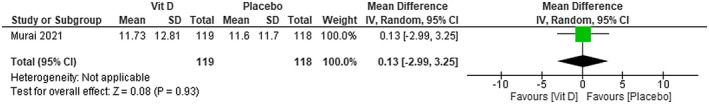
Change in CRP (ng/mL) in patients with COVID‐19 under vitamin D treatment and placebo
We were not able to find any difference on procalcitonin levels between using cholecalciferol plus standard care in patients with COVID‐19 compared with placebo plus standard care (MD 0.39; 95% CI −0.28 to 1.06)—low certainty of evidence (Figure 13).
FIGURE 13.
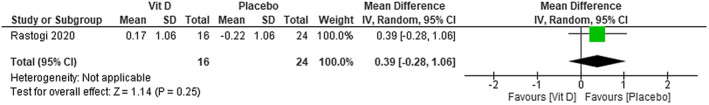
Change in procalcitonin (mg/L) in patients with COVID‐19 under cholecalciferol treatment and placebo
The patients that used cholecalciferol plus standard care achieved more SARS‐CoV‐2 negativity compared with the patients in the placebo plus standard care group. The RR is 3.00; 95% CI [1.26 to 7.14] (Figure 14)—low certainty of evidence.
FIGURE 14.
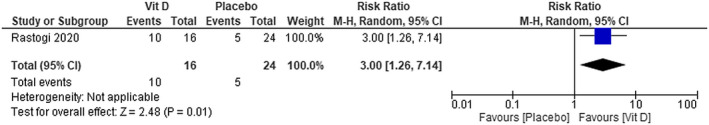
SARS‐CoV‐2 viral clearance in patients with COVID‐19 under cholecalciferol treatment and placebo
4. DISCUSSION
This is the first systematic review applying the principles of evidence‐based medicine to find out a possible benefit of the use of vitamin D in patients with COVID‐19. We found low certainty of evidence from one RCT demonstrating that the use of calcifediol in association with standard care is effective for the reduction of ICU need in hospitalised patients with COVID‐19. Additionally, we found low certainty from other RCT showing that the use of cholecalciferol seems to reduce fibrinogen levels and lead to faster SARS‐CoV‐2 negativity compared with patients under standard care alone. We also found low to moderate certainty of evidence that a single high dose of vitamin D does not seem to be effective for reducing mortality, length of hospital stay, ICU admissions and D‐dimer or CRP levels when used in patients with moderate to severe COVID‐19.
Our results are in line with the hypothesis of several studies that have suggested that vitamin D may play a role in SARS‐CoV‐2 infection. 44 However, it may depend on the degree of severity of the disease. From a mechanistic standpoint, the hypothesis that vitamin D favourably modulates host responses to SARS‐CoV‐2 has relevant plausibility, as there may be many potential links between respiratory viral infections such as COVID‐19 and vitamin D status.
The main biological reason for the use of vitamin D in patients with COVID‐19 lies in its possible immunological effects through a reduction in the inflammatory response. Vitamin D has been reported to decrease the production of Th1 cells. 45 Therefore, it may help suppress the progression of inflammation by reducing the production of inflammatory cytokines. Of note, the release of inflammatory biomarkers leads to cytokine storms and the worst prognosis in patients with COVID‐19. 46 Additionally, vitamin D has been reported to exert a protective effect on alveolar epithelial cells, to preserve endothelial integrity, as well as to induce expression of Angiotensin‐converting enzyme 2 (ACE‐2). 47 ACE‐2 expression has also been suggested to play an important role in the pathophysiological mechanisms of SARS‐CoV‐2 infection. 48 All cells of the immune system and also of the lung epithelium can express the vitamin D receptor and may be important targets for the vitamin D endocrine system. 49 Any of these mechanisms could explain the association of vitamin D deficiency with the severity of COVID‐19.
Although plausibility is apparent, only three RCTs on the effects of vitamin D in patients with COVID‐19 are currently available. Of note, the currently available evidence is provided by three distinct studies, with heterogeneous populations and indicated fragmented results. Furthermore, for two of the studies, 12 , 13 there were some concerns or a high risk of bias. The clinical heterogeneity and different outcomes assessed across studies did not allow us to pool data from the included studies. The use of cholecalciferol seems to indicate a small effect on fibrinogen levels and viral clearance in mildly symptomatic or asymptomatic COVID‐19 patients, but not on D‐dimer, CRP and procalcitonin levels. Although the pro‐inflammatory and prothrombotic status of COVID‐19 has been increasingly investigated and the monitoring of coagulation parameters such as fibrinogen and D‐dimer has become protocols for inpatients, the slight increase in fibrinogen levels showed in this review does not help in current clinical decision making. Additionally, the use of calcifediol seems to reduce ICU need, but not mortality in hospitalised patients with COVID‐19. The difference between groups on ICU need, but not on mortality, or in other clinically relevant outcomes raise questions not only on the magnitude of the effect of vitamin D but also on the clinical utility of its use. The absence of difference on important clinical outcomes, when used in patients with moderate to severe COVID‐19, shed some light on the need for further studies evaluating the effects of different doses of vitamin D in the various degrees of severity and clinical characteristics of COVID‐19 patients.
Even though the current evidence has been assessed through rigorous methodological appraisal, the reported results should be interpreted with caution while analysing these data in decision‐making processes, as the included studies 12 , 13 have several limitations. Regarding the Entreans‐Castillo study, 12 it should be underscored that it is not a placebo‐controlled trial. Secondly, the authors did not evaluate the possible role of obesity as a risk factor for a worse prognosis. Thirdly, it is striking to note that serum calcifediol concentrations at baseline were not reported. Therefore, we cannot analyse whether baseline vitamin D status modifies these results. Also, calcifediol, known as calcidiol, 25‐hydroxy‐cholecalciferol or 25‐hydroxy‐vitamin D, is the main circulating metabolite of vitamin D3. The use of calcifediol in this study instead of native vitamin D should also be underscored, as calcifediol may have a more reliable intestinal absorption and can rapidly restore serum concentrations of 25OHD compared with vitamin D. 49 Additionally, as there are only three RCTs—a low power to detect differences between groups. For instance, for mortality, only two events were observed and a greater probability of type II error exists. Finally, the adverse events were not evaluated in the included clinical trial. Therefore, we cannot evaluate the safety of vitamin D in patients with COVID‐19. In Rastogi et al, 13 only mildly symptomatic and asymptomatic individuals were enrolled in the study which limits the generalizability of the results to symptomatic or severe cases of COVID‐19. In terms of blinding, the placebo used in the study was not exactly matched with regards to the taste and consistency with the cholecalciferol nano‐formulation. This can increase the chances of performance and detection bias and it may compromise the quality of the results. Finally, although the study did not mention any reports of toxicity among patients, the dose of cholecalciferol used in the study is high compared with conventional treatment with vitamin D, which may raise concerns over the medium and long‐term toxic effects of vitamin D. It is especially important because extreme vitamin D supplementation may induce hypercalcemia, hypercalciuria and hyperphosphatemia, which are considered to be the initial signs of vitamin D intoxication and may result in impaired organ function even in hypovitaminosis D. 50 In Murai et al’s study, 14 relatively low sample size was used. Therefore, the trial could have had inadequate power to exclude small, but clinically meaningful differences between the groups. Of interest, the percentage of patients with vitamin D deficiency included in this trial was relatively low, which seems to be important clinical information to be further analysed.
These questions may be answered by the 28 ongoing studies 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 evaluating the effects of vitamin D in patients with COVID‐19 found through our comprehensive search strategies. We believe that these RCTs will provide high‐quality evidence and a representative sample from which more precise effects will be estimated. Furthermore, these studies may be able to provide reliable evidence upon which to base clinical decision‐making processes, covering the gaps on the effects of vitamin D used alone or combined to other treatments, best dosage, the timing of usage and characteristics of patients who are more likely to benefit from vitamin D treatment.
5. CONCLUSIONS
As a practical implication, the use of vitamin D in association with standard care seems to provide some benefit to patients with COVID‐19. However, the evidence is currently insufficient to support the routine use of vitamin D for the management of COVID‐19, as its effectiveness seems to depend on the dosage, on the baseline vitamin D levels, and on the degree of severity of the disease. So far, the safety of vitamin D in patients with SARS‐CoV‐2 infection is still considered uncertain and no conclusions can be drawn. The results of the ongoing high‐quality RCTs are necessary to provide more precise and reliable information on the proper use of vitamin D in patients with COVID‐19 and to guide clinical decision‐making processes.
DISCLOSURE
None.
AUTHOR CONTRIBUTIONS
AR, ACPNP: collected the data and performed the statistical analysis and assessed the risk of bias of the included studies; MESP: search strategy; AR, ACPNP, JMA and ALRP: wrote the discussion and conclusion; AR, ACPN and ANA: drafted the final review and reviewed the text.
Supporting information
Supplementary Material
da Rocha AP, Atallah AN, Aldrighi JM, Pires ALR, dos Santos Puga ME, Pinto ACPN. Insufficient evidence for vitamin D use in COVID‐19: A rapid systematic review. Int J Clin Pract. 2021;75:e14649. 10.1111/ijcp.14649
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary material.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19). Weekly Epidemiological Update. Data as received by WHO from national authorities, as of 27 September 2020, 10 am CEST 2020. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200928‐weekly‐epi‐update.pdf?sfvrsn=9e354665_6
- 2. Mardani R, Alamdary A, Mousavi Nasab SD, Gholami R, Ahmadi N, Gholami A. Association of vitamin D with the modulation of the disease severity in COVID‐19. Virus Res. 2020;289:198148. 10.1016/j.virusres.2020.198148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hastie CE, Pell JP, Sattar N. Vitamin D and COVID‐19 infection and mortality in UK Biobank. Eur J Nutr. 2020;26:1‐4. 10.1007/s00394-020-02372-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panarese A, Shahini E. Letter: Covid‐19, and vitamin D. Aliment Pharmacol Ther. 2020;51:993‐999. 10.1111/apt.15752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ. 2017;356:i6583. 10.1136/bmj.i6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions (2nd edn). John Wiley & Sons; 2019. [Google Scholar]
- 7. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clinical and Experimental Research. 2020;32(7):1195‐1198. 10.1007/s40520-020-01570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao H‐L, Huang Y‐M, Huang Y. Mortality in older patients with COVID‐19. J Am Geriatr Soc. 2020;68:1685‐1687. 10.1111/jgs.16649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boettger SF, Angersbach B, Klimek CN, et al. Prevalence and predictors of vitamin D‐deficiency in frail older hospitalized patients. BMC Geriatr. 2018;219. 10.1186/s12877-018-0919-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garritty C, Gartlehner G, Kamel C, et al. Cochrane Rapid Reviews Interim Guidance from the Cochrane Rapid Reviews Methods Group 2020. https://methods.cochrane.org/rapidreviews/sites/methods.cochrane.org.rapidreviews/files/public/uploads/cochrane_rr_‐_guidance‐23mar2020‐v1.pdf
- 11. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Entreans‐Castillo M, Costa LME, Barrios JMV, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID‐19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. 10.1016/j.jsbmb.2020.105751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rastogi A, Bhansali A, Khare N, et al. Short term, high‐dose vitamin D supplementation for COVID‐19 disease: a randomised, placebo‐controlled, study (SHADE study). Postgrad Med J. 2020;12. 10.1136/postgradmedj-2020-139065 [DOI] [PubMed] [Google Scholar]
- 14. Murai IH, Fernandes AL, Sales LP, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID‐19: a randomized clinical trial. JAMA, 325, 1053‐1060. 10.1001/jama.2020.26848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NCT04344041. Cholecalciferol to Improve the Outcomes of COVID‐19 Patients (CARED). https://clinicaltrials.gov/ct2/show/NCT04411446?term=NCT04411446&draw=2&rank=1
- 16. Beigmohammadi MT, Bitarafan S, Hoseindokht A, et al. Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus‐19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):614. 10.1186/s13063-020-04547-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. NCT04344041. COvid‐19 and vitamin D supplementation: a multicenter randomized controlled trial of high dose versus standard dose vitamin D3 in high‐risk COVID‐19 patients (CoVitTrial). https://clinicaltrials.gov/ct2/show/NCT04344041
- 18. NCT04351490. Impact of zinc and vitamin D3 supplementation on the survival of aged patients infected with COVID‐19. https://clinicaltrials.gov/show/NCT04351490
- 19. NCT04395768. International alliance study of therapies to prevent progression of COVID‐19. https://clinicaltrials.gov/ct2/show/NCT04395768
- 20. NCT04476745. The effect of D3 on selected cytokines involved in cytokine storm in the Covid‐19 uninfected Jordanian people. https://clinicaltrials.gov/show/NCT04476745
- 21. NCT04363840. The LEAD COVID‐19 trial: low‐risk, early aspirin and vitamin D to reduce COVID‐19 hospitalizations. https://clinicaltrials.gov/show/NCT04363840
- 22. NCT04482686. Trial of combination therapy to treat COVID‐19 infection. https://clinicaltrials.gov/show/NCT04482686
- 23. NCT04385940. Vitamin D and COVID‐19 management. https://clinicaltrials.gov/show/NCT04385940
- 24. NCT04334005. Vitamin D on prevention and treatment of COVID‐19. https://clinicaltrials.gov/show/NCT04334005
- 25. NCT04525820. High dose vitamin‐D substitution in patients with COVID‐19: a randomized controlled, multi center study. https://clinicaltrials.gov/show/NCT04525820 [DOI] [PMC free article] [PubMed]
- 26. CTRI/2020‐001960‐28/ES. Efficacy of vitamin D treatment in patients diagnosed with pneumonia who require hospital admission and have vitamin D deficiency and a positive diagnosis for SARS‐Cov‐2 (COVID‐19); 2020. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2020‐001960‐28/ES
- 27. CTRI/2020/06/026189. To compare the safety and efficacy of Vitamin D, with Magnesium in mild to moderate Covid 19 patients; 2020. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/06/026189
- 28. 2020‐002312‐43/ES. Clinical trial, randomized, open‐label, to evaluate the efficacy of high‐dose vitamin D in patients with COVID‐19 pneumonia; 2020. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2020‐002312‐43/ES
- 29. IRCT20200401046909N2. Evaluation of the efficacy of oral 25‐hydroxyvitamin D3 on COVID‐19; 2020. https://covid‐19.cochrane.org/studies/crs‐13803024
- 30. IRCT20200411047024N1. Evaluation effect of treatment with IM D3 on clinical, paraclinical symptoms and signs and inhospital outcome at patient admitted with covid19; 2020. https://www.irct.ir/trial/47093
- 31. 2020‐001960‐28. Efficacy of vitamin D treatment in patients diagnosed with pneumonia who require hospital admission and have vitamin D deficiency and a positive diagnosis for SARS‐Cov‐2 (COVID‐19). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=covid‐19+and+Vitamin+D
- 32. NCT04476680. Reducing asymptomatic infection with vitamin D in coronavirus disease; 2020. https://clinicaltrials.gov/show/NCT04476680
- 33. NCT04502667. Efficacy of vitamin D treatment in pediatric patients hospitalized by COVID‐19; 2020. https://clinicaltrials.gov/show/NCT04502667
- 34. NCT04552951. Effect of vitamin D on morbidity and mortality of the COVID‐19; 2020. https://clinicaltrials.gov/show/NCT04552951
- 35. NCT04579640. Trial of vitamin D to reduce risk and severity of COVID‐19 and other acute respiratory infections (CORONAVIT); 2020. https://clinicaltrials.gov/show/NCT04579640
- 36.NCT04621058. Effectiveness of vitamin D supplements in reducing complications of COVID19; 2020. https://clinicaltrials.gov/showNCT04621058
- 37. NCT04636086. Effect of vitamin D on hospitalized adults with COVID‐19 infection; 2020. https://clinicaltrials.gov/showNCT04636086
- 38. NCT04641195. Vitamin D and zinc supplementation for improving treatment outcomes among COVID‐19 patients in India; 2020. https://clinicaltrials.gov/showNCT04641195
- 39. NCT04733625. The effect of vitamin D therapy on morbidity and mortality in patients with SARS‐CoV 2 infection; 2021. https://clinicaltrials.gov/showNCT04733625
- 40. NCT04536298. The vitamin D and COVID‐19 trial (VIVID). A pragmatic cluster‐randomized design. [DOI] [PMC free article] [PubMed]
- 41. IRCT20110726007117N11 . Effect of vitamin D supplementation in diagnosed cases of 2019 Novel Coronavirus; a randomized clinical trial. Iran Reg Clin Trials. 2020. https://en.irct.ir/trial/48287 [Google Scholar]
- 42. NCT04386850. Oral 25‐hydroxyvitamin D3 and COVID‐19; 2020. https://clinicaltrials.gov/ct2/show/NCT04386850
- 43. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quesada‐Gomez JM, Entrenas‐Castillo M, Bouillon R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS‐CoV‐2 infections: Revised Ms SBMB 2020_166. J Steroid Biochem Mol Biol. 2020;202:105719. 10.1016/j.jsbmb.2020.105719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ardizzone S, Cassinotti A, Trabattoni D, et al. Immunomodulatory effects of 1,25‐dihydroxyvitamin D3 on TH1/TH2 cytokines in inflammatory bowel disease: an in vitro study. Int J Immunopathol Pharmacol. 2009;22:63‐71. 10.1177/039463200902200108 [DOI] [PubMed] [Google Scholar]
- 46. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID‐19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. 10.3389/fimmu.2020.01708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mandal AKJ, Baktash V, Hosack T, Missouris CG. Vitamin D status and COVID 19 in older adults. Aging Clin Exp Res. 2020;32:2425‐2426. 10.1007/s40520-020-01716-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ni W, Yang X, Yang D, et al. Role of angiotensin‐converting enzyme 2 (ACE2) in COVID‐19. Crit Care. 2020;24:422. 10.1186/s13054-020-03120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stamp TCB. Intestinal absorption of 25‐hydroxycholecalciferol. The Lancet. 1974;121:123. [DOI] [PubMed] [Google Scholar]
- 50. Razzaque MS. Can adverse effects of excessive vitamin D supplementation occur without developing hypervitaminosis D? J Steroid Biochem Mol Biol. 2018;180:86. 10.1016/j.jsbmb.2017.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary material.


