Abstract
Cancer vaccines (CVs) represent a long-sought therapeutic and prophylactic immunotherapy strategy to obtain antigen (Ag)-specific T-cell responses and potentially achieve long-term clinical benefit. However, historically, most CV clinical trials have resulted in disappointing outcomes, despite promising signs of immunogenicity across most formulations. In the past decade, technological advances regarding vaccine delivery platforms, tools for immunogenomic profiling, and Ag/epitope selection have occurred. Consequently, the ability of CVs to induce tumor-specific and, in some cases, remarkable clinical responses have been observed in early-phase clinical trials. It is notable that the record-breaking speed of vaccine development in response to the coronavirus disease-2019 pandemic mainly relied on manufacturing infrastructures and technological platforms already developed for CVs. In turn, research, clinical data, and infrastructures put in place for the severe acute respiratory syndrome coronavirus 2 pandemic can further speed CV development processes. This review outlines the main technological advancements as well as major issues to tackle in the development of CVs. Possible applications for unmet clinical needs will be described, putting into perspective the future of cancer vaccinology.
Key words: cancer, vaccines, immunotherapies
Introduction
Cancer immunotherapies (CIs) represent one of the most promising fields in oncology.1 They aim to enhance immune system recognition of tumor cells, possibly leading to disease control or survival benefit.
CIs include cell therapies, antibodies, cytokines, oncolytic viruses (OVs), and cancer vaccines (CVs).2 , 3 Historically, early CI attempts had exploited the use of systemic cytokines, which were associated with unfavorable toxicity profiles, limiting their clinical applications.4 Over the years, growing knowledge in molecular biology, genomics, and cancer immunology has prompted the discovery of novel targets and therapeutic approaches.2 , 5 , 6 Notable examples are chimeric antigen receptor T (CART) cells and immune checkpoint blockade (ICB). Both strategies provided clinical benefits in different malignancies,7, 8, 9, 10 leading to their approval by regulatory agencies as well as to the 2018 Nobel Prize in Medicine.7 , 11, 12, 13, 14 Regardless, different CIs are burdened by various escape mechanisms, including antigen (Ag)/human leukocyte antigen (HLA) loss, metastatic seeding of immunological sanctuaries, and unpredictable all-or-none or dissociated responses.15, 16, 17
CVs have also been tested with the aim of unleashing cancer-specific responses and establishing long-term immunological memory. However, results have been relatively disappointing, with most formulations failing to show clinical benefit and only one CV, Sipuleucel-T (Provenge), being approved by the Food and Drug Administration (FDA) in 2010.18, 19, 20
In the past year, the record-breaking speed of vaccine development in response to the coronavirus disease (COVID-19) pandemic relied on manufacturing infrastructure and platforms previously built for CVs.21 New vaccine delivery systems, such as gene-based platforms, have been introduced for the first time into large-scale vaccination campaigns. Their favorable safety, immunogenicity, and efficacy profiles have been highlighted by the record-time approval of BNT162b2 and mRNA-1273, the first two FDA-approved vaccines against COVID-19.22, 23, 24, 25, 26, 27
This work will focus on the major unresolved issues in cancer vaccinology, providing insights concerning technological improvements of different platforms, addressing open areas for clinical translation.
Biological and clinical issues to tackle
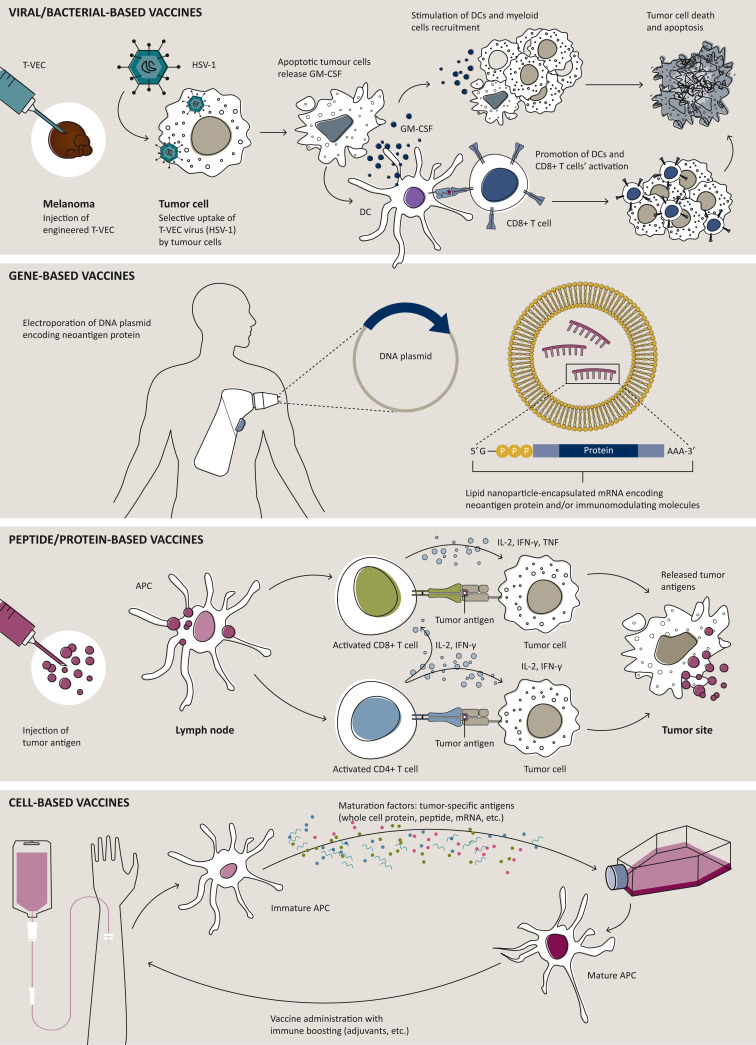
CV platforms are classically divided into four types: viral/bacterial, gene, peptide, and cell based, as depicted in Figure 1 .
Figure 1.
Main vaccine formulations developed for cancer therapy.
Four types of vaccine platforms have been developed for therapeutic purposes: viral/bacterial-based, gene-based, peptide-based, and cell-based vaccines.27 Examples of each different strategies are depicted. APC, antigen-presenting cell; DC, dendritic cell; HSV-1, herpes simplex virus 1; IFN-γ, interferon-γ; IL-2, interleukin-2; mRNA, messenger RNA; TNF, tumor necrosis factor; T-VEC, talimogene laherparepvec.
Most CV clinical trials so far have utilized cellular-, viral-, or peptide-based platforms, thanks to pre-existing knowledge regarding safety, immunogenicity, and manufacture.18 , 28 Until late 2014, 451 CV clinical trials had been conducted, with gene-based formulations representing <5%.29 , 30 Remarkably, the ratio of phase III : II CV clinical trials was as low as 1:21, highlighting a tight bottleneck in drug development processes.30 In addition, most phase III trials ultimately failed to demonstrate efficacy data.29 For example, the MAGE-A3 as Adjuvant Non-Small Cell LunG CanceR ImmunoTherapy trial failed to show increased disease-free survival in patients with surgically resected non-small-cell lung cancer (NSCLC) receiving a recombinant anti-MAGE-A3 protein vaccine compared to placebo.31 Similarly, the TeloVac trial did not show improved overall survival (OS) in locally advanced/metastatic pancreatic cancer patients by adding GV1001, a peptide-based vaccine against telomerase, compared to standard-of-care chemotherapy.32 Altogether, these results possibly originated from non-optimized vaccination strategies, non-ideal Ag selection, or greatly unexplored combinatorial strategies.
Nonetheless, despite numerous other CVs failing to prove efficacy in phase III trials, the entire field never came to a halt.33 Indeed, strong evidence originating from virtually all trials across different platforms supported favorable toxicity profiles and immunogenicity.30 These were the two foundational pillars on to which cancer vaccinology resumed, envisioning novel platforms and trial designs to eventually attain positive efficacy results.29 , 34 After widespread disenchantments, a rejuvenation of the entire field has been witnessed, driven by key aspects warranting optimization to meet expectations.35
Antigen choice
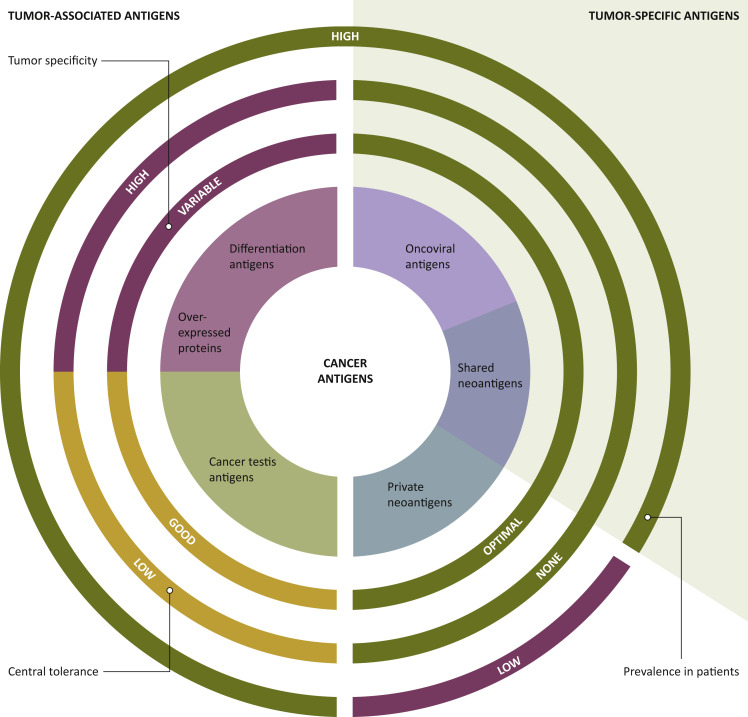
There are two classes of Ags to target: tumor-associated antigens (TAAs) and tumor-specific antigens. The former are non-mutated proteins, being either overexpressed (i.e. human epidermal growth factor receptor 2, or telomerase), part of tissue differentiation (i.e. tyrosinase, Melan-A), or cancer germline (i.e. MAGEs, NY-ESO), and not necessarily tumor specific. In contrast, the latter, comprising oncoviral Ags and neoantigens (NeoAgs), are tumor specific, as shown in Figure 2 . NeoAgs arise from nonsynonymous mutations, which can be functionally relevant. Indeed, ‘driver’ mutations confer cell-growth advantages and are clonally selected, as opposed to ‘passenger’ mutations, which do not affect the replication rate, and hence are less susceptible to clonal selection.36 , 37 Past CV trials preferentially targeted TAAs over NeoAg since the latter were harder to identify.33 However, since TAAs are non-mutated proteins, immune responses tap into T-cell repertoires that are subject to central/peripheral tolerance.22
Figure 2.
Targets for tumor vaccines fall into two general classes: tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs).
TAAs are self-antigens that are either preferentially or abnormally expressed in tumor cells but may be expressed at some level in normal cells, as well. T cells that bind with high affinity to TAAs are typically deleted from the immune repertoire by central and peripheral tolerance mechanisms. TSAs, comprising oncoviral antigens and neoantigens, are tumor specific. Consequently, they are generally highly immunogenic, due to lack of central tolerance. TSAs associated to oncogenic viruses have been identified in virus-induced cancers such as human papillomavirus (HPV)-associated cervical cancer, hepatitis B virus-associated hepatocellular carcinoma, and human herpesvirus 8-associated Kaposi sarcoma. Tumor neoantigens are products of somatic mutations acquired during carcinogenesis. Neoantigens (NeoAgs) encoded by oncogenic driver mutations may be prevalent across patients and tumor types, so they are referred to as shared NeoAgs. However, the majority of NeoAgs are unique to individual patients' tumors (private NeoAgs). To date, through integration of tumor sequencing with the prediction of major histocompatibility complex (MHC)-binding epitopes, it is possible to tailor tumor NeoAg selection on the single patient level.27 Tumor specificity ‘optimal’ (antigen present only in cancer cells), ‘good’ (antigen preferentially expressed in cancer cells), or ‘variable’ (antigen overexpressed/shared with healthy tissues). Central tolerance ‘high’ (antigen physiologically expressed in healthy tissues), ‘low’ (central tolerance present but antigens restricted to immune-privileged sites), or ‘none’ (no evidence of immunological tolerance).18,22
In contrast, phase I/II clinical trials have shown that immune responses against NeoAg are not subjected to central or peripheral tolerance, alongside favorable toxicity profiles.38, 39, 40, 41 In the past years, faster and cheaper availability of next-generation sequencing technology and more refined bioinformatic prediction tools enabled clinical testing of personalized CVs targeting tumor-specific NeoAg, yielding promising early clinical data.42
Tumor heterogeneity
Tumors are not single biological entities, as they evolve under diverse intrinsic and extrinsic pressures mainly imposed by treatment, immunity, metabolism, and the tumor microenvironment (TME).43 , 44 Importantly, tumors with a high degree of intratumor heterogeneity (ITH) have been linked to worse prognosis.45, 46, 47, 48, 49, 50 Genetic and/or non-genetic mechanisms feed different types of ITH: spatial, temporal, immunological, and behavioral ITH.43 As a major determinant of therapy resistance, ITH should be a fundamental aspect to consider to better guide therapeutic strategies.44 , 51 Theoretically, one approach to reduce ITH would be to conduct CV trials in patients with low-tumor burden and in frontline settings.52 Similarly to immune checkpoint blockers (ICBs), CVs may elicit more robust immune responses in treatment-naïve patients.53 , 54 Moreover, a bolder and promising proposal is to use CVs for immunoprevention of cancer, particularly in individuals with preneoplastic lesions.55 , 56
Importantly, gene-based vaccines have the potential to better target ITH compared to the other platforms by eliciting Ag-specific immune responses against different epitopes.57 , 58 This could be achieved by inserting sequences encoding for different NeoAgs within each vaccination shot.57, 58, 59 In addition, gene-based platforms potentially allow for indefinite rounds of vaccinations, following tumor evolution over time.60 In this setting, the emergence of immunodominance, whereby immune responses preferentially focus on a fraction of the possible epitopes of an unknown protein, could probably blunt the effect of chasing ITH by inserting multiple sequences for diverse NeoAgs, and the eventual emergence of such phenomenon shall be precisely assessed.61 , 62
Immunomodulation: from ‘cold’ to ‘hot’ TME
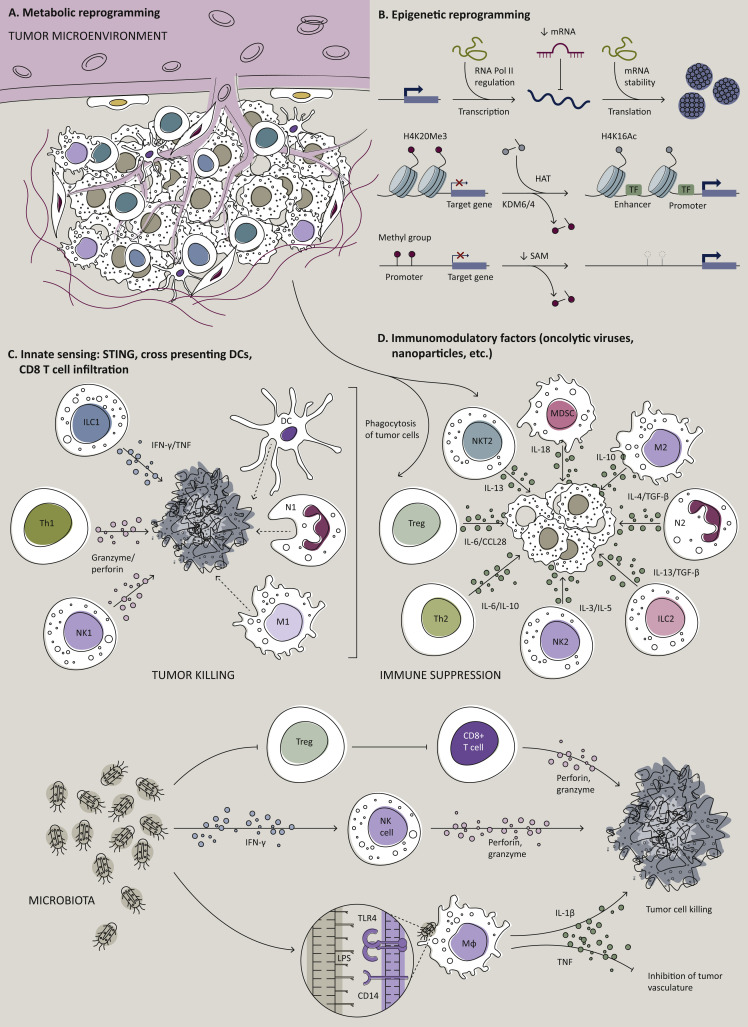
A major hurdle undermining therapeutic CV efficacy is immune-evasive mechanisms, particularly in solid tumors.63 Recognized mechanisms are T-cell exhaustion in inflamed tumors, lack of T-cell infiltration in immune-excluded tumors, and defective Ag presentation processes in immune desert tumors.64 Recently, novel technologies to unravel TME complexity and heterogeneity have been developed, such as mass cytometry and single-cell ‘-omics’ (i.e. epigenomics, transcriptomics, metabolomics, proteomics).65 , 66 However, in this context, spatial localization of a given cell within the TME is often lost during sample preparation and sequencing procedure.67 Thus, development of novel systems-based approaches of simultaneous single-cell analyses combined with spatial microarchitectural information remains a primary technological challenge.68, 69, 70 Potential strategies to heat up the TME and improve antitumor immunity are summarized in Figure 3 .71, 72, 73, 74
Figure 3.
Potential strategies to heat up the TME.
(A) Targeting cellular metabolism and certain metabolites within the TME to reduce immunosuppressive regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), or to generate metabolically fit T cells with better mitochondrial activity to protect against the tumor. Image captions: °, immune cell; ˆ, stroma cell; ∗, cancer cell. (B) Targeting epigenetic modulators to either promote immunogenicity of tumor cells or to re-educate TAMs, MDSCs, or Tregs for the support of T-cell effector functions.71 (C) Induced activation of the innate immune sensing system with stimulator of STING agonists or boosting cross-presenting DCs to promote tumor Ag-specific T-cell trafficking or function within the TME. (D) Creating an inflamed TME via OVs or nanoparticle delivery of key immunomodulatory factors.71,72 In this regard, OVs deserve a special mention, as they are capable of tumor-specific replication, which can provide a therapeutic opportunity.72 Briefly, OVs are naturally occurring or genetically engineered viral species, able to selectively kill cancer cells without damaging healthy tissue.72 Their mechanism of action is multimodal, as the injection of OVs in primary/accessible tumors induces immunogenic cell death of tumor cells, promoting the build-up of an inflamed TME.71 In fact, OVs support natural killer (NK) cell and T-cell immune responses, ultimately improving the lysis of OV-infected cancer cells. Moreover, the activation of antiviral innate immunity, such as type I IFNs and IFN-stimulated genes, promotes the release of damage- and pathogen-associated molecular patterns, the exposure of viral/tumor Ag, as well as the polarization of TAMs towards antitumor M1 phenotype within the TME.73,74 The consequent OV-mediated upregulation of immune checkpoints (i.e. programmed death-ligand 1 and programmed death-ligand 2, PD-L1 and PD-L2, respectively) provides a rationale for combination immunotherapy of OVs plus ICB. CCL28, chemokine (C–C motif) ligand 28; DC, dendritic cell; HAT, histone acetyltransferase; IFN, interferon; IL, interleukin; ILC1/2, innate lymphoid cells 1/2; KDM, histone lysine demethylase; lncRNA, long non-coding RNA; LPS, lipopolysaccharides; M1, classically activated macrophages; M2, alternatively activated macrophages; MDSC, myeloid-derived suppressor cell; miRNA, microRNA; N1, antitumorigenic neutrophil; N2, pro-tumorigenic neutrophil; NK, natural killer; OV, oncolytic virus; RNA Pol II, RNA polymerase II; SAM, S-adenosyl methionine; STING, stimulator of interferon genes; TAM, tumor-associated macrophage; TF, transcription factor; TGF- β, transforming growth factor-β; Th, T helper cell; TLR, Toll-like receptor; TNF, tumor necrosis factor; Treg, regulatory T cell.
Of note, interactions between tumor cells and their TME can destroy normal tissue homeostasis and shift the TME toward the metastasis-promoting state. In addition, metastatic tumors growing in different organs may consist of significantly different immune infiltrates as compared with the primary tumor site, triggering differential responses to immunotherapies.75 For example, the comparison of the immune infiltrates of patient-matched primary versus metastatic breast cancers (BCs) evidenced a higher content of tumor-associated macrophages (TAMs) in metastatic sites, suggesting TAMs as a potential therapeutic target for metastatic BCs.76
In addition, many studies have focused on how tumor metabolism imposes an immunosuppressive TME status.77 Recognized TME metabolic derangements are, for example, low pH, hypoxia, aerobic glycolysis, fatty acid biosynthesis, accumulation of lactate and kynurenines as well as deprivation of glucose, glutamine, and tryptophan.78 Targeting such metabolic alterations has been explored in preclinical models, while human translation studies have mainly investigated the role of indoleamine-2,3-dioxygenase (IDO) inhibition.79 IDO is a rate-limiting enzyme upregulated in response to interferon-γ that catabolizes tryptophan, blunting T-cell responses.80 IDO inhibitors have shown limited efficacy when utilized as single agents, thereby numerous clinical trials are ongoing investigating combinations with ICBs or CVs.81 , 82
Another key determinant of cancer–immune interactions is the concomitant use of drugs with intrinsic TME modulator (TMEm) functions.83 One example is cyclin-dependent kinase inhibitors, which have been linked with improved Ag-presenting processes and pro-inflammatory cytokine secretion, ultimately favoring immune escape via programmed death-ligand 1 upregulation.84 In order to fully uncover immunological aftermaths of diverse drugs, their TMEm functions need to be tested and validated in disease-specific preclinical models.85 For example, the CD73–adenosine axis has recently been recognized as a key druggable immunomodulatory pathway activated in glioblastoma and pancreatic ductal adenocarcinoma murine models, paving the way for clinical testing (i.e. ARC-8, NCT04104672).86
Ultimately, recent studies suggest that the gut microbiome plays a critical role in modulating immune responses, also affecting response to ICB therapies. For example, commensal Prevotella heparinolytica has been shown to promote interleukin-17 (IL-17)-producing cells, accelerating myeloma progression in a preclinical model.88 Moreover, gut colonization with Firmicutes and Faecalibacterium genus was associated with improved clinical response rates and colitis in ICB-treated melanoma patients.89 Similarly, the microbiota composition was shown to influence antitumor efficacy of NeoAg CVs.90 In the field of pharmacobiotics, Bifidobacterium longum supplementation on anti-programmed cell death protein 1 (PD-1) therapy was recently suggested to curb tumor growth in a BC murine model.91 Importantly, the impact of the microbiome needs to be assessed across various tumor subtypes, as its effects are likely to differ.
Technology advancements and pitfalls
Platform-related improvements
Substantial technological improvements have affected mainly gene- and viral-based platforms. Gene-based vaccines have historically lagged behind, mainly due to scarce safety data, large-scale manufacturing experience, reduced immunogenicity of early formulations, their instability, and poor uptake/specificity.92 However, their use has blossomed in recent years, mainly due to three major areas of technological advancements: structure optimization, novel delivery systems, and refined epitope prediction tools.93, 94, 95, 96 Messenger RNA (mRNA) production has been standardized, with simpler manufacture processes obviating the need for cell culture or viral vector production.97 Additionally, mRNA-based platforms in the pipeline allow for quick sequence adaptations in response to emerging resistance mutations.92 Lastly, another advantage of gene-based platforms is that they do not need exogenous, immunogenic cargos, potentially allowing for indefinite dosing/booster shots.92 Concerning RNA-structural optimization, recent technological advances aim at avoiding detrimental immune activation as well as increasing safety, biodistribution, and immunogenicity profiles.98 Major breakthroughs have been sequence optimization, allowing for enhanced transcription and in vivo stability, Good Manufacturing Practice grade purification systems to avoid toxic leftovers, and the insertion of modified nucleotides with higher translation capacity and lower immunogenicity [via Toll-like receptor 7 (TLR7) avoidance], such as N1-methyl-pseudouridine (1mΨ).98 Such modifications may expand the clinical indications of RNA-based vaccines: for example, a non-inflammatory, m1Ψ-based, vaccine structure design recently showed disease protection in a preclinical model of experimental autoimmune encephalitis.99
In addition, mRNA sequences can be utilized not only to encode for tumor-specific Ags, but also immunomodulators (i.e. cytokines, ligands), monoclonal/bispecific antibodies, small interfering RNA, CART constructs, or combinations.93 Indeed, several phase I/II trials are currently testing such approaches.93 For example, mRNA-2752, a lipid nanoparticle (LNP) loaded with OX40 ligand (OX40L, also known as tumor necrosis factor superfamily, member 4 ligand), IL-23, and IL-36γ, is being tested against several malignancies in combination with durvalumab, demonstrating an acceptable safety profile, pro-inflammatory cytokine release, together with some cases of tumor shrinkage (NCT03739931).100
In parallel, improvements have recently been made also in the field of viral vector vaccines,101 which typically utilize either live or non-replicating vectors.102 Major innovations include the introduction of different viral vectors, such as adenoviruses (Ad) (i.e. non-human primate, NHP), parvoviruses (i.e. adeno-associated viruses), and poxviruses [i.e. Modified Vaccinia Ankara (MVA)].103 Such platforms allow for remarkable versatility, carrying the genetic information for Ag expression, and induce potent T-cell responses.104 , 105 A major limitation is the high prevalence of pre-existing immunity against the vector itself, possibly reducing overall efficacy by limiting multiple vaccinations.102 To overcome this, prime/boost approaches based on two different viruses immunologically non-cross-reacting (‘heterologous prime/boost’) have shown promising results in humans.106, 107, 108 Alternatively, use of serotypes with low prevalence is also advised.109 Moreover, complex manufacturing pipelines based on cell-culture systems and the possibility of residual viral replication also remain open areas of research and technological development.102 , 110
Moreover, viral-based approaches also pertain OVs.72 Historically, they have been used as in situ vaccination agents to elicit immune responses against multiple, unpredicted epitopes, given their natural ability to replicate within cancer cells.72 Notably, the only OV being granted regulatory approval has been talimogene laherparepvec.111 Subsequently, efforts have been made to arm OVs with immunomodulating agents, to couple them with immune-stimulating agents and/or to elicit Ag-specific responses.112 , 115 , 116 Remarkable responses and tumor immune infiltration have been recently documented with herpes simplex virus 1 G207 in pediatric high-grade gliomas.113 Moreover, the use of a genetically modified Maraba virus (MR1) has been validated in preclinical models to boost immune responses when administered after Ad-based vaccination, posing the rationale to test Ad:MR1 prime-boost combinations in humans (NCT02285816 and NCT02879760).114 Ultimately, strategies aiming at eliciting Ag-specific responses via OVs exploit virion coating with peptides of interest, exploiting either electrostatic forces (i.e. negatively charged virions and positively charged poly-lysine peptides) or membrane anchoring.72 , 112
Novel delivery vehicles
Innovative compounds have been introduced in clinical trials, especially for RNA-based platforms, such as protamine combined, lipoplexes (LPX), or lipid nanoparticles (LNPs).58 , 117 , 118 Among them, LNPs stand out as a major nanomedicine advancement, as witnessed by their implementation in the development of COVID-19 vaccines. In particular, BNT162b2 and mRNA-1273 exploit LNPs as vectors for spike protein-encoding mRNAs and are currently being administered in worldwide vaccination campaigns.23 , 24 For the first time, such gene-based vaccines have been linked with remarkable safety profiles in the general population, as well as in special subgroups such as cancer patients, pregnant women, and the elderly.26 , 119 , 120 Remarkably, antibody persistence was also detected up to 6 months after the completion of the second vaccination boost.25 , 161 Briefly, LNPs protect RNA sequence from degradation and allow for stringent spatial–temporal control. In addition, their lipid/moiety composition could be further modified to promote cell-/organ-specific targeting and adjuvant properties, further expanding the potential use of gene-based vaccines.97 , 121
Bioinformatics and novel antigen prediction tools
NeoAgs may be exploited not only to indirectly estimate the likelihood of response to ICBs in certain tumors, but also to design personalized therapeutic CVs.122 , 123 To do so, standardized bioinformatic tools able to identify and prioritize possible tumor-specific mutations have been developed.123 However, not all mutations result in neoepitopes that are recognized by the immune system, owing to HLA restriction/immunodominance.59 , 62 Therefore, HLA typing is also required to foresee potentially immunogenic epitopes.42 HLA class I-binding epitopes are predicted through algorithms and computational approaches trained on peptide-binding affinity data.42 , 126 Such algorithms have also been tested on mass spectrometry (MS) data of peptides presented on specific mono-allelic HLA-expressing cell lines to increase accuracy.123, 124, 125 , 127 , 128 Besides MS, methods for high-throughput detection of mutation-associated epitopes, such as mass cytometry and T-cell receptor clonotyping, are also being successfully implemented.42 Additionally, recent advances in big data analysis and artificial intelligence are contributing to improve neoepitope prediction.129, 130, 131, 132 In particular, deep learning approaches have been applied to large HLA peptide and genomic datasets from various human tumors (e.g. NetMHCpan, NetMHCIIpan) to create a computational model of Ag presentation.129 Moreover, large-scale cancer proteomic data-sharing efforts such as the Clinical Proteomic Tumor Analysis Consortium, the Tumor Neoantigen Selection Alliance, and the HLA Ligand Atlas of healthy human tissues will facilitate the enumeration of targetable tumor NeoAg.133 , 134
Several obstacles currently make the design of therapies targeting NeoAg difficult. For example, among the vast number of putative NeoAgs, only a small fraction is ultimately validated, efficiently presented or shown to be immunogenic.135 In fact, prediction tools are more specific for major histocompatibility complex-I (MHC-I) compared to MHC-II molecules, possibly due to a longer sequence and open ends of the latter.42 Also, additional evidence suggests that many tumor-specific epitopes may arise from non-translated sequences, for which most in silico tools have not yet been optimized.136 Lastly, further studies to improve understanding of the factors that can affect NeoAg expression, presentation, and immunogenicity are necessary.42
Getting cancer vaccines to the clinic
Gene-based CVs
Clinical results regarding nucleoside-based CVs have been heterogeneous, due to the large number of phase I/II trials enrolling a limited number of patients, diverse primary endpoints, and fast-emerging technological advancements.92
In general, gene-based vaccines comprise ∼22% of vaccines in preclinical development (37/166) and ∼37% of those in clinical development (10/27). Importantly, two of them received FDA licensing for COVID-19.137 , 138 In this context, two landmark clinical trials targeting TAAs and NeoAgs provided first evidence of efficacy as therapeutic approaches for cancer.40 , 135 Indeed, in the phase I Lipo-MERIT trial (NCT02410733), 89 advanced, ICB-treated melanoma patients received mRNA-based CV against up to four TAAs.139 Remarkably, Th1-skewed, polyclonal T-cell responses following vaccination were observed, along with synergy with anti-PD-1 in ICB-experienced patients, ultimately resulting in durable response rates (35% in the combination group). Notably, the RNA was optimized to achieve highest expression in immature dendritic cells96 and the liposomal delivery system elicited TLR-7-mediated type I interferon responses, easing T-cell expansion.140 The phase I Individualized Cancer Immunotherapies (IVAC) MUTANOME trial (NCT02035956), testing an RNA-based platform targeting 2 TAAs and up to 10 NeoAgs in 13 advanced melanoma patients, showed the emergence of T-cell responses in vaccinated patients, with a reduction in the cumulative rate of metastatic events.40 Of note, polyclonal T-cell responses were detected in all patients in both CD4 and CD8 compartments, and evidence of synergy with ICB.40
Altogether, these trials provided evidence about heavily pre-treated, high-tumor burden, patient populations, highlighting the potential of gene-based platforms and their synergism with traditional immunotherapies.
Viral vector CVs
Several viral vectors have been evaluated in CV clinical studies.141 For example, a gorilla adenovirus (GAd)-derived, NeoAg-based CV was recently shown to synergize with ICB in preclinical tumor models, leading up to disease eradication.142 , 143 Importantly, viral vectors can be armed with multiple Ags of interest, such as prostate specific antigen/Mucin-1/brachyury in metastatic castration-resistant prostate cancer patients (NCT03481816), or with regulated immunomodulator expression, such as gene switches for IL-12 delivery in a preclinical model of glioma.144 , 145 In addition, two NHP Ad vectors are in clinical development for the delivery of NeoAg CVs: chimpanzee (ChAd68) and GAd20. Preliminary results in patients with advanced tumors have demonstrated robust and consistent induction of CD8 T cells against multiple NeoAgs upon vaccination with ChAd68 (Granite, NCT03639714, NCT03953235).
The above-mentioned induction of anti-vector immunity has been overcome by heterologous prime/boost. Such trials elicited higher immune responses than repeated vaccination with an individual viral vector.146 Both self-amplifying RNA and MVA technologies are currently being used to boost NHP Ad vectors in clinical trials (NCT03639714). In this regard, the NHP/MVA prime/boost regimen with two vectors (GAd20 and MVA) is currently evaluated with a NeoAg-based vaccine for high microsatellite instable (MSI-H) tumors (NCT04041310). Instead, the Nous-209 vaccine is based on concomitant administration of four viral vectors encoding for 209 shared NeoAg peptides among patients with MSI-H tumors.146
Adenovirus-vectored vaccines are also being tested to elicit responses in the central nervous system. In this context, a phase I, dose-escalation study was conducted with DNX-2401 (Delta-24-RGD) in 37 patients with recurrent high-grade glioma, resulting in 20% survival at >3 years.147 Overall, the entire field of viral-based CV is advancing thanks to the exploitation of novel viral species and innovative strategies with other vaccination approaches, prompting their application in the oncologic setting.
Peptide-based CVs
Historically, most peptide-based CVs tested so far in the clinic showed variable signs of immunogenicity and clinical activity.148 Two major improvements in this field have been the introduction of novel adjuvants as well as the use of synthetic long peptides (SLPs).149, 150, 151 As opposed to short peptides, SLPs do not directly bind to major histocompatibility complex (MHC) class I molecules; indeed they require Ag-presenting processing for presentation to cytotoxic T lymphocytes with proper immune-stimulatory co-receptors.152 Moreover, SLPs also allow for multi-epitope targeting, as shown for TAS0314, a peptide containing four TAAs from Squamous Cell Carcinoma Antigen Recognized By T-Cells (SART)2 and SART3 proteins, in a preclinical model of SART293-101-expressing melanoma.153 Other formulations of this approach are currently undergoing evaluation.154
In addition, peptide-based CV formulations can also target patient-specific NeoAg. In this scenario, one of the most advanced CV products is represented by NeoVax, comprising up to 20 different SLPs with the immunostimulatory adjuvant poly-ICLC (a synthetic double-stranded RNA viral mimic that acts as a TLR3 agonist).38 Clinical trials in advanced melanoma and glioblastoma have both demonstrated the emergence of polyfunctional, specific, Th1-skewed responses post-vaccination (NCT01970358 and NCT02287428).38 , 155 In the melanoma trial, four patients out of six had no recurrence up to 25 months after vaccination, while the two relapsing patients showed complete tumor regression after ICB therapy.38 These studies highlighted the potential of such peptide-based CV formulations, which are currently being tested also in combination with other immunotherapies (NCT02950766 and NCT03929029).42 Moreover, another promising trial is represented by the phase Ib NT-002, assessing a personalized NeoAg CV, NEO-PV-01, targeting up to 20 NeoAgs predicted by bioinformatic analysis, as a first-line therapy for advanced non-squamous NSCLC with carboplatin, pemetrexed, and pembrolizumab (NCT03380871).156, 157, 158 The authors reported Ag-specific and durable (up to 1 year) immune responses, with ∼55% of vaccine peptides eliciting measurable immune responses. Remarkably, overall response rates in the intention-to-treat and the vaccination populations were 37% and 57%, respectively.157
Overall, concerning peptide-based CV formulations, research in the field of adjuvants as well as in the discovery of ideal antigenic targets is still needed to further improve immunogenicity and, ultimately, clinical efficacy.
Biotech and industrial perspectives
A key aspect of CV development efforts is the capacity of making early and objective treatment choices in order to select ideal candidates, a specific platform, eventual combinatorial agents, and vaccination schedules.159
For RNA-based CVs, different aspects still need to be thoroughly assessed to boost their efficacy. One is their design, as LNP : mRNA mass ratio can be adjusted (from 10 : 1 to 30 : 1), implying, for example, a significant amount of LNP for multi-Ag candidates in a given dose.42 , 98 Moreover, differences in safety and immunogenicity profiles between non-replicating mRNA and self-amplifying mRNA vaccine sequences are largely unknown, and may have implications to improve sequence optimizations upon iterative development schemes.97 In addition, early-phase clinical trials need to precisely capture the inflammatory components of the different mRNA vaccine formulations, given that several intracellular immune sensors are activated by RNA, in order to optimize the benefit (immunogenicity, efficacy) while reducing the risk (safety) profiles.160 In this regard, safety and tolerability may limit multi-Ag approaches, and here preclinical studies will be crucial for development. Lastly, limited data exist on repeated administrations of mRNA vaccines in humans.159 As the entire field accrues more data from human studies and current COVID-19 vaccination programs, potential long-term safety and immunogenicity issues will need to be accurately collected and critically discussed.118 , 138
Considering biotech and industrial implications, viral-based CVs face different issues. Importantly, manufacturing pipelines are more complex and require laborious cell-culture methods imposing complex purification and microbiological constraints.162 Moreover, replication-defective viruses need thorough (pre-)clinical validation regarding their replication capacity and the absence of systemic disease manifestations in frail sub-populations, such as immunocompromised individuals.163 In addition, vaccine-related disease enhancement has been described in some preclinical models for severe acute respiratory syndrome coronavirus or respiratory syncytial virus vaccines and must be always considered.164 , 165 Moreover, punctual reports concerning toxicities as novel viruses are introduced in clinical practice must be thoroughly monitored by regulatory agencies.166
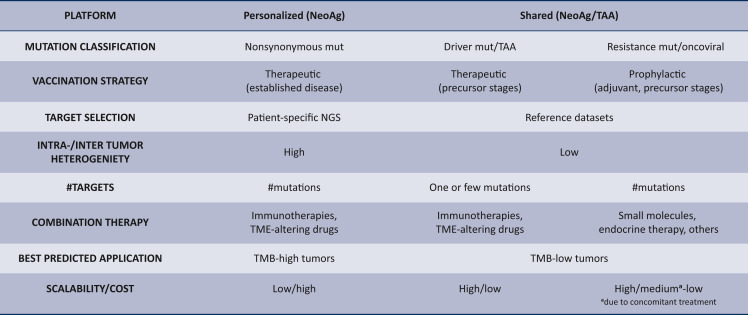
Key aspects to consider in the development of a vaccination strategy, given a certain kind of mutation obtained from sequencing studies, are summarized in Figure 4 . For example, tumor mutational burden (TMB)-high tumors should benefit more from multiple, personalized rounds of vaccinations against different predicted neo-epitopes, possibly in combination with ICBs or TME-altering drugs.92 Instead, off-the-shelf vaccination strategies should be envisioned in TMB-low tumors both prophylactically, against viral-associated epitopes or mutations conferring resistance to chronic concomitant therapies, or therapeutically, against known driver mutations. In general, these considerations must be continuously updated and re-evaluated considering the fast-changing technologic advances and data procurement.
Figure 4.
Proposed antigen (Ag)-based cancer vaccination strategies.
Different mutations/biological dependencies may instruct various vaccination strategies and combinatorial agents. #, multiple; NeoAg, neoantigens; NGS, next-generation sequencing; TAA, tumor-associated antigens; TMB, tumor mutation burden; TME, tumor microenvironment.
Future perspectives
Since most CV clinical trials are small scale, data originating from them will need to be standardized in order to allow comparability and build large-scale reference datasets regarding immunogenicity, biomarkers, and efficacy readouts.18 In this way, patient stratification could identify subgroups potentially gaining benefit from CV programs.167 In parallel, attempts to translate predictive biomarkers identified from ongoing research in ICB-treated patients may also help identifying patients most likely to respond to CVs. For example, a recent meta-analysis of transcriptomic and clinical data from >1000 ICB-treated patients across various malignancies identified clonal TMB, C-X-C Motif Chemokine Ligand (CXCL) 9/CXCL13 expression, Cyclin D1 amplification, and TNF Receptor Associated Factor 2 loss being predictive of ICB response.168
Another concern is to match vaccination strategies with tumor biology/genetics. In fact, CVs can either be utilized as ready-to-use, off-the-shelf drugs, or as personalized products based on sequencing data.18 , 169 Cancer biology and data originating from large longitudinal sequencing studies in multiple malignancies must instruct different vaccination strategies in different clinical settings.168 For example, vaccination strategies relying on products tailored on sequencing data should be most suited for patients carrying biomarkers predicting positive response to ICBs.168 Conversely, tumors showing high oncogene addiction are characterized by driver mutations, which fall in specific loci and harbor few recurrent genetic alterations.18 Theoretically, these tumors could benefit from vaccination strategies aimed at targeting such recurrent driver mutations, possibly in the (neo)adjuvant setting, by means of ready-to-use, off-the-shelf CV products released on histologic information. In this regard, in 2019 Moderna & Merck opened a phase I clinical trial with the aim of targeting the four most common KRAS alterations in NSCLC, pancreatic, and CRC patients by means of an mRNA-based vaccine with or without pembrolizumab (NCT03948763). Importantly, the use of off-the-shelf CV products can also be applied in the prophylactic setting in patients under chronic treatment with targeted or endocrine therapies, to avoid recurrent mutations causing loss of response, as well as in patients with genetic cancer syndromes (i.e. familial adenomatous polyposis, Lynch syndrome).170, 171, 172 Moreover, driver mutations, and their therapy-resistance mutation, may not be the solely targets in oncogene-driven tumors. In fact, oncogenic pathways often co-operate with other mutant proteins to promote disease progression.173 , 174 For example, KRAS exploits mutant TP53 in fostering disease growth in a preclinical model of pancreatic ductal adenocarcinoma.87
The choice of combinatorial agents must consider tumor biology and TME-specific derangements, as previously discussed, together with the clinical setting in which these are introduced. This is because any combinatorial drug comes at the cost of possible added side-effects, and the toxicity/benefit ratio varies from patient to patient and from early to advanced settings. For example, in early stages, combinatorial regimens should aim at increasing CV immunogenicity and foster the formation and persistence memory T-cell subsets. In the metastatic setting, instead, combinatorial drugs should achieve disease control in the short to medium term, allowing CVs to stimulate T-cell-specific immunity and/or re-invigorate ICB-driven responses.
Of note, growing evidence suggests a higher benefit of ICB therapies in circulating tumor DNA (ctDNA)-positive patients in several malignancies, with a favorable prognostic role of ctDNA seroconversion rates.175 , 176 Consolidated data concerning eventual prognostic/predictive roles of this biomarker could possibly instruct for the use of ctDNA seroconversion rate as a primary endpoint of (neo)adjuvant CV clinical trials, alongside long-term survival data (i.e. progression-free survival, OS) supporting eventual regulatory approvals.
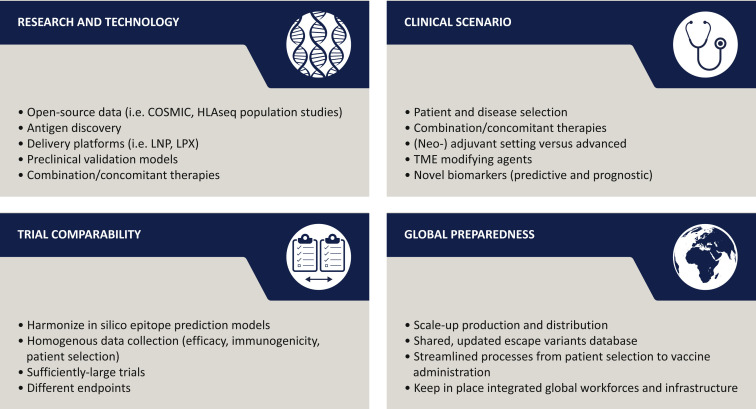
Finally, in order to build positive momentum in the cancer vaccination field, four aspects should be strengthened: research and technology, clinical scenario, trial comparability, and global preparedness (Figure 5 ).
Figure 5.
Key issues to boost applicability and improve clinical efficacy of future cancer vaccine programs.
Main areas of development for cancer vaccinology are the rapid introduction of technological advancements, the identification of clear populations that could benefit from CV programs, efforts to allow for comparability of different clinical trials, and the establishment of a global workforce able to sustain possible demand and supply chain. COSMIC, Catalogue of Somatic Mutations In Cancer; LNP, lipid nanoparticle; LPX, lipoplexes; TME, tumor microenvironment.
Conclusions
Suboptimal clinical trial designs, the use of CVs as single agents, sometimes with weak Ags, as well as the enrolment of advanced, heavily pre-treated patients, have been just some of the reasons that led to poor clinical trial results so far.177 Nonetheless, enormous progress has been made in both oncology and vaccinology.27, 28, 29
Firstly, unprecedented in-depth, running, characterization of cancer genetics, including genetic determinants of therapy resistance, and the introduction of novel immunotherapies or TME-altering drug to combine CVs with in future clinical trials have broadened the spectrum of both TAA or NeoAg targets.178 Moreover, bioinformatic prediction tools are becoming more refined with the growing availability of tumor mutations alongside HLA sequencing population libraries (i.e. IPD-IMGT/HLA database).169, 179, 180, 181 Secondly, technological advances in the vaccinology field are occurring, especially regarding formulations (gene, viral, peptide based) and delivery systems, contributing to the time-record introduction of effective vaccines in the COVID-19 pandemic.21 In this scenario, research and innovation efforts to address COVID-19 provided large-scale evidence about the favorable safety and immunogenicity profiles of these vaccine platform technologies and point to the need to accompany CVs with interventions at the level of the suppressive TME. This momentum could, in turn, speed up the development of CVs employing novel technologies, which are showing promising, although immature, signs of efficacy in early phase I trials.42 , 98
Importantly, the choice of ideal endpoints to allow for a hypothetical regulatory approval of such agents remains a matter of debate, as whether safety profiles should be considered according to a platform-based approach or to the single vaccine product. For these reasons, testing as per classical phase I-III schedule is still to be addressed.
Overall, there is rising optimism that technological advancements, data accumulating from worldwide vaccination campaigns, strengthened production processes and, importantly, clinical results from ongoing phase II/III trials will clarify the ultimate role of CVs in cancer treatment in the ensuing years.
Acknowledgements
GA contributed to the literature search, conception, and design of the article and drafted the first version of the manuscript. CC, PT, and LA contributed to the literature search, conception, and design of the article and provided critical revisions of the manuscript. GC contributed to conception and design of the article and provided critical revisions of the manuscript and supervision. Figure 1, Figure 2, Figure 3 were created with biorender.com. All the authors provided critical revisions of the manuscript and final approval to the submitted work.
Funding
None declared.
Disclosure
JC reports consulting/advisor fees or honoraria from: Roche, Celgene, Cellestia, AstraZeneca, Biothera Pharmaceutical, Merus, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Servier, Merck Sharp & Dohme, GSK, Novartis, Eisai, Pfizer, Samsung Bioepis, Ariad Pharmaceuticals, Baxalta GMBH/Servier Affaires, Bayer healthcare, Guardanth health, Piqur Therapeutics, Puma C, and Queen Mary University of London. PR serves as consultant for MaxiVax, Enterome, and Inotrem. EAM is on the SAB for AstraZeneca/Medimmune, Celgene, Genentech, Genomic Health, Merck, Peregrine Pharmaceuticals, SELLAS Lifescience, and Tapimmune and has clinical trial support to her former institution (MDACC) from AstraZeneca/Medimmune, EMD-Serono, Galena Biopharma, and Genentech as well as Genentech support to a SU2C grant, as well as sponsored research support to the laboratory from GSK and Eli Lilly. MLD has stock ownership of VentiRX and Epithany. MLD has received grant funding from EMD Serono, VentiRx, Seattle Genetics, and Celgene. GC served as consultant or advisor for Roche, Lilly, and Bristol-Myers Squibb; served on the speaker's bureau for Roche, Pfizer, and Lilly; received travel funding from Pfizer and Roche; and received honoraria from Roche, Pfizer, Lilly, Novartis, and SEAGEN, all outside the submitted work. EAM has been compensated for participation on SABs for Exact Sciences, Merch and Roche, and uncompensated participation on steering committees for BMS, Lilly, and Roche. The remaining authors have declared no conflicts of interest.
References
- 1.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 2.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berraondo P., Sanmamed M.F., Ochoa M.C., et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120(1):6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 6.Marshall H.T., Djamgoz M.B.A. Immuno-oncology: emerging targets and combination therapies. Front Oncol. 2018;8:315. doi: 10.3389/fonc.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neelapu S.S., Locke F.L., Bartlett N.L., et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude S.L., Laetsch T.W., Buechner J., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi F.S., O'Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougan M., Dranoff G., Dougan S.K. Cancer immunotherapy: beyond checkpoint blockade. Annu Rev Cancer Biol. 2019;3(1):55–75. doi: 10.1146/annurev-cancerbio-030518-055552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.First-Ever CAR T-cell Therapy Approved in U.S. Cancer Discovery [Internet] https://cancerdiscovery.aacrjournals.org/content/7/10/OF1 Available at. [DOI] [PubMed]
- 14.The Nobel Prize in Physiology or Medicine 2018 [Internet]. NobelPrize.org. https://www.nobelprize.org/prizes/medicine/2018/press-release/ [cited 2021 Apr 26]. Available at.
- 15.Majzner R.G., Mackall C.L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8(10):1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 16.Paulson K.G., Voillet V., McAfee M.S., et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat Commun. 2018;9(1):3868. doi: 10.1038/s41467-018-06300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vago L., Perna S.K., Zanussi M., et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 18.Jou J., Harrington K.J., Zocca M.-B., Ehrnrooth E., Cohen E.E.W. The changing landscape of therapeutic cancer vaccines—novel platforms and neoantigen identification. Clin Cancer Res. 2021;27(3):689–703. doi: 10.1158/1078-0432.CCR-20-0245. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantoff P.W., Higano C.S., Shore N.D., et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 21.Wherry E.J., Jaffee E.M., Warren N., D'Souza G., Ribas A. How did we get a COVID-19 vaccine in less than 1 year? Clin Cancer Res. 2021;27(8):2136–2138. doi: 10.1158/1078-0432.CCR-21-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingsworth R.E., Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4(1):1–10. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimabukuro T.T., Kim S.Y., Myers T.R., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18(3):168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shemesh C.S., Hsu J.C., Hosseini I., et al. Personalized cancer vaccines: clinical landscape, challenges, and opportunities. Mol Ther. 2021;29(2):555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan A.C., Goubier A., Kohrt H.E. A quantitative analysis of therapeutic cancer vaccines in phase 2 or phase 3 trial. J Immunother Cancer. 2015;3:48. doi: 10.1186/s40425-015-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vansteenkiste J.F., Cho B.C., Vanakesa T., et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(6):822–835. doi: 10.1016/S1470-2045(16)00099-1. [DOI] [PubMed] [Google Scholar]
- 32.Middleton G., Silcocks P., Cox T., et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15(8):829–840. doi: 10.1016/S1470-2045(14)70236-0. [DOI] [PubMed] [Google Scholar]
- 33.Mocellin S., Mandruzzato S., Bronte V., Lise M., Nitti D. Part I: Vaccines for solid tumours. Lancet Oncol. 2004;5(11):681–689. doi: 10.1016/S1470-2045(04)01610-9. [DOI] [PubMed] [Google Scholar]
- 34.Morse M.A., Chui S., Hobeika A., Lyerly H.K., Clay T. Recent developments in therapeutic cancer vaccines. Nat Clin Pract Oncol. 2005;2(2):108–113. doi: 10.1038/ncponc0098. [DOI] [PubMed] [Google Scholar]
- 35.Romero P., Banchereau J., Bhardwaj N., et al. The Human Vaccines Project: a roadmap for cancer vaccine development. Sci Transl Med. 2016;8(334):334ps9. doi: 10.1126/scitranslmed.aaf0685. [DOI] [PubMed] [Google Scholar]
- 36.Bozic I., Antal T., Ohtsuki H., et al. Accumulation of driver and passenger mutations during tumor progression. Proc Natl Acad Sci U S A. 2010;107(43):18545–18550. doi: 10.1073/pnas.1010978107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell P.J., Getz G., Korbel J.O., et al. Pan-cancer analysis of whole genomes. Nature. 2020;578(7793):82–93. doi: 10.1038/s41586-020-1969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ott P.A., Hu Z., Keskin D.B., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carreno B.M., Magrini V., Becker-Hapak M., et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahin U., Derhovanessian E., Miller M., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 41.Hilf N., Kuttruff-Coqui S., Frenzel K., et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 42.Blass E., Ott P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18(4):215–229. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitale I., Shema E., Loi S., Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med. 2021;27(2):212–224. doi: 10.1038/s41591-021-01233-9. [DOI] [PubMed] [Google Scholar]
- 44.Dagogo-Jack I., Shaw A.T. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 45.Vitale I., Sistigu A., Manic G., Rudqvist N.-P., Trajanoski Z., Galluzzi L. Mutational and antigenic landscape in tumor progression and cancer immunotherapy. Trends Cell Biol. 2019;29(5):396–416. doi: 10.1016/j.tcb.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Hua X., Zhao W., Pesatori A.C., et al. Genetic and epigenetic intratumor heterogeneity impacts prognosis of lung adenocarcinoma. Nat Commun. 2020;11(1):2459. doi: 10.1038/s41467-020-16295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel A.P., Tirosh I., Trombetta J.J., et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin D.-C., Mayakonda A., Dinh H.Q., et al. Genomic and epigenomic heterogeneity of hepatocellular carcinoma. Cancer Res. 2017;77(9):2255–2265. doi: 10.1158/0008-5472.CAN-16-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li S., Garrett-Bakelman F.E., Chung S.S., et al. Distinct evolution and dynamics of epigenetic and genetic heterogeneity in acute myeloid leukemia. Nat Med. 2016;22(7):792–799. doi: 10.1038/nm.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dentro S.C., Leshchiner I., Haase K., et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell. 2021;184(8):2239–2254.e39. doi: 10.1016/j.cell.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marusyk A., Janiszewska M., Polyak K. Intratumor heterogeneity: the rosetta stone of therapy resistance. Cancer Cell. 2020;37(4):471–484. doi: 10.1016/j.ccell.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim S.I., Cassella C.R., Byrne K.T. Tumor burden and immunotherapy: impact on immune infiltration and therapeutic outcomes. Front Immunol. 2021;11:629722. doi: 10.3389/fimmu.2020.629722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marra A., Viale G., Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med. 2019;17(1):90. doi: 10.1186/s12916-019-1326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiang A.C., Herbst R.S. Frontline immunotherapy for NSCLC—the tale of the tail. Nat Rev Clin Oncol. 2020;17(2):73–74. doi: 10.1038/s41571-019-0317-y. [DOI] [PubMed] [Google Scholar]
- 55.Enokida T., Moreira A., Bhardwaj N. Vaccines for immunoprevention of cancer. J Clin Invest. 2021;131(9):e146956. doi: 10.1172/JCI146956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lollini P.-L., Nicoletti G., Landuzzi L., De Giovanni C., Nanni P. Immunoprevention and immunotherapy of mammary carcinoma. Breast J. 2010;16(suppl 1):S39–S41. doi: 10.1111/j.1524-4741.2010.01002.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L. Multi-epitope vaccines: a promising strategy against tumors and viral infections. Cell Mol Immunol. 2018;15(2):182–184. doi: 10.1038/cmi.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beck J.D., Reidenbach D., Salomon N., et al. mRNA therapeutics in cancer immunotherapy. Mol Cancer. 2021;20(1):69. doi: 10.1186/s12943-021-01348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akram A., Inman R.D. Immunodominance: a pivotal principle in host response to viral infections. Clin Immunol. 2012;143(2):99–115. doi: 10.1016/j.clim.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 60.Lopes A., Vandermeulen G., Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res. 2019;38(1):146. doi: 10.1186/s13046-019-1154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tokuyasu T.A., Huang J.-D. A primer on recent developments in cancer immunotherapy, with a focus on neoantigen vaccines. J Cancer Metastasis Treat. 2018;4:2. [Google Scholar]
- 62.Schreiber H., Wu T.H., Nachman J., Kast W.M. Immunodominance and tumor escape. Semin Cancer Biol. 2002;12(1):25–31. doi: 10.1006/scbi.2001.0401. [DOI] [PubMed] [Google Scholar]
- 63.Giraldo N.A., Sanchez-Salas R., Peske J.D., et al. The clinical role of the TME in solid cancer. Br J Cancer. 2019;120(1):45–53. doi: 10.1038/s41416-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hegde P.S., Chen D.S. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Hu Y., An Q., Sheu K., Trejo B., Fan S., Guo Y. Single cell multi-omics technology: methodology and application. Front Cell Dev Biol. 2018;6:28. doi: 10.3389/fcell.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Single-cell omics [Internet] https://www.nature.com/collections/sxnwgntqsk/ Available at.
- 67.Santegoets S.J., van Ham V.J., Ehsan I., et al. The anatomical location shapes the immune infiltrate in tumors of same etiology and affects survival. Clin Cancer Res. 2019;25(1):240–252. doi: 10.1158/1078-0432.CCR-18-1749. [DOI] [PubMed] [Google Scholar]
- 68.Allam M., Cai S., Coskun A.F. Multiplex bioimaging of single-cell spatial profiles for precision cancer diagnostics and therapeutics. NPJ Precis Oncol. 2020;4(1):1–14. doi: 10.1038/s41698-020-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gohil S.H., Iorgulescu J.B., Braun D.A., Keskin D.B., Livak K.J. Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(4):244–256. doi: 10.1038/s41571-020-00449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.González-Silva L., Quevedo L., Varela I. Tumor functional heterogeneity unraveled by scRNA-seq technologies. Trends Cancer. 2020;6(1):13–19. doi: 10.1016/j.trecan.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Duan Q., Zhang H., Zheng J., Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6(7):605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 72.Chiocca E.A., Rabkin S.D. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(4):295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 74.Escobar G., Barbarossa L., Barbiera G., et al. Interferon gene therapy reprograms the leukemia microenvironment inducing protective immunity to multiple tumor antigens. Nat Commun. 2018;9(1):2896. doi: 10.1038/s41467-018-05315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quail D., Joyce J. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu L., Narloch J.L., Onkar S., et al. Metastatic breast cancers have reduced immune cell recruitment but harbor increased macrophages relative to their matched primary tumors. J Immunother Cancer. 2019;7(1):265. doi: 10.1186/s40425-019-0755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bader J.E., Voss K., Rathmell J.C. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 2020;78(6):1019–1033. doi: 10.1016/j.molcel.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Labadie B.W., Bao R., Luke J.J. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan–kynurenine–aryl hydrocarbon axis. Clin Cancer Res. 2019;25(5):1462–1471. doi: 10.1158/1078-0432.CCR-18-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhai L., Spranger S., Binder D.C., et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res. 2015;21(24):5427–5433. doi: 10.1158/1078-0432.CCR-15-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naour J.L., Galluzzi L., Zitvogel L., Kroemer G., Vacchelli E. Trial watch: IDO inhibitors in cancer therapy. OncoImmunology. 2020;9(1):1777625. doi: 10.1080/2162402X.2020.1777625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Redman J.M., Steinberg S.M., Gulley J.L. Quick efficacy seeking trial (QuEST1): a novel combination immunotherapy study designed for rapid clinical signal assessment metastatic castration-resistant prostate cancer. J Immunother Cancer. 2018;6(1):91. doi: 10.1186/s40425-018-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petroni G., Buqué A., Zitvogel L., Kroemer G., Galluzzi L. Immunomodulation by targeted anticancer agents. Cancer Cell. 2021;39(3):310–345. doi: 10.1016/j.ccell.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J., Bu X., Wang H., et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553(7686):91–95. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olson B., Li Y., Lin Y., Liu E.T., Patnaik A. Mouse models for cancer immunotherapy research. Cancer Discov. 2018;8(11):1358–1365. doi: 10.1158/2159-8290.CD-18-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bendell J.C., Manji G.A., Pant S., et al. A phase I study to evaluate the safety and tolerability of AB680 combination therapy in participants with gastrointestinal malignancies. JCO. 2020;38(suppl 4):TPS788. [Google Scholar]
- 87.Kim M.P., Li X., Deng J., et al. Oncogenic KRAS recruits an expansive transcriptional network through mutant p53 to drive pancreatic cancer metastasis. Cancer Discov. 2021;11(8):2094–2111. doi: 10.1158/2159-8290.CD-20-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calcinotto A., Brevi A., Chesi M., et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat Commun. 2018;9(1):4832. doi: 10.1038/s41467-018-07305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaput N., Lepage P., Coutzac C., et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 90.Lione L., Salvatori E., Petrazzuolo A., et al. Antitumor efficacy of a neoantigen cancer vaccine delivered by electroporation is influenced by microbiota composition. Oncoimmunology. 2021;10(1):1898832. doi: 10.1080/2162402X.2021.1898832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hyeyoon K., Rira O., Sangjun P., Geun Eog J., Myeong S.P., Sung-Eun K. Bifidobacterium longum RAPO enhances efficacy of anti-PD-1 immunotherapy in a mouse model of triple-negative breast cancer [Internet] https://www.abstractsonline.com/pp8/#!/9325/presentation/1136 Available at.
- 92.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Hoecke L., Verbeke R., Dewitte H., et al. mRNA in cancer immunotherapy: beyond a source of antigen. Mol Cancer. 2021;20(1):48. doi: 10.1186/s12943-021-01329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kauffman K.J., Webber M.J., Anderson D.G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release. 2016;240:227–234. doi: 10.1016/j.jconrel.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 95.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holtkamp S., Kreiter S., Selmi A., et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108(13):4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 97.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1):41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pardi N., Hogan M.J., Weissman D. Recent advances in mRNA vaccine technology. Curr Opin Immunol. 2020;65:14–20. doi: 10.1016/j.coi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 99.Krienke C., Kolb L., Diken E., et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371(6525):145–153. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 100.Patel M.R., Bauer T.M., Jimeno A., et al. A phase I study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ, for intratumoral (iTu) injection alone and in combination with durvalumab. JCO. 2020;38(suppl 15):3092. [Google Scholar]
- 101.Zhang C., Zhou D. Adenoviral vector-based strategies against infectious disease and cancer. Hum Vaccin Immunother. 2016;12(8):2064–2074. doi: 10.1080/21645515.2016.1165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramezanpour B., Haan I., Osterhaus A., Claassen E. Vector-based genetically modified vaccines: exploiting Jenner's legacy. Vaccine. 2016;34(50):6436–6448. doi: 10.1016/j.vaccine.2016.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ertl H.C. Viral vectors as vaccine carriers. Curr Opin Virol. 2016;21:1–8. doi: 10.1016/j.coviro.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 105.Afkhami S., Yao Y., Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol Ther Methods Clin Dev. 2016;3:16030. doi: 10.1038/mtm.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DiPaola R.S., Plante M., Kaufman H., et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cappuccini F., Bryant R., Pollock E., et al. Safety and immunogenicity of novel 5T4 viral vectored vaccination regimens in early stage prostate cancer: a phase I clinical trial. J Immunother Cancer. 2020;8(1):e000928. doi: 10.1136/jitc-2020-000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baden L.R., Karita E., Mutua G., et al. Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention: a randomized trial. Ann Intern Med. 2016;164(5):313–322. doi: 10.7326/M15-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monath T.P., Fast P.E., Modjarrad K., et al. rVSVΔG-ZEBOV-GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with ebola zaire glycoprotein: standardized template with key considerations for a risk/benefit assessment. Vaccine X. 2019;1:100009. doi: 10.1016/j.jvacx.2019.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andtbacka R.H.I., Collichio F., Harrington K.J., et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III–IV melanoma. J Immunother Cancer. 2019;7(1):145. doi: 10.1186/s40425-019-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ylösmäki E., Cerullo V. Design and application of oncolytic viruses for cancer immunotherapy. Curr Opin Biotechnol. 2020;65:25–36. doi: 10.1016/j.copbio.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 113.Friedman G.K., Johnston J.M., Bag A.K., et al. Oncolytic HSV-1 G207 immunovirotherapy for pediatric high-grade gliomas. N Engl J Med. 2021;384(17):1613–1622. doi: 10.1056/NEJMoa2024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brun J., McManus D., Lefebvre C., et al. Identification of genetically modified maraba virus as an oncolytic rhabdovirus. Mol Ther. 2010;18(8):1440–1449. doi: 10.1038/mt.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ylösmäki E., Ylösmäki L., Fusciello M., et al. Characterization of a novel OX40 ligand and CD40 ligand-expressing oncolytic adenovirus used in the PeptiCRAd cancer vaccine platform. Mol Ther Oncol. 2021;20:459–469. doi: 10.1016/j.omto.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tähtinen S., Feola S., Capasso C., et al. Exploiting preexisting immunity to enhance oncolytic cancer immunotherapy. Cancer Res. 2020;80(12):2575–2585. doi: 10.1158/0008-5472.CAN-19-2062. [DOI] [PubMed] [Google Scholar]
- 117.Papachristofilou A., Hipp M.M., Klinkhardt U., et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J Immunother Cancer. 2019;7(1):38. doi: 10.1186/s40425-019-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rosa S.S., Prazeres D.M.F., Azevedo A.M., Marques M.P.C. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021;39(16):2190–2200. doi: 10.1016/j.vaccine.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anderson E.J., Rouphael N.G., Widge A.T., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Swaminathan G., Thoryk E.A., Cox K.S., et al. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine. 2016;34(1):110–119. doi: 10.1016/j.vaccine.2015.10.132. [DOI] [PubMed] [Google Scholar]
- 122.McGrail D.J., Pilié P.G., Rashid N.U., et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661–672. doi: 10.1016/j.annonc.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Saxena M., van der Burg S.H., Melief C.J.M., Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 124.Minati R., Perreault C., Thibault P. A roadmap toward the definition of actionable tumor-specific antigens. Front Immunol. 2020;11:583287. doi: 10.3389/fimmu.2020.583287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Mattos-Arruda L., Vazquez M., Finotello F., et al. Neoantigen prediction and computational perspectives towards clinical benefit: recommendations from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(8):978–990. doi: 10.1016/j.annonc.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fritsch E.F., Rajasagi M., Ott P.A., Brusic V., Hacohen N., Wu C.J. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunol Res. 2014;2(6):522–529. doi: 10.1158/2326-6066.CIR-13-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abelin J.G., Keskin D.B., Sarkizova S., et al. Mass spectrometry profiling of HLA-associated peptidomes in mono-allelic cells enables more accurate epitope prediction. Immunity. 2017;46(2):315–326. doi: 10.1016/j.immuni.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pak H., Michaux J., Huber F., et al. Sensitive immunopeptidomics by leveraging available large-scale multi-HLA spectral libraries, data-independent acquisition and MS/MS prediction. Mol Cell Proteomics. 2021;20:100080. doi: 10.1016/j.mcpro.2021.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bulik-Sullivan B., Busby J., Palmer C.D., et al. Deep learning using tumor HLA peptide mass spectrometry datasets improves neoantigen identification. Nat Biotechnol. 2019;37:55–63. doi: 10.1038/nbt.4313. [DOI] [PubMed] [Google Scholar]
- 130.Gartner J.J., Parkhurst M.R., Gros A., et al. A machine learning model for ranking candidate HLA class I neoantigens based on known neoepitopes from multiple human tumor types. Nat Cancer. 2021;2:563–574. doi: 10.1038/s43018-021-00197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ong E., Wong M.U., Huffman A., He Y. COVID-19 coronavirus vaccine design using reverse vaccinology and machine learning. Front Immunol. 2020;11:1581. doi: 10.3389/fimmu.2020.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keshavarzi Arshadi A., Webb J., Salem M., et al. Artificial intelligence for COVID-19 drug discovery and vaccine development. Front Artif Intell. 2020;3:65. doi: 10.3389/frai.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gupta R.G., Li F., Roszik J., Lizée G. Exploiting tumor neoantigens to target cancer evolution: current challenges and promising therapeutic approaches. Cancer Discov. 2021;11:1024–1039. doi: 10.1158/2159-8290.CD-20-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marcu A., Bichmann L., Kuchenbecker L., et al. HLA Ligand Atlas: a benign reference of HLA-presented peptides to improve T-cell-based cancer immunotherapy. J Immunother Cancer. 2021;9(4):e002071. doi: 10.1136/jitc-2020-002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yadav M., Jhunjhunwala S., Phung Q.T., et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 136.Laumont C.M., Vincent K., Hesnard L., et al. Noncoding regions are the main source of targetable tumor-specific antigens. Sci Transl Med. 2018;10(470):eaau5516. doi: 10.1126/scitranslmed.aau5516. [DOI] [PubMed] [Google Scholar]
- 137.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Corti C., Crimini E., Tarantino P., et al. Current perspectives: SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Cancer. 2021;148:P316–P327. doi: 10.1016/j.ejca.2021.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sahin U., Oehm P., Derhovanessian E., et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 140.Kranz L.M., Diken M., Haas H., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 141.Larocca C., Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011;17(5):359–371. doi: 10.1097/PPO.0b013e3182325e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Capone S., Raggioli A., Gentile M., et al. Immunogenicity of a new gorilla adenovirus vaccine candidate for COVID-19. Mol Ther. 2021;29(8):2412–2423. doi: 10.1016/j.ymthe.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.D'Alise A.M., Leoni G., Cotugno G., et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade. Nat Commun. 2019;10(1):2688. doi: 10.1038/s41467-019-10594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bilusic M., McMahon S., Madan R.A., et al. Phase I study of a multitargeted recombinant Ad5 PSA/MUC-1/brachyury-based immunotherapy vaccine in patients with metastatic castration-resistant prostate cancer (mCRPC) J Immunother Cancer. 2021;9(3):e002374. doi: 10.1136/jitc-2021-002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barrett J.A., Cai H., Miao J., et al. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System® (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 2018;25(5-6):106–116. doi: 10.1038/s41417-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sasso E., D'Alise A.M., Zambrano N., Scarselli E., Folgori A., Nicosia A. New viral vectors for infectious diseases and cancer. Semin Immunol. 2020;50:101430. doi: 10.1016/j.smim.2020.101430. [DOI] [PubMed] [Google Scholar]
- 147.Lang F.F., Conrad C., Gomez-Manzano C., et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Calvo Tardón M., Allard M., Dutoit V., Dietrich P.-Y., Walker P.R. Peptides as cancer vaccines. Curr Opin Pharmacol. 2019;47:20–26. doi: 10.1016/j.coph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 149.Aucouturier J., Dupuis L., Deville S., Ascarateil S., Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1(1):111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 150.Zom G.G., Filippov D.V., van der Marel G.A., Overkleeft H.S., Melief C.J., Ossendorp F. Two in one: improving synthetic long peptide vaccines by combining antigen and adjuvant in one molecule. Oncoimmunology. 2014;3(7):e947892. doi: 10.4161/21624011.2014.947892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maynard S.K., Marshall J.D., MacGill R.S., et al. Vaccination with synthetic long peptide formulated with CpG in an oil-in-water emulsion induces robust E7-specific CD8 T cell responses and TC-1 tumor eradication. BMC Cancer. 2019;19:540. doi: 10.1186/s12885-019-5725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Melief C.J.M., van der Burg S.H. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8(5):351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 153.Tanaka Y., Wada H., Goto R., et al. TAS0314, a novel multi-epitope long peptide vaccine, showed synergistic antitumor immunity with PD-1/PD-L1 blockade in HLA-A∗2402 mice. Sci Rep. 2020;10(1):17284. doi: 10.1038/s41598-020-74187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kondo S., Shimizu T., Koyama T., et al. First-in-human study of the cancer peptide vaccine TAS0313 in patients with advanced solid tumors. Cancer Sci. 2021;112(4):1514–1523. doi: 10.1111/cas.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Keskin D.B., Anandappa A.J., Sun J., et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ott P.A., Hu-Lieskovan S., Chmielowski B., et al. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell. 2020;183(2):347–362.e24. doi: 10.1016/j.cell.2020.08.053. [DOI] [PubMed] [Google Scholar]
- 157.Awad M.M., Govindan R., Spigel D.R., et al. A personal neoantigen vaccine NEO-PV-01 in combination with chemotherapy and pembrolizumab induces broad de novo immune responses in first-line non-squamous NSCLC: Associations with clinical outcomes [Internet] https://www.abstractsonline.com/pp8/#!/9325/presentation/1131 Available at.
- 158.Hu Z., Leet D.E., Allesøe R.L., et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat Med. 2021;27(3):515–525. doi: 10.1038/s41591-020-01206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jackson N.A.C., Kester K.E., Casimiro D., Gurunathan S., DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5(1):11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tatematsu M., Funami K., Seya T., Matsumoto M. Extracellular RNA sensing by pattern recognition receptors. J Innate Immun. 2018;10(5-6):398–406. doi: 10.1159/000494034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Doria-Rose N., Suthar M.S., Makowski M., et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gomez P.L., Robinson J.M. Plotkin's Vaccines. 7th ed. Elsevier; 2018. Vaccine manufacturing; pp. 51–60.e1. [Google Scholar]
- 163.Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18(6):546–556. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Diamond M.S., Pierson T.C. The challenges of vaccine development against a new virus during a pandemic. Cell Host Microbe. 2020;27(5):699–703. doi: 10.1016/j.chom.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bolles M., Deming D., Long K., et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85(23):12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chandler R.E. Optimizing safety surveillance for COVID-19 vaccines. Nat Rev Immunol. 2020;20(8):451–452. doi: 10.1038/s41577-020-0372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee E.Y., Kulkarni R.P. Circulating biomarkers predictive of tumor response to cancer immunotherapy. Expert Rev Mol Diagn. 2019;19(10):895–904. doi: 10.1080/14737159.2019.1659728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Litchfield K., Reading J.L., Puttick C., et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021;184(3):596–614.e14. doi: 10.1016/j.cell.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tate J.G., Bamford S., Jubb H.C., et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47(D1):D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bedard P.L., Hyman D.M., Davids M.S., Siu L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395(10229):1078–1088. doi: 10.1016/S0140-6736(20)30164-1. [DOI] [PubMed] [Google Scholar]
- 171.Jeselsohn R., Buchwalter G., De Angelis C., Brown M., Schiff R. ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12(10):573–583. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Punta M., Jennings V.A., Melcher A.A., Lise S. The immunogenic potential of recurrent cancer drug resistance mutations: an in silico study. Front Immunol. 2020;11:524968. doi: 10.3389/fimmu.2020.524968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sanchez-Vega F., Mina M., Armenia J., et al. Oncogenic signaling pathways in The Cancer Genome Atlas. Cell. 2018;173(2):321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sever R., Brugge J.S. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5(4):a006098. doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Syeda M.M., Wiggins J.M., Corless B.C., et al. Circulating tumour DNA in patients with advanced melanoma treated with dabrafenib or dabrafenib plus trametinib: a clinical validation study. Lancet Oncol. 2021;22(3):370–380. doi: 10.1016/S1470-2045(20)30726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ococks E., Frankell A.M., Soler N.M., et al. Longitudinal tracking of 97 esophageal adenocarcinomas using liquid biopsy sampling. Ann Oncol. 2021;32(4):522–532. doi: 10.1016/j.annonc.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 177.Melief C.J.M., van Hall T., Arens R., Ossendorp F., van der Burg S.H. Therapeutic cancer vaccines. J Clin Invest. 2015;125(9):3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Berger M.F., Mardis E.R. The emerging clinical relevance of genomics in cancer medicine. Nat Rev Clin Oncol. 2018;15(6):353–365. doi: 10.1038/s41571-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Robinson J., Barker D.J., Georgiou X., Cooper M.A., Flicek P., Marsh S.G.E. IPD-IMGT/HLA database. Nucleic Acids Res. 2020;48(D1):D948–D955. doi: 10.1093/nar/gkz950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Peters B., Nielsen M., Sette A. T cell epitope predictions. Annu Rev Immunol. 2020;38:123–145. doi: 10.1146/annurev-immunol-082119-124838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sarkizova S., Klaeger S., Le P.M., et al. A large peptidome dataset improves HLA class I epitope prediction across most of the human population. Nat Biotechnol. 2020;38(2):199–209. doi: 10.1038/s41587-019-0322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]