Abstract
Aim
During the pandemic of coronavirus disease 2019 (COVID‐19), the physicians are using various off‐label therapeutics to manage COVID‐19. We undertook a cross‐sectional survey to study the current variation in therapeutic strategies for managing severe COVID‐19 in India.
Methods
From January 4 to January 18, 2021, an online cross‐sectional survey was conducted among physicians involved in the management of severe COVID‐19. The survey had three sections: 1. Antiviral agents, 2. Immunomodulators, and 3. Adjuvant therapies.
Results
1055 respondents (from 24 states and five union territories), of which 64.2% were consultants, 54.3% working in private hospitals, and 39.1% were from critical care medicine completed the survey. Remdesivir (95.2%), antithrombotics (94.2%), corticosteroids (90.3%), vitamins (89.7%) and empirical antibiotics (85.6%) were the commonly used therapeutics. Ivermectin (33%), convalescent plasma (28.6%) and favipiravir (17.6%) were other antiviral agents used. Methylprednisolone (50.2%) and dexamethasone (44.1%) were preferred corticosteroids and at a dose equivalent of 8 mg of dexamethasone phosphate (70.2%). There was significant variation among physicians from different medical specialities in the use of favipiravir, corticosteroids, empirical antibiotics and vitamins.
Conclusion
There is a considerable variation in the physicians’ choice of therapeutic strategies for the management of severe COVID‐19 in India, as compared with the available evidence.
What’s known
India contributed to 10.9% of the global patient load of COVID‐19 and second in position after United States by the number of confirmed patients (as of 15 February 2021).
The Ministry of Health and Family Welfare (MOHFW), India, released the last guidelines on the clinical management of COVID‐19 on 3 July 2020.
The rapid growth of COVID‐19 pandemic propelled off‐label use of various drugs by physicians with little or no evidence.
What’s new
The common therapeutic agents in the management of severe COVID‐19 in India, include remdesivir, antithrombotics, corticosteroids, vitamins and empirical antibiotics.
There is a significant variation among physicians of different medical specialities in the use of favipiravir, corticosteroids, empirical antibiotics and vitamins for severe COVID‐19.
There is a considerable variation in the physicians’ choice of therapeutic strategies for severe COVID‐19 in India as compared with the available evidence.
1. INTRODUCTION
The pandemic of coronavirus disease 2019 (COVID‐19) had a significant impact on the healthcare systems globally and more so in third world countries, such as India. 1 India contributed to 19.2% of the global patient load of COVID‐19 and second in position after the United States by the number of confirmed patients (as of 15 February 2021). However, the mortality is lower, with a patient fatality rate of 1.15% compared with 2.1% worldwide. 2 There has been some debate on the low patient fatality rate, and it depends on various factors including the average age of the population, patient reporting and testing strategies. 3 , 4 , 5
More than 80% of the patients with COVID‐19 have mild to moderate symptoms and may not require hospitalisation. However, 15%‐20% of patients may develop severe COVID‐19, which is defined as the presence of pneumonia and clinical signs of respiratory distress, or peripheral oxygen saturation (SpO2) <90% on room air, a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2:Fio2) of <300 mm Hg, or >50% lung infiltrates. 6 Patients usually develop severe COVID‐19 one week after the onset of symptoms and can deteriorate rapidly, requiring hospital and intensive care unit (ICU) admissions. 7 The reported mortality in severe COVID‐19 is 34%‐50%, with a decline in the last few months. 8 , 9 , 10 The mortality in severe COVID‐19 is dependent on various factors such as age, male sex, organ dysfunction, the severity of respiratory failure (lower PaO2/Fio2 ratio), and the requirement of invasive mechanical ventilation. 9 , 10 , 11
The rapid growth of COVID‐19 pandemic propelled off‐label use of various drugs by physicians with little or no evidence. The Ministry of Health and Family Welfare (MOHFW), India, released the last guidelines on the clinical management of COVID‐19 on 3 July 2020. 12 There has been significant research on the various treatment options for moderate‐severe COVID‐19 in the last few months. The landmark RECOVERY trial has revealed that dexamethasone can reduce significant mortality in patients on oxygen support or invasive mechanical ventilation while conferring no benefit in those not requiring oxygen. 13 In the absence of revision in the guidelines, there is an expected variation in the pharmacological management of COVID‐19. We undertook a nationwide cross‐sectional survey over two weeks in January 2021 to study the variation in the therapeutic strategies of severe COVID‐19 in India.
2. MATERIALS AND METHODS
An online cross‐sectional survey on the pharmacological treatment of severe COVID‐19 using “Google forms” was conducted from 4 January to 18 January 2021. Eighteen physicians from Critical Care Medicine, Anaesthesiology, Pulmonary Medicine and Internal Medicine involved in managing severe COVID‐19 and representing prominent states were invited into the steering group. We searched “Google Scholar” and “PubMed” electronic databases using search terms “therapeutics,” “pharmacological treatment,” “management,” AND “COVID‐19,” “SARS‐CoV‐2.” We reviewed all English language, published research, including national guidance from MOHFW, India, World Health Organisation (WHO) on adult humans with COVID‐19, published during 2020. With the help of a heterogenous steering group, we also included other off‐label medications currently being used in India. The survey was then prepared using several qualitative or quantitative clinical practice points on the pharmacological treatment of severe COVID‐19. A dry run of the survey was conducted among the steering group for any typographical mistakes and feedback. The survey was circulated to physicians through e‐mail and social media channels and consent was taken from all respondents. A concerted effort was taken to invoke participation from all the states, public and private hospitals, and across all medical specialties involved in managing severe COVID‐19.
The survey had 35 multiple‐choice questions (including four on demographic details of the respondents). (Appendix 1: Copy of the original survey) The demographic data included the type and place of hospital of engagement, designation, and medical speciality for all respondents. The survey was divided into three sections: 1. Antiviral agents 2. Immunomodulators 3. Adjuvant therapies. The WHO criteria for the severity assessment of COVID‐19 was used in this cross‐sectional survey. 14 The respondents were allowed to select multiple options, based on either disease severity or clinical indications for the various therapeutic agents. The comments and feedback from participants were taken through the open comment section after questions. Duplication of entries was prevented through e‐mail lock in the Google forms. The study was registered with Clinical trials.gov (Identifier: NCT04691921).
The descriptive analysis was used for absolute numbers and percentage (%). The pharmacological therapeutics were analysed based on the severity of illness, hospital type, working speciality and designation of the respondents in the hospital using the chi‐square test. P‐value less than 0.05 was taken as significant. IBM SPSS (version 26.0, Armonk, NY: IBM Corp.) was used for analysis.
3. RESULTS
Over two weeks, 1063 complete responses were received, and1055 were finally analysed (responses from outside of India were removed). The respondents from 24 (85.7%) Indian states and five (62.5%) union territories participated in the survey, representing approximately 99.3% of Indian population (Figure 1). 64.2% of the respondents were consultants or senior consultants, 54.3% working in private hospitals and 31.9% in medical college. The top three medical specialities were critical care medicine (39.1%), anaesthesia (26.4%) and pulmonary medicine (11%) (Appendix 2, Figure 1).
FIGURE 1.
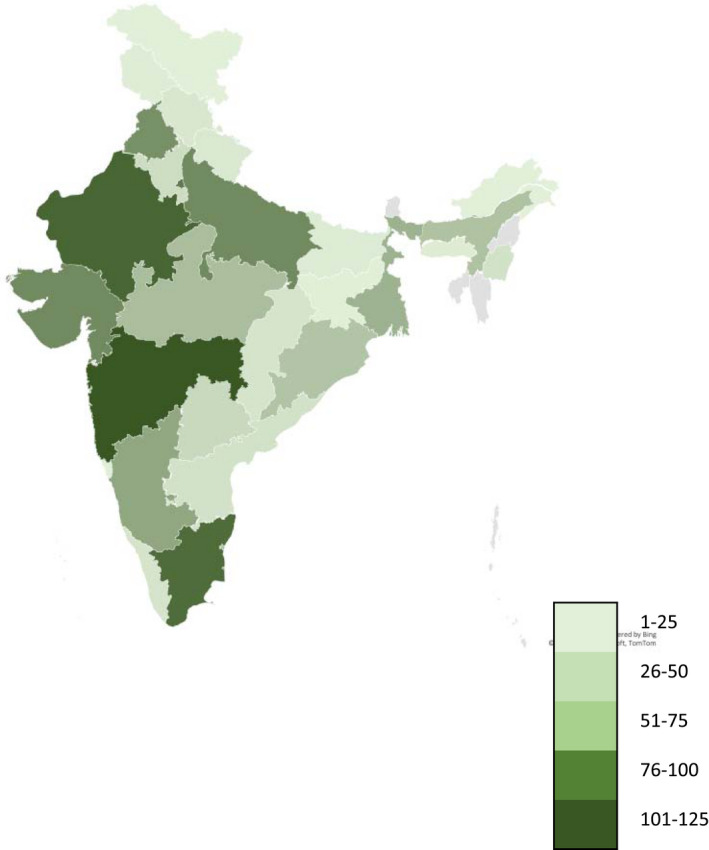
Geographical distribution of the survey respondents. Colour scale represents the number of participants from respective state or union territory
Remdesivir (95.2%) and corticosteroids (90.3%), each for 5‐10 days, were the most common therapeutics chosen by respondents. (Figure 2) The majority of the responders were using empirical antibiotics (85.6%), vitamins (89.7%) and antithrombotics (94.2%) as adjuvant therapies in severe COVID‐19. 71.6% used remdesivir for severe COVID‐19, while 65.2% and 53.6% for moderate and critical COVID‐19, respectively (Tables 1 and 2). The other primary antiviral agent being used included ivermectin (33%), convalescent plasma (28.6%) and favipiravir (17.6%). 31.5% and 47% of respondents respectively, used ivermectin and convalescent plasma irrespective of the disease severity, while 80% used favipiravir for moderate illness. Tocilizumab is the other immunomodulator used by 40% of respondents, and even considered for non‐severe disease. Regarding corticosteroids, methylprednisolone (50.2%) and dexamethasone (44.1%) were the commonly prescribed agents with a preferred dose equivalent to 8 mg of dexamethasone phosphate (70.2%). (Table 2).
FIGURE 2.
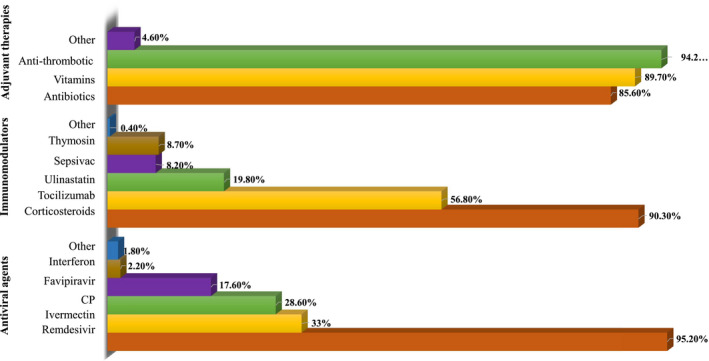
Percentage of respondents using various therapeutics to manage severe COVID‐19. CP, convalescent plasma
TABLE 1.
Percentage of use of different pharmacological agents based on the severity and respiratory support for COVID‐19
| Drugs | All patients irrespective of severity | Moderate COVID‐19 | Severe COVID‐19 | Critical COVID‐19 | Oxygen requirement for Spo2>90% | On IMV | Do not use |
|---|---|---|---|---|---|---|---|
| Antiviral agents | |||||||
| Remdesivir | 8.3% | 65.2% | 71.6% | 53.6% | 64.3% | 50.2% | 8.3% |
| Favipiravir | 3.5% | 30.4% | 4.5% | 2.3% | 8.1% | 1.8% | 61.8% |
| Ivermectin | 31.5% | 26.4% | 10.5% | 7.3% | 10.2% | 6.1% | 41.9% |
| Convalescent plasma | 47.3% | 20.9% | 33.1% | 27.9% | 19.9% | 17.7% | 1.6% |
| Interferon | 0.7% | 2.2% | 6.7% | 7% | 3.3% | 4.5% | 88.2% |
| Immunomodulators | |||||||
| Corticosteroids | 18.6% | 57.1% | 64.6% | 55.3% | 70.1% | 51.1% | 0.6% |
| Tocilizumab | 40.4% | 31.3% | 31.1% | 12.8% | 38.1% | 19.1% | 34.2% |
| Itolizumab | 9.3% | 2.3% | 8.3% | 8.7% | 12.2% | 4.9% | 82.5% |
| Ulinastatin | 1.1% | 8.9% | 17.7% | 16.3% | 9.9% | 11.1% | 75.2% |
| Sepsivac | 0.9% | 3.6% | 8.4% | 8.3% | 3.9% | 4.9% | 85.6% |
| Thymosin | 0.7% | 4.8% | 8.6% | 8.1% | 5.1% | 5.4% | 87.4% |
| Adjuvant therapies | |||||||
| On the basis of laboratory markers | |||||||
| Empirical antibiotics | 28.2% | 33% | 47.5% | 46.1% | 33% | 7.5% | |
| Thromboprophylaxis anticoagulants | 28.9% | 48.1% | 58.3% | 55.3% | 56.4% | 0.5% | |
| Anti‐platelets | 13.3% | 18.7% | 29.2% | 26.6% | 51.9% | ||
| Multi‐vitamins | 69.7% | 18.3% | 21.1% | 20.5% | 8.2% | 7.7% | |
Abbreviation: IMV, invasive mechanical ventilation.
TABLE 2.
Percentage of responses on duration or dose of different pharmacological agents
| Remdesivir | 5‐10 d depending on severity | 5 d | 10 d | >10 d | |
| 56.6% | 40.6% | 2.6% | 0.2% | ||
| Favipiravir | <7 d | 7‐14 d | >14 d | ||
| 28.6% | 69.6% | 1.8% | |||
| Ivermectin | 1 dose | 1‐3 d | >3days | ||
| 4.1% | 65.2% | 30.7% | |||
| Interferon | <5 d | 5‐10 d | >10 d | ||
| 58.6% | 38.2% | 3.3% | |||
| Convalescent plasma | 1 session | 2 session | >2 session | ||
| 66.3% | 26.9% | 6.9% | |||
| Corticosteroids duration | <5 d | 5‐10 d | >10 d | >10 d based on laboratory markers a | |
| 9.6% | 45.9% | 13.3% | 50.2% | ||
| Corticosteroids type | Hydrocortisone | Dexamethasone | Methylprednisolone | Prednisolone | Any corticosteroid |
| 1.9% | 44.1% | 50.2% | 1.5% | 2.3% | |
| Corticosteroids dose b | 8 mg | 9‐16 mg | >16 mg | ||
| 70.1% | 20.6% | 9.3% | |||
| Tocilizumab | 1 dose | 2 doses | >2 doses | ||
| 39.5% | 57.2% | 3.3% | |||
| Itolizumab | 1 dose | 2 doses | >2 doses | ||
| 47.2% | 45.3% | 7.5% |
Extended duration based on the clinical response and inflammatory biomarkers.
Corticosteroid dose in equivalent of dexamethasone phosphate.
There was significant variation in the use of favipiravir (P < .001), and ulinastatin (P = .034) among respondents based on their designation in the hospital. (Figures 3 and 4) There was also a significant variation (P = .005) in the use of antiplatelets based on hospital type in severe COVID‐19 (Figure 5). Antiviral agents {favipiravir (P = .005)}, corticosteroids (P = .035), and the use of adjuvant therapies {antibiotics (P < .001), and vitamins (P < .001)} were significantly variable, based on the working speciality of respondents (Figures 3, 4, 5).
FIGURE 3.
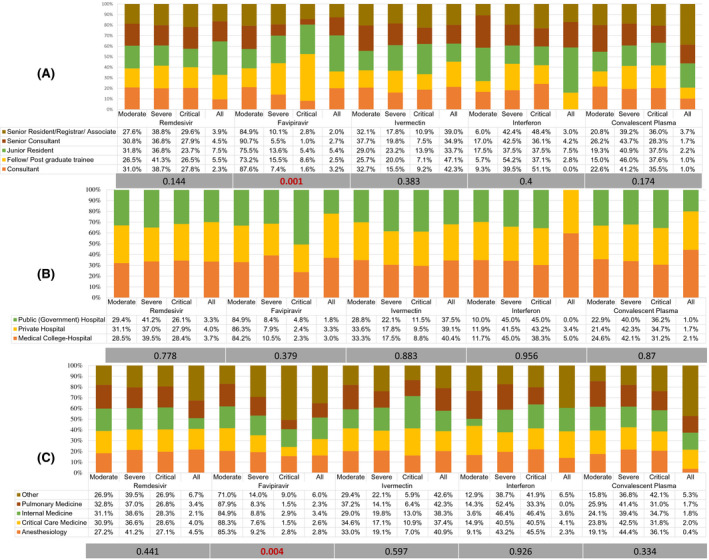
Percentage distribution of the use of antiviral agents by participating physicians according to the severity level of COVID‐19. A, Based on hospital designation of participants. B, Based on participants' hospital type. C, Based on speciality of participants. Grey bar represents the p‐value using the chi‐square test for variation
FIGURE 4.
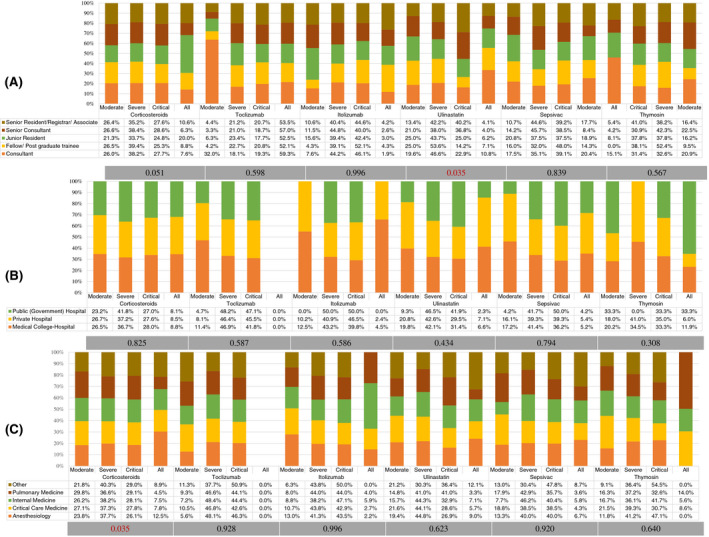
Percentage distribution of the use of immunomodulators by participating physicians according to the severity level of COVID‐19. A, Based on hospital designation of participants. B, Based on participants' hospital type. C, Based on speciality of participants. Grey bar represents the p‐value using the chi‐square test for variation
FIGURE 5.
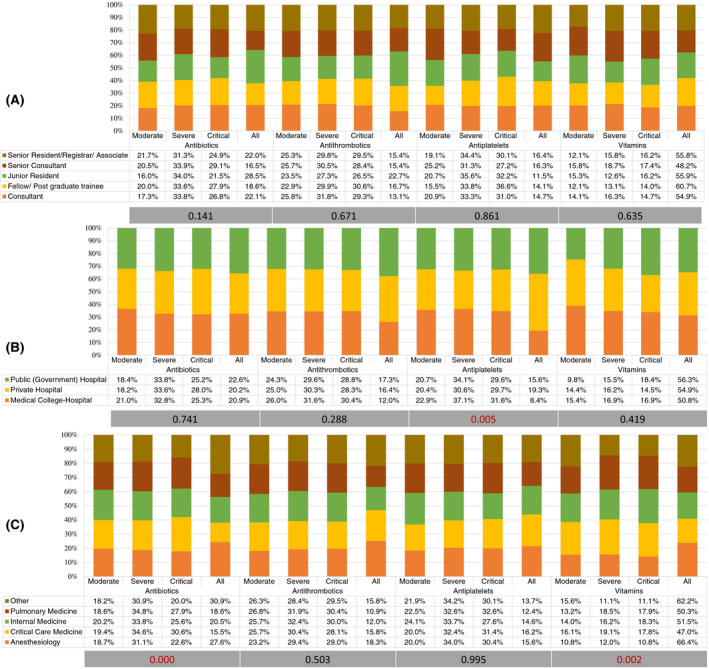
Percentage distribution of the use of adjuvant therapies by participating physicians according to the severity level of COVID‐19. A, Based on hospital designation of participants. B, Based on participants' hospital type. C, Based on speciality of participants. Grey bar represents the p‐value using the chi‐square test for variation
4. DISCUSSION
The arduous clinical work during pandemic and rapidly evolving evidence made it difficult for physicians to keep abreast of the latest research. The deluge of information through print or social media, the pre‐peer review release of the research, and political interference in the science have further perplexed physicians. Researchers globally have made substantial progress in the last few months to understand the pathophysiology and explore various therapeutic options for severe COVID‐19. 13 , 14 , 15 , 16 We undertook this survey in January 2021, after the first wave of the pandemic of COVID‐19 started receding in India, to study the variation in therapeutic strategies. Remdesivir, corticosteroids and prophylactic antithrombotics were the most common therapeutic agents used by the physicians to manage severe COVID‐19. There was considerable use of empirical antibiotics and vitamins by the physicians. There was also significant variation among physicians based on their working specialities in the use of therapeutics such as favipiravir, corticosteroids, empirical antibiotics, and vitamins to manage COVID‐19.
4.1. Antiviral agents
Most of the responding physicians used remdesivir (95.2%) as the preferred antiviral agent in severe COVID‐19 for 5‐10 days (56.6%). However, 53.6% agreed to use remdesivir in critical COVID‐19 or patients requiring invasive mechanical ventilation (50.2%). Recently, a meta‐analysis of three randomised controlled trials (RCT) with 7600 patients concluded remdesivir did not reduce 28‐day mortality in COVID‐19. 17 However, on subgroups analysis, remdesivir showed to reduce mortality in patients requiring supplemental oxygen {relative risk (RR) 0.80} but not in mechanically ventilated patients (RR 1.16). 17
Thirty‐three per cent of the respondents used ivermectin in severe COVID‐19 for 1‐3 days (65.2%). However, 52% of respondents admitted using ivermectin in COVID‐19 irrespective of the disease severity. The available evidence on ivermectin is based on small studies, non‐severe disease, different doses or schedule or mostly non‐peer‐reviewed studies. National Institute of Health (NIH), after reviewing the available evidence, recently revised its recommendation on ivermectin from “against” to “insufficient evidence” for the use of ivermectin in COVID‐19. 18 The ongoing trials of ivermectin in severe COVID‐19 [NCT04646109, NCT04602507, NCT04746365] may provide some evidence in the future. Most of the respondents (82.4%) [senior consultants (90.7%) and consultants (87.6%)] were using favipiravir in moderate COVID‐19. (Figure 3) However, a significantly higher number of postgraduate trainees (8.6%) and junior residents (5.4%) admitted using favipiravir in critical COVID‐19. Similarly, there was a significantly higher use of favipiravir in critical COVID‐19 among physicians from non‐frontline medical specialities (9.0%). The use of favipiravir is discrepant with MOHFW guidelines on clinical management protocol for COVID‐19 and available evidence. 12 , 19
Two‐third of the respondents who agreed to use convalescent plasma in severe COVID‐19 used a single session or dose. The largest RCT on convalescent plasma from India in non‐critical COVID‐19 failed to show any mortality benefit. 20 In a recent metanalysis of 10 published and pre‐published RCT, the use of convalescent plasma in COVID‐19 was not associated with a decrease in mortality or any other benefit. 21
4.2. Immunomodulators
The majority (90.3%) of the respondents agreed to the use of corticosteroids in severe COVID‐19. There was, however, a divide, between methylprednisolone (50.2%) and dexamethasone (44.1%) as the preferred choice of corticosteroid. The equivalent dose and duration of corticosteroids also caused a split up among respondents; 29.9% opted for higher doses (>8 mg), and 50.2% for a longer duration (>10 days) based on the clinical response and inflammatory biomarkers. A significantly higher number of anaesthesiologists (12.5%, P = .035) admitted using corticosteroids in COVID‐19, irrespective of the disease severity. The role of corticosteroids in reducing mortality in severe COVID‐19 has already been proven. 13 , 22 However, controversy still exists on the choice, dose and duration of corticosteroids. The surviving sepsis guidelines on COVID‐19 recommended dexamethasone over other corticosteroids at 6 mg/d dose for ten days to manage severe and critical COVID‐19 based on a weak recommendation with shallow quality evidence. 23
More than half of respondents (56.8%) admitted using tocilizumab in severe COVID‐19 in two doses (57.2%) of maximum 8 mg/kg each. Thirty‐one per cent respondents used tocilizumab in critical COVID‐19 or patients requiring invasive mechanical ventilation (38%). The evidence for tocilizumab is conflicting so far, with various RCTs not finding any mortality benefit. 24 However, National Institute of Health and Care Excellence (NICE), revised its guidance based on the preliminary results of the REMAP‐CAP study. It approved the use of tocilizumab in severe or critical COVID‐19 and in those receiving respiratory or cardiovascular organ support in ICU. 25 , 26
19.8% of the respondents, especially consultants, were using ulinastatin (a urinary trypsin inhibitor) in severe COVID‐19. 8.2% of respondents also agreed to use sepsivac (heat‐killed Mycobacterium W), and 8.7% thymosin (purified peptide from bovine thymic tissues) as immunomodulators for severe COVID‐19. There are no prospective studies available on these therapeutics in COVID‐19, and some of them are undergoing trials at present in India. 27
4.3. Adjuvant therapies
Considerable respondents (85.6% and 89.7%, respectively) admitted using empirical antibiotics and vitamins (vitamin C and vitamin D) in severe COVID‐19. Azithromycin or doxycycline was commonly prescribed antibiotics with severity of COVID‐19 (58.3%) or increased inflammatory markers (56.4%) were preferred indications. There was significant variation in the use of adjuvant therapies with specialists from critical care medicine preferred use of antibiotics in severe and critical COVID‐19, while anaesthesiologists preferred vitamins in all patients of COVID‐19.
Bacterial co‐infections are uncommon in COVID‐19 and vary from 3.5% at the time of admission to 15% among hospitalised patients. 28 A recent guideline on antimicrobials in COVID‐19, recommended a restrictive approach and empirical antibiotics to be considered only if clinical, imaging and biochemical markers suggest a bacterial co‐infection. The de‐escalation of antibiotics within 48 hours is suggested if representative cultures or antigen tests excluded bacterial co‐infection. 29 The authors did not recommend any empirical antibiotic regimen and advised to use community or hospital‐acquired pneumonia guidelines while starting antibiotics and using local antimicrobial surveillance. 29 Recently, the RECOVERY trial found no role of azithromycin in the treatment of hospitalised COVID‐19 patients. 30 The prepublication release from the PRINCIPAL trial using azithromycin or doxycycline for 14 days showed no significant benefit in time to recovery or hospitalisation. 31 NIH does not recommend vitamin C in COVID‐19 because of insufficient data. 32 There are ongoing trials on intravenous vitamin C in COVID‐19 (NCT04323514, NCT04401150, NCT04357782, NCT04264533, NCT02735707). The currently available evidence on vitamin D in COVID‐19 is also controversial and cannot be used for its standard recommendation. 33 The high usage of prophylactic antithrombotic by respondents in this survey for severe COVID‐19 is in line with recently published surviving sepsis campaign guidelines. 23
Forty‐eight per cent of the respondents admitted using prophylactic antiplatelets in severe or critical COVID‐19 with the significantly higher number of physicians from public or medical college‐hospitals in severe COVID‐19. In a retrospective study of 412 patients, aspirin use was associated with reduced use of invasive mechanical ventilation, ICU admission and hospital mortality. 34 However, this association has not been proved in any prospective study so far and cannot be recommended as a standard therapy. NIH recommends the continuation of antiplatelets in patients on chronic antiplatelet therapy before COVID‐19. 35
4.4. Strength and limitations
The strength of this study is its widespread representation from most states and union territories, public or private hospitals, and physicians from different rank or medicine specialities. Secondly, the large number (over 1000) of participation in a short time frame of two weeks helped capture practice variation over a wide range of therapeutic strategies. Thirdly, the study was conducted just after the number of patients started receding after the first wave of pandemic, which may have helped capture practices afresh over the last few months. Finally, we tried to include all possible therapeutics being used in India to manage severe COVID‐19.
There are few limitations in this study. The data on the age and gender of responders was not collected. However, the decision making of physicians is closely linked to their designation in the hospital rather than age. The inherent limitations of a cross‐sectional survey, such as respondents recall bias, a measure of point prevalence and failure to follow the trend is also applicable to this survey. We provided open space for comments after few questions to collect practice variations throughout the pandemic. Finally, we did not include therapeutic anticoagulation in this survey.
5. CONCLUSION
Remdesivir, corticosteroids, and prophylactic antithrombotics are the most used therapeutic strategies in managing severe COVID‐19 in India. There is considerable use of empirical antibiotics and therapeutic vitamins by physicians in severe COVID‐19. There is variation among physicians on the use of pharmaceutical agents in comparison to available evidence. Periodical update on the national guidelines is required to educate physicians on the rapidly evolving evidence.
DISCLOSURE
All authors declare no conflict of interest related to this article.
Supporting information
Appendix 1
Appendix 2
ACKNOWLEDGEMENTS
We acknowledge the contribution of Rohit Ravindra Dussane in the statistical analysis of the results.
Jagiasi B, Nasa P, Chanchalani G, et al. Variation in therapeutic strategies for the management of severe COVID‐19 in India: A nationwide cross‐sectional survey. Int J Clin Pract. 2021;75:e14574. 10.1111/ijcp.14574
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
REFERENCES
- 1. Gopalan HS, Misra A. COVID‐19 pandemic and challenges for socio‐economic issues, healthcare and National Health Programs in India. Diabetes Metab Syndr. 2020;14:757‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20:533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323:1775‐1776. [DOI] [PubMed] [Google Scholar]
- 4. He W, Yi GY, Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID‐19: meta‐analysis and sensitivity analysis. J Med Virol. 2020;92:2543‐2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chatterjee P. Is India missing COVID‐19 deaths? Lancet. 2020;396:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 7. Berlin DA, Gulick RM, Martinez FJ. Severe covid‐19. N Engl J Med. 2020;383:2451‐2460. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. Anaesthesia. 2020;75:1340‐1349. [DOI] [PubMed] [Google Scholar]
- 9. Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID‐19 requiring invasive mechanical ventilation. a meta‐analysis. Am J Respir Crit Care Med. 2021;203:54‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Asch DA, Sheils NE, Islam MN, et al. Variation in US hospital mortality rates for patients admitted with COVID‐19 during the first 6 months of the pandemic. JAMA Intern Med. 2020;22:e208193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Government of India, Ministry of Health and Family Welfare Directorate General of Health Services . Clinical management protocol: COVID‐19. https://www.mohfw.gov.in/pdf/UpdatedClinicalManagementProtocolforCOVID19dated03072020.pdf. Accessed February 12, 2021.
- 13. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid‐19 ‐ preliminary report. N Engl J Med. 2021;384:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organisation . Clinical management of COVID‐19. Interim guidance; 2020. https://www.who.int/publications/i/item/clinical‐management‐of‐covid‐19. Accessed February 11, 2021.
- 15. Borczuk AC, Salvatore SP, Seshan SV, et al. COVID‐19 pulmonary pathology: a multi‐institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33:2156‐2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid‐19 ‐ final report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO Solidarity Trial Consortium , Pan H, Peto R, Henao‐Restrepo AM, et al. Repurposed antiviral drugs for covid‐19 ‐ Interim WHO Solidarity trial results. N Engl J Med. 2021;384:497‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NIH . The COVID‐19 Treatment Guidelines Panel’s Statement on the Use of Ivermectin for the Treatment of COVID‐19. https://www.covid19treatmentguidelines.nih.gov/statement‐on‐ivermectin/. Accessed February 9, 2021.
- 19. Joshi S, Parkar J, Ansari A, et al. Role of favipiravir in the treatment of COVID‐19. Int J Infect Dis. 2021;102:501‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate covid‐19 in adults in India:open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID‐19: a systematic review and meta‐analysis. JAMA. 2021;325:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) Working Group , Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324:1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID‐19) in the ICU: first update. Crit Care Med. 2021;49:e219‐e234. [DOI] [PubMed] [Google Scholar]
- 24. Parr JB. Time to Reassess Tocilizumab's Role in COVID‐19 Pneumonia. JAMA Intern Med. 2021;181:12‐15. [DOI] [PubMed] [Google Scholar]
- 25. Wise J. Covid‐19: Arthritis drugs improve survival in intensive care patients, shows study. BMJ. 2021;372:n61. PMID: 33419743 [DOI] [PubMed] [Google Scholar]
- 26. NICE . COVID‐19 rapid evidence summary: Tocilizumab for COVID‐19. https://www.nice.org.uk/advice/es33/chapter/Product‐overview. Accessed February 9, 2021.
- 27. Charan J, Kaur R, Bhardwaj P, et al. Snapshot of COVID‐19 related clinical trials in India. Indian J Clin Biochem. 2020;35:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sieswerda E, de Boer MGJ, Bonten MMJ, et al. Recommendations for antibacterial therapy in adults with COVID‐19 ‐ an evidence based guideline. Clin Microbiol Infect. 2021;27:61‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. RECOVERY Collaborative Group . Azithromycin in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet. 2021;397:605‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. News . PRINCIPLE trial finds antibiotics azithromycin and doxycycline not generally effective treatments for COVID‐19. https://www.ox.ac.uk/news/2021‐01‐25‐principle‐trial‐finds‐antibiotics‐azithromycin‐and‐doxycycline‐not‐generally. Accessed February 10, 2021.
- 32. NIH . COVID‐19 Treatment Guidelines. Vitamin C. https://www.covid19treatmentguidelines.nih.gov/adjunctive‐therapy/vitamin‐c/. Accessed on February 10, 2021.
- 33. Murdaca G, Pioggia G, Negrini S. Vitamin D and Covid‐19: an update on evidence and potential therapeutic implications. Clin Mol Allergy. 2020;18:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in‐hospital mortality in hospitalized patients with COVID‐19. Anesth Analg. 2020;132:930‐941. [DOI] [PubMed] [Google Scholar]
- 35. NIH . COVID‐19 Treatment Guidelines. Antithrombotic Therapy in Patients with COVID‐19. https://www.covid19treatmentguidelines.NIH.gov/adjunctive‐therapy/antithrombotic‐therapy/. Accessed February 10, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1
Appendix 2
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.


