Abstract
Patients with hematologic malignancies have an increased risk of severe COVID‐19 infection. Vaccination against COVID‐19 is especially important in these patients, but whether they develop an immune response following vaccination is unknown. We studied serologic responses to the BNT162b2 vaccine in this population. A lower proportion of patients were seropositive following vaccination (75%) than in a comparison group (99%; p < 0.001), and median (interquartile range [IQR]) antibody titers in patients were lower (90 [12.4–185.5] and 173 [133–232] AU/ml, respectively; p < 0.001). Older age, higher lactate dehydrogenase, and number of treatment lines correlated with lower seropositivity likelihood and antibody titers, while absolute lymphocyte count, globulin level, and time from last treatment to vaccination correlated with higher seropositivity likelihood and antibody titers. Chronic lymphocytic leukemia patients had the lowest seropositivity rate followed by indolent lymphoma. Patients recently treated with chemo‐immunotherapy, anti‐CD20 antibodies, BCL2, BTK or JAK2 inhibitors had significantly less seropositive responses and lower median (IQR) antibody titers (29%, 1.9 [1.9–12] AU/ml; 0%, 1.9 [1.9–1.9] AU/ml; 25%, 1.9 [1.9–25] AU/ml; 40%, 1.9 [1.9–92.8] AU/ml; and 42%, 10.9 [5.7–66.4] AU/ml, respectively; p < 0.001). Serological response to BNT162b2 vaccine in patients with hematologic malignancies is considerably impaired, and they could remain at risk for severe COVID‐19 infection and death.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has spread at an alarming rate, leading to a death toll of over 3.0 million worldwide. 1 Patients suffering from hematologic malignancies are severely immunocompromised and are considered particularly vulnerable and prone to severe disease due to SARS‐CoV‐2 infection. Risk of death for these patients is close to 35%, which is twice that of subjects without hematologic malignancies infected with COVID‐19. 2 , 3 Patients diagnosed recently, those suffering from acute myeloid leukemia (AML) and those receiving active antineoplastic treatment with monoclonal antibodies (MoAbs) are at a particularly high risk. 4 , 5
When compared to patients with solid tumors and healthy controls, patients with hematologic malignancies infected with SARS‐CoV‐2 were shown to have prolonged viral shedding and delayed seroconversion. Some of these patients, in particular those recently treated with anti‐CD20 MoAbs or stem cell transplantation (SCT), were unable to mount an antibody response at all. 6 Even when an antibody response develops, reinfection with COVID‐19 is still a possibility. This was observed in healthy young individuals, seropositive for SARS‐CoV‐2 immunoglobulin G (IgG), at a rate as high as 10% in close quarters. Reinfection was associated with lower baseline IgG titers. 7
Efforts to develop effective vaccines against SARS‐CoV‐2 have led to the rapid approval of several vaccines including the BNT162b2 COVID‐19 vaccine, which has been available in Israel since December 20, 2020. The company‐sponsored trial in the general population, 8 as well as real‐world data from Israel 9 have shown high efficacy in preventing severe clinical disease. Vaccinating patients with hematologic malignancies is especially important due to their vulnerability to severe COVID‐19, but whether they develop an immune response following vaccination remains unknown. Furthermore, patients with hematologic malignancies were previously shown to have poor responses to vaccinations, such as against influenza, herpes zoster, pneumococcal infection and hepatitis B. 10 , 11 , 12 , 13 , 14
We therefore aimed to study the serological response following the recommended two‐dose BNT162b2 COVID‐19 vaccine in a wide range of hematologic malignancies patients.
2. METHODS
Patients with hematologic malignancies treated at Shamir Medical Center in Israel, having received two doses of vaccination, were approached for participation in the study.
Data were collected between February 7 and April 8, 2021, and compared with an age‐matched group of subjects with no hematologic malignancy (comparator group). Participants with solid cancer or immune diseases were not excluded. All participants completed a questionnaire pertaining to possible exposure to COVID‐19, prior PCR testing for COVID‐19 and results thereof, concurrent medical conditions and immunosuppressive therapy. Data regarding hematologic diagnoses, as well as current and prior treatments were retrieved from the electronic medical records.
Blood samples for COVID‐19 serology were collected between 30 and 60 days following the second vaccine dose. Patients with prior COVID‐19 infection were excluded from the study, as were patients having serology testing done within 14 days of vaccination. The study was approved by the local institutional review board and all participants signed an informed consent form.
2.1. COVID‐19 antibody test
Serologic testing for SARS‐Cov2 IgG was performed using the Liaison SARS‐CoV‐2 S1/S2 IgG test (DiaSorin, Saluggia, Italy), a chemiluminescence immunoassay for the quantitative determination of anti‐S1‐ and anti‐S2‐specific IgG antibodies to SARS‐CoV‐2 in human serum or plasma samples. Clinical sensitivity and specificity of this assay are 97.4% and 98.5%, respectively. Samples were considered negative for antibody titers <12 AU/ml. 15 , 16
2.2. Statistical analysis
Categorical variables are reported as frequency and percentage. Continuous variables were evaluated for normal distribution using histogram and Q–Q plot. All continuous variables were skewed, therefore they are reported as median and interquartile range (IQR). Chi‐squared and Fisher's exact tests were used to compare categorical variables between patients and comparison group, and Mann‐Whitney test was applied to compare continuous variables. As several baseline characteristics of patients and comparison group were found to be different, we used a case‐matched analysis to exclude the influence of comorbidities on seronegativity rates. Matching between patients and subjects from the comparison group was done according to the following criteria: same gender and comorbidities (diabetes mellitus, hypertension, obesity, history of non‐hematologic cancer, cardiac, lung, renal and autoimmune disease), age ± 3 years, and time between the second vaccine and serology testing ±7 days. Categorical variables were compared between the matched groups using McNemar's test and continuous variables using the Wilcoxon signed‐rank test.
In patients with hematologic malignancies, chi‐squared test and Fisher's exact test were used to compare between categorical variables and Kruskal–Wallis and Mann‐Whitney test were used to compare between continuous variables. Spearman's correlation coefficient was used to evaluate associations between continuous variables.
Chi‐squared Automatic Interaction Detection (CHAID) and Classification and Regression Tree (CART) modeling were used to identify characteristics of the study population, significantly associated with COVID‐19 seronegativity. The following variables were available for the classification trees: age, gender, diagnosis, comorbidities, pre‐vaccination lactate dehydrogenase (LDH), absolute lymphocyte count (ALC) and globulin level, current treatment and treatment given in the previous 6, 24, and 60 months.
All statistical tests were two‐sided and p < 0.05 was considered statistically significant.
IBM SPSS Statistics for Windows, v.24.0 (IBM Corp. Armonk, NY, USA) was used for the statistical analysis.
3. RESULTS
Serologic samples were collected from 427 individuals. Four subjects were excluded due to an interval of less than 14 days between vaccination and sampling (three patients and one control). Therefore, 423 subjects are included in this analysis: 315 patients with hematologic malignancies and 108 in the comparison group. Characteristics of study subjects are given in Table 1. The median (IQR) age of the study cohort was 70 (61–77) years, with no significant difference in age between patients and the comparison group. The patient cohort included more males and more patients with renal disease compared with the comparison group (p = 0.026 and 0.046, respectively). The time period from the second vaccine to serology testing did not differ between the two groups (p = 0.61).
TABLE 1.
Study cohort characteristics
| Entire cohort | Patients with hematologic malignancies | Comparison group | p | |
|---|---|---|---|---|
| N (%) | 423 | 315 (74.5) | 108 (25.5) | |
| Age (median [IQR]) | 70 (61–77) | 71 (61–78) | 69 (58–74) | 0.062 |
| Gender, male (N [%]) | 223 (53) | 176 (56) | 47 (44) | 0.026 |
| Comorbidities (N [%]) | ||||
| Cardiac | 58 (14) | 43 (14) | 15 (14) | 0.951 |
| Hypertension | 131 (31) | 97 (31) | 34 (32) | 0.854 |
| Diabetes mellitus | 80 (19) | 58 (18) | 22 (20) | 0.654 |
| Lung | 24 (6) | 20 (6) | 4 (4) | 0.305 |
| Renal | 13 (3) | 13 (4) | 0 | 0.046 |
| Obesity | 36 (9) | 25 (8) | 11 (10) | 0.47 |
| Autoimmune | 11 (3) | 8 (3) | 3 (3) | > 0.999 |
| Other cancer | 34 (8) | 25 (8) | 9 (8) | 0.896 |
| Post‐vaccination COVID19 serology | ||||
| Positive, ≥ 12 AU/ml (N [%]) | 342 (81) | 235 (75) | 107 (99) | < 0.001 |
| Negative, < 12 AU/ml (N [%]) | 81 (19) | 80 (25) | 1 (1) | |
| Antibody titer (AU) (median [IQR]) | 118 (30–186) | 85 (11–172) | 157 (130–221) | < 0.001 |
| Time from vaccine to serology essay (days) (median [IQR]) | 32 (28–39) | 32 (29–40) | 33.5 (28–39) | 0.61 |
| Matched analysis (N) | 69 | 69 | ||
| Seropositive, ≥ 12 AU/ml (N [%]) | 52 (75) | 68 (99) | < 0.001 | |
| Seronegative, < 12 AU/ml (N [%]) | 17 (25) | 1 (1) | ||
| Antibodies titer (AU) (median [IQR]) | 90 (12–185) | 173 (133–232) | < 0.001 |
In all, 74.6% of patients with hematologic malignancies developed a positive humoral response (seropositivity) with a median (IQR) antibody titer of 85 (10.7–172) AU/ml as opposed to the comparison group in whom 99.1% were seropositive following vaccinations, with a median antibody titer of 157 (130–221) AU/ml (p < 0.001 for both comparisons).
In case‐matched analysis, 69 patients/comparison group paired for age, gender, comorbidities, and time from vaccination to serology assay were analyzed (Table 1). COVID‐19 seropositivity developed in 75% of patients and 99% of the matched comparison group, with median (IQR) antibody titers of 90 (12.4–185.5) and 173 (133–232) AU/ml, respectively (p < 0.001 for both), much the same as for the entire cohort.
3.1. COVID‐19 seropositivity in hematologic malignancy patients
Features of hematologic malignancies as well as serologic data are presented in Table 2. Seropositive patients were significantly younger compared with seronegative patients (median [IQR] age: 69 [58–77] compared with 73 [67–82] years, respectively; p < 0.001). Gender and rates of comorbidity did not differ between seropositive and seronegative patients (data not shown). Pre‐vaccination laboratory parameters significantly associated with seropositivity are shown in Figure 1. Seropositive patients had significantly higher ALC (median [IQR] = 1.5 [1.1–2.1] compared with 1 [0.6–1.88] × 103/μl; p < 0.001), total globulin levels (29 [26–31] compared with 26 [22–30] g/L; p = 0.003) and lower LDH (378 [316–444] compared with 427 [325–574] U/L; p = 0.015) compared with seronegative patients.
TABLE 2.
Post‐vaccination serology in hematologic malignancy patients
| Entire patient cohort | Covid‐19 serology | p a | Covid‐19 antibody titer (AU/ml) | p b | ||
|---|---|---|---|---|---|---|
| N (%) | Positive | Negative | Median (IQR) | |||
| Diagnosis N (%) | <0.001 | <0.001 | ||||
| Aggressive NHL | 51 (16) | 36 (71) | 15 (29) | 103 (1.9‐ 182) | ||
| Indolent NHL | 40 (13) | 24 (60) | 16 (40) | 30.6 (1.9‐ 158.3) | ||
| Hodgkin lymphoma | 16 (5) | 15 (94) | 1 (6) | 177 (159.5‐ 310) | ||
| Multiple myeloma | 53 (17) | 40 (76) | 13 (24) | 46.8 (11.1‐ 124.5) | ||
| CLL | 34 (11) | 16 (47) | 18 (53) | 3.45 (1.9‐ 43) | ||
| Acute leukemia | 15 (5) | 12 (80) | 3 (20) | 118 (12.8‐ 190) | ||
| MDS | 16 (5) | 15 (94) | 1 (6) | 139 (78.1‐ 215.3) | ||
| MPN | 68 (22) | 57 (84) | 11 (16) | 100.5 (47.5‐ 166) | ||
| CML | 22 (7) | 20 (91) | 2 (9) | 155 (77.3‐ 201.5) | ||
| Disease status | 0.44 | 0.005 | ||||
| Active | 185 (59) | 136 (74) | 49 (26) | 65.7 (8.9‐ 148.5) | ||
| Remission | 128 (41) | 99 (77) | 29 (23) | 122 (33.2‐ 189) | ||
| Current treatment | <0.001 | <0.001 | ||||
| None | 151 (48) | 130 (86) | 21 (14) | 139 (42.9‐ 211) | ||
| Chemotherapy | 10 (3) | 6 (60) | 4 (40) | 13.8 (4.1‐ 76.5) | ||
| Chemo‐immunotherapy | 28 (9) | 8 (29) | 20 (71) | 1.9 (1.9‐ 12) | ||
| Single agent anti CD20 Ab | 2 (0.5) | 0 | 2 (100) | 1.9 (1.9‐ 1.9) | ||
| Other monoclonal Ab (MoAb) | 3 (1) | 3 (100) | 0 | 168 (159‐ 276) | ||
| Proteasome inhibitors (PI) | 6 (2) | 5 (83) | 1 (17) | 78.4 (6‐ 190.3) | ||
| IMIDs | 12 (4) | 11 (92) | 1 (8) | 84.7 (27.8‐ 143.5) | ||
| BCR‐ABL TKI | 20 (6) | 18 (90) | 2 (10) | 155 (69.4‐ 194.5) | ||
| BCL2 inhibitors | 4 (2) | 1 (25) | 3 (75) | 1.9 (1.9‐ 25) | ||
| JAK2 inhibitors | 12 (4) | 5 (42) | 7 (58) | 10.9 (5.7‐ 66.4) | ||
| BTK inhibitors | 5 (1.5) | 2 (40) | 3 (60) | 1.9 (1‐9‐ 92.8) | ||
| PI/IMID/MoAb combination | 22 (7) | 14 (64) | 8 (36) | 39.3 (5.9‐ 78.4) | ||
| Others | 40 (13) | 32 (80) | 8 (20) | 97 (47.1‐ 124.5) | ||
| Auto‐ SCT | 0.48 | 0.4 | ||||
| Yes | 21 (7) | 17 (81) | 4 (19) | 95.4 (10.4‐ 214) | ||
| No | 286 (93) | 211 (74) | 75 (26) | 80.3 (10.4‐ 168.3) | ||
| Lines of treatment | 0.001 | 0.003 | ||||
| 0 | 55 (17) | 52 (95) | 3 (5) | 117 (47.7‐ 215) | ||
| 1 | 184 (58) | 135 (73) | 49 (27) | 88.2 (10.5‐ 170.3) | ||
| ≥2 | 76 (24) | 48 (63) | 28 (37) | 51.1 (1.9‐ 159.8) | ||
| Time of vaccine from last treatment (months) | 0.001 | <0.001 | ||||
| 0‐6 | 175 (55) | 114 (65) | 61 (35) | 56 (1.9‐ 119) | ||
| >6‐12 | 9 (3) | 8 (89) | 1 (11) | 100 (26.4‐ 193.5) | ||
| >12‐24 | 19 (6) | 14 (74) | 5 (26) | 127 (11.4‐ 288) | ||
| >24‐60 | 31 (10) | 27 (87) | 4 (13) | 178 (54.3‐ 226) | ||
| >60 | 21 (7) | 16 (76) | 5 (24) | 149 (15.2‐ 285) | ||
| No treatment | 59 (19) | 55 (93) | 4 (7) | 119 (47.7‐ 200) | ||
Abbreviations: Ab, antibody; BCL2, B‐cell lymphoma 2; BTK, bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; IMIDs, immune modulatory drugs; JAK2, janus kinase 2; MDS, myelodysplastic syndrome; MoAb, monoclonal antibodies; MPN, myeloproliferative neoplasms; NHL, non‐Hodgkin lymphoma; PIs, proteasome inhibitors; SCT, stem cell transplantation; TKI, tyrosine kinase inhibitor.
Comparison between seropositive and seronegative rates between subcategories.
Comparison between COVID‐19 Ab titers between subcategories.
FIGURE 1.
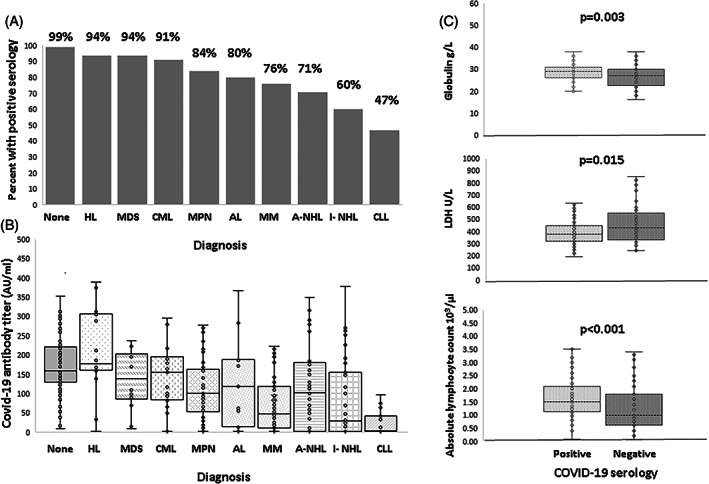
Serologic results according to hematologic diagnosis and laboratory results. (A) Seropositivity rates (%) in hematologic malignancy‐specific diagnosis and the comparator group. (B) Post‐vaccination antibody titers in hematologic malignancy‐specific diagnosis and the comparator group. (C) Seropositive patients had a significantly higher globulin level (upper panel), lower lactate dehydrogenase (LDH, middle panel), and higher absolute lymphocyte count (lower panel). HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; MPN, myeloproliferative neoplasms; AL, acute leukemia; MM, multiple myeloma; A‐NHL, aggressive non‐Hodgkin lymphoma; I‐ NHL, indolent non‐Hodgkin lymphoma; CLL, chronic lymphocytic leukemia
Chronic lymphocytic leukemia (CLL) patients had the lowest rate of seropositivity post‐vaccination (47%), followed by non‐Hodgkin lymphoma (NHL, 71% of aggressive and 60% of indolent lymphoma) and multiple myeloma (MM, 76%), while patients with chronic myeloid leukemia (CML, 91%), BCR‐ABL‐negative myeloproliferative neoplasms (MPN, 84%), myelodysplastic syndrome (MDS, 94%) and Hodgkin lymphoma (94%) had the highest rates (p < 0.001) (Table 2; Figure 2). At the time of vaccination, 59% of patients with hematologic malignancies had active disease and 52% were receiving treatment. Patients who had never received treatment were more likely to obtain seropositivity than those receiving one, or two or more therapeutic lines (95% compared with 73% and 63%, respectively; p = 0.001). Time from end of treatment to COVID‐19 vaccination influenced the rate of seropositivity, with patients receiving treatment 0–6 months prior to vaccination having the lowest rate of seropositivity (66%, p < 0.001 compared with no treatment). Type of treatment at vaccination significantly affected the rate of seropositivity. Patients receiving chemo‐immunotherapy (CIT), single‐agent anti‐CD20 therapy, BCL2 inhibitors, BTK inhibitors, as well as JAK2 inhibitors had the lowest rate of seropositivity (29%, 0%, 25%, 40%, and 42%, respectively). Type of treatment also remained significant when comparing between treatments given up to 6, 24 and 60 months prior to vaccination (p < 0.001 for comparison of seropositive proportions between treatment types at each time point). Patients who underwent auto‐SCT had the same rate of seropositivity as those who did not undergo SCT (p = 0.48).
FIGURE 2.
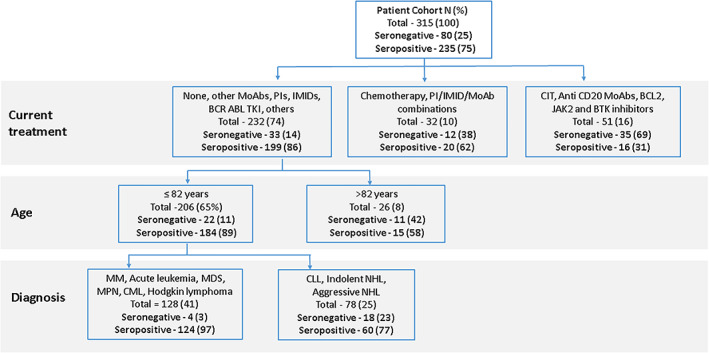
Classification tree for seronegativity using current treatment, age, and diagnosis. The first division for discriminating patients is based on current treatment. The second division is based on age >82 years or ≤82 years. The third division for patients aged ≤82 years is based on diagnosis. MoAb, monoclonal antibodies; PIs, proteasome inhibitors; IMIDs, immune modulatory drugs; TKI, tyrosine kinase inhibitors; CIT, chemo‐immunotherapy; BCL2, B‐cell lymphoma 2; JAK2, janus kinase 2; BTK, bruton tyrosine kinase; MM, multiple myeloma; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; NHL, non‐Hodgkin lymphoma [Color figure can be viewed at wileyonlinelibrary.com]
3.2. COVID‐19 antibody titers in hematologic malignancy patients
We found a negative correlation between post‐vaccination COVID‐19 antibody titers and age (R = −0.36, p < 0.001), as well as pre‐vaccination levels of LDH (R = −0.139, p = 0.02). Antibody titers were positively correlated with pre‐vaccination ALC counts (R = 0.219, p < 0.001) and total globulin levels (R = 0.188, p = 0.002).
Median antibody titers in the different types of hematologic malignancies are given in Table 2. Patients with CLL had the lowest antibody titers, with median [IQR] titers (3.45 [1.9–43] AU/ml) in the seronegative range, followed by indolent NHL (30.6 [1.9–158.3] AU/ml) and MM (46.8 [11.1–124.5] AU/ml). While there was no statistically significant difference in the rate of seropositivity in patients with active disease compared with those in remission, median [IQR] antibody titers were significantly lower in patients with active disease (65.7 [8.9–148.5] compared with 122 [33.2–189] AU/ml, p = 0.005).
Antibody titers were negatively correlated with the number of treatment lines (R = −0.192, p < 0.001), and positively correlated with the time lapsed from last treatment to vaccination (R = 0.31, p < 0.001).
For patients on current treatment, significantly lower antibody titers were found in patients treated with chemotherapy, CIT, single‐agent anti‐CD20 Ab, BCL2, BTK and JAK2 inhibitors. Patients receiving other MoAb, proteasome inhibitor/immune modulatory drug/MoAb (PI/IMID/MoAb) combinations and BCR‐ABL tyrosine kinase inhibitors (TKI) had a relatively preserved post‐vaccination antibody titer (Table 2).
Type of treatment also significantly influenced post‐vaccination antibody titers, when comparing between types of treatment given in the last 6, 24, and 60 months (data not shown).
3.3. Classification trees
CHAID and CART are decision tree techniques for partitioning data into homogeneous groups, creating a tree where each leaf (node) is the predicted target category. Categories that are not significantly different are merged into a single node. Possible cross‐tabulations for each categorical predictor are performed until the best outcome is achieved and no further splitting can be performed. The relationships between the split variables and the associated related factor within the tree are visualized.
Classification trees were applied in an attempt to identify subgroups of patients who are at risk for seronegativity following vaccination with the BNT162b2 COVID‐19 vaccine. Figure 2 demonstrates a classification tree incorporating age, type of current treatment, and diagnosis. As seen, the first division for discriminating patients is based on current treatment. Patients treated with CIT, single‐agent anti‐CD20 Ab, BCL2, BTK, or JAK2 inhibitors had close to a 70% chance of seronegativity, and those treated with chemotherapy or PI/IMID/MoAb combinations had an almost 40% chance of seronegativity. The second division is based on age. Patients >82 years old treated with other MoAbs, single‐agent PIs, IMIDs, BCR‐ABL TKI and other treatments or receiving no treatment had 42% chance of seronegativity. In the third division, based on diagnosis, patients aged 82 years or younger with a diagnosis of NHL or CLL had a 23% chance of seronegativity.
A second classification tree shown in Figure 3 discriminated serology results by current treatment, ALC, and treatment given within the 60 months prior to vaccination. In the first division, based on current treatment, patients treated with CIT, single‐agent anti‐CD20 Ab, BCL2, BTK, or JAK2 inhibitors had the highest risk for seronegativity (69%). The second division based on ALC further discriminated a subgroup of those patients with an ALC ≤1.150 × 103/μl and an extremely high seronegativity rate of 87%.
FIGURE 3.
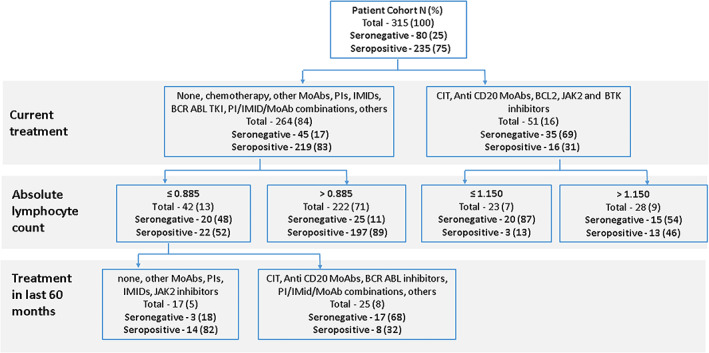
Classification tree for seronegativity using current treatment, absolute lymphocyte count and treatment in the past 60 months. The first division for discriminating patients is based on current treatment. The second division is based on the absolute lymphocyte count. The third division for patients with a lymphocyte count ≤0.885 is based on treatment given in the previous 60 months. MoAb, monoclonal antibodies; PIs, proteasome inhibitors; IMIDs, immune modulatory drugs; TKI, tyrosine kinase inhibitors; CIT, chemo‐immunotherapy; BCL2, B‐cell lymphoma 2; JAK2, janus kinase 2; BTK, bruton tyrosine kinase [Color figure can be viewed at wileyonlinelibrary.com]
For patients currently treated with chemotherapy, PI/IMID/MoAb combinations, other MoAbs, single‐agent PIs, IMIDs, BCR‐ABL TKI, other treatments or no treatment, and an ALC ≤0.885 × 103/μl, a third division according to treatment given in the last 60 months (including chemotherapy, CIT, BCR‐ABL TKI, PI/IMID/MoAb combinations, or other treatments), defined another subgroup with a high rate of seronegativity of 68%.
3.4. Clinical outcome
All study participants were approached via a telephone call at the end of the study to inquire about the possibility of SARS‐CoV‐2 infection. At a mean follow‐up of 63 days (range 19–94) following administration of the second vaccine, none of the study subjects developed clinical disease.
4. DISCUSSION
This is the first study consisting of a large cohort of patients with hematologic malignancies having received two doses of the BNT162b2 COVID‐19 vaccine. We found that a large proportion of these patients are seronegative following vaccination, and that anti‐SARS‐CoV‐2 antibody titers are low in seropositive patients, compared with the comparison group. Age, type of treatment, and diagnosis were the main factors influencing seroconversion in our cohort.
Vaccination is the most effective tool in slowing the spread of COVID‐19 and preventing serious illness; however, as previously shown, patients with hematologic malignancies have poor responses to vaccinations and could be at a disadvantage regarding COVID‐19 vaccination. This was demonstrated for influenza and pneumococcal vaccine compared with healthy controls and those with solid tumors, as well as for lymphoma patients treated with anti‐CD20 Abs. 10 , 11 In addition, these patients are almost universally excluded from trials testing novel vaccines, as is the case in trials assessing vaccines against SARS‐COV‐2, and therefore data on the protective effect of vaccination in this population are lacking.
Initial publications demonstrated immunogenicity of the BNT162b2 mRNA COVID‐19 vaccine in a dose‐dependent manner in both young and elderly healthy adults. The mean titer of neutralizing antibodies (28–35 days post‐vaccination) was higher than the mean titer of a panel of SARS‐CoV‐2 convalescent serum samples. 17 Thereafter the C4591001 Clinical Trial Group publication prompted FDA approval of the vaccine. In this pivotal placebo‐controlled trial, safety and efficacy were demonstrated in a cohort of over 43 500 participants. BNT162b2 was 95% effective in preventing COVID‐19 across subgroups defined by age, sex, race, ethnicity, baseline body‐mass index, and coexisting conditions. 8
Vaccination of the Israeli population with the BNT162b2 COVID‐19 vaccine began on December 20, 2020 and has progressed rapidly. To date over 4.9 million adults (> 16 years old), comprising 53.4% of the general population, have received two doses of vaccine. Over 85% of individuals, aged 60 and above, have been vaccinated with two doses. 18 These parameters have led to a rapid decline in the cumulative incidence of COVID‐19‐documented infection, symptomatic infection, hospitalization, severe disease, and death. Vaccine effectiveness for documented infections, symptomatic illness, hospitalization, and severe disease was reported at 92%, 94%, 87% and 92%, respectively, in this real‐world setting. 9 Furthermore, the Israeli Ministry of Health has issued a “green light passport” for people who have completed two vaccinations. This alleviates some of the restrictions put in place during the COVID‐19 pandemic, but could put those who remain seronegative at a potentially higher risk for infection. Antibody titers are also known to decrease with time, and thus patients starting out with lower titers might rapidly become seronegative, again placing them at potential risk.
Only a few reports on post‐vaccination seroconversion in immunocompromised populations are available. A recent study has shown that the majority of solid organ transplant recipients failed to mount an appreciable antibody response to the first dose of mRNA‐based COVID‐19 vaccine. 19 Herishanu et al. 20 have just published results of serologic responses to BNT162b2 COVID‐19 vaccine in a cohort of CLL patients showing that responses in these patients were poor. Only 39.5% of patients mounted a seropositive response (compared with 47% in our cohort) and response rates were even lower for those receiving treatment with BTK inhibitors, BCL2 inhibitors, or anti‐CD20 MoAbs, which is similar to our results. This is also consistent with a low rate of seroconversion reported in CLL patients with PCR‐positive SARS‐CoV‐2 infection, more than half of whom were on active treatment with either BCR inhibitors or a combination of a BCL2 inhibitor and anti CD20 MoAb. 21 In a recently published cohort of MM patients, seropositivity was demonstrated at a rate of 56% following the first dose of COVID‐19 vaccine, with patients not in complete response or very good partial response at a higher risk for seronegativity as well as those with immunoparesis and more prior treatment lines. Any therapy but no specific treatment was associated with seronegativity. 22 Comparably we demonstrated seropositivity in 76% of MM patients following vaccination with two doses.
Of special interest, treatment with ruxolitinib, the main JAK2 inhibitor used in our patient cohort, was associated with one of the lowest seropositivity rates and low antibody titers (42% and 10.9 [IQR: 5.7–66.4] AU/ml, respectively). This JAK1/JAK2 inhibitor has broad anti‐inflammatory activity. As the severe respiratory disease due to COVID‐19 has features consistent with cytokine release syndrome, ruxolitinib was studied in these patients. Treatment with ruxolitinib in severely ill COVID‐19 patients led to a reduction in COVID hyperinflammation scores and clinical improvement, although it was not statistically significant in a randomized control trial. 23 , 24 Therefore, it is plausible that ruxolitinib treatment could blunt the immune response following COVID‐19 vaccination, leading to seronegativity of treated patients.
Our large cohort included a variety of hematologic malignancies and resulted in several novel findings. Patients with CLL and indolent lymphoma developed the lowest antibody titers following vaccination. Patients on PI/IMID/MoAb combinations develop lower antibody titers than those on single‐agent PIs/IMIDs. Patients on JAK2 inhibitors had a high rate of seronegativity following vaccination. Acute leukemia, MDS, CML, BCR‐ABL negative MPN and Hodgkin lymphoma patients had a relatively preserved serologic response to COVID‐19 vaccination. Of note, most of the acute leukemia patients in our cohort were AML patients treated with the combination of azacytidine and venetoclax.
Anti‐COVID‐19 antibodies are an important component of immunity against SARS‐CoV‐2 infection, as is demonstrated by the efficacy of convalescent plasma in disease attenuation in the general population, as well as immune‐compromised populations. 25 Further, COVID‐19 seropositivity correlates with a very low risk of symptomatic reinfection as was demonstrated in a large cohort of healthcare workers. 26 Serologic response to vaccine, however, is not synonymous with protection against SARS‐CoV‐2 infection. It has been shown that exposure to SARS‐COV‐2 can induce a cellular immune response without seroconversion. 27 Also, most participants in the early BNT162b2 COVID‐19 vaccine trials mounted a virus‐specific CD4+ and CD8+ T‐cell immune response, which could convey long‐lasting memory immunity against COVID‐19, in addition to a robust serological response. 28 Such responses were demonstrated in SARS‐CoV‐1 survivors lasting 6–11 years. 29 Thus, it is plausible that patients diagnosed with hematologic malignancies may still benefit from vaccination through a cellular immune response, even when seronegative for antibodies to SARS‐CoV‐2.
Newer approaches to vaccinate patients with reduced immunological responses could include different vaccine design or dosing schedules, 30 as well as combining different coronavirus vaccines, 31 and these should be further studied.
Limitations of the current study include relatively small patient subgroups in some disease and treatment categories, which could lead to a confounding effect of diagnosis and treatment type. The distribution of antibody titers was extremely skewed, resulting in difficult to perform linear regression even after natural log transformation, and very large CI. Finally, post‐vaccination follow‐up of the study cohort was very short and we could not demonstrate a correlation between seronegativity or low antibody titers and clinical disease.
To conclude, older patients, those diagnosed with CLL, NHL, and MM, and those receiving CIT, single‐agent anti‐CD20 therapy, BCL2 inhibitors, BTK inhibitors, as well as JAK2 inhibitors are at risk for seronegativity following vaccination and thus are potentially still susceptible to COVID‐19 infection.
CONFLICT OF INTEREST
All authors declare there are no relevant disclosures or conflicting financial interests.
AUTHOR CONTRIBUTIONS
Katrin Herzog Tzarfati and Maya Koren‐Michowitz initiated and designed the study, collected, and analyzed data, and wrote the paper. Odit Gutwein, Arie Apel, Naomi Rahimi‐Levene, Maya Sadovnik, and Lotem Harel participated in data collection. Adina Bar Chaim and Patricia Benveniste‐Levkovitz were responsible for serological testing.
ACKNOWLEDGMENTS
We thank Dr Tomer Ziv‐Baran for statistical analysis support.
Herzog Tzarfati K, Gutwein O, Apel A, et al. BNT162b2 COVID‐19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195‐1203. 10.1002/ajh.26284
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. COVID‐19 dashboard by the Center for Systems Science and Engineering at John Hopkins University of Medicine. https://coronavirus.jhu.edu/map.html. Accessed April 20, 2021.
- 2. Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136:2881‐2892. 10.1182/blood.2020008824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID‐19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737‐e745. 10.1016/S2352-3026(20)30251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García‐Suárez J, De La Cruz J, Cedillo Á, et al. Impact of hematologic malignancy and type of cancer therapy on COVID‐19 severity and mortality: lessons from a large population‐based registry study. J Hematol Oncol. 2020;13:133. 10.1186/s13045-020-00970-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdul‐Jawad S, Baù L, Alaguthurai T, et al. Acute immune signatures and their legacies in severe acute respiratory syndrome coronavirus‐2 infected cancer patients. Cancer Cell. 2021;39:257‐275.e256. 10.1016/j.ccell.2021.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Letizia AG, Ramos I, Obla A, et al. SARS‐CoV‐2 transmission among marine recruits during quarantine. N Engl J Med. 2020;383:2407‐2416. 10.1056/NEJMoa2029717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412‐1423. 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yri OE, Torfoss D, Hungnes O, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118:6769‐6771. 10.1182/blood-2011-08-372649 [DOI] [PubMed] [Google Scholar]
- 11. La Torre G, Mannocci A, Colamesta V, et al. Influenza and pneumococcal vaccination in hematological malignancies: a systematic review of efficacy, effectiveness, and safety. Mediterr J Hematol Infect Dis. 2016;8:e2016044. 10.4084/mjhid.2016.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pullukcu H, Ertem E, Karaca Y, et al. Efficacy of accelerated hepatitis B vaccination program in patients being actively treated for hematologic malignancies. Int J Infect Dis. 2008;12:166‐170. 10.1016/j.ijid.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 13. Mullane KM, Morrison VA, Camacho LH, et al. Safety and efficacy of inactivated varicella zoster virus vaccine in immunocompromised patients with malignancies: a two‐arm, randomised, double‐blind, phase 3 trial. Lancet Infect Dis. 2019;19:1001‐1012. 10.1016/s1473-3099(19)30310-x [DOI] [PubMed] [Google Scholar]
- 14. Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137:185‐189. 10.1182/blood.2020008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DiaSorin's LIAISON® SARS‐CoV‐2 diagnostic solutions. https://www.diasorin.com/en/node/11792. Accessed April 20, 2021.
- 16. Oved K, Olmer L, Shemer‐Avni Y, et al. Multi‐center nationwide comparison of seven serology assays reveals a SARS‐CoV‐2 non‐responding seronegative subpopulation. EClinicalMedicine. 2020;29:100651. 10.1016/j.eclinm.2020.100651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walsh EE, Olmer L, Shemer‐Avni Y, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383:2439‐2450. 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. COVID‐19 Data Dashboard , Israeli Ministry of Health. https://datadashboard.health.gov.il/COVID-19/general. Accessed April 20, 2021.
- 19. Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS‐CoV‐2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784‐1786. 10.1001/jama.2021.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herishanu Y, Werbel WA, Avery RK, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. 10.1182/blood.2021011568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roeker LE, Knorr DA, Pessin MS, et al. Anti‐SARS‐CoV‐2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34:3047‐3049. 10.1038/s41375-020-01030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bird S, Panopoulou A, Shea RL, Tsui M, Saso R, et al. Response to first vaccination against SARS‐CoV‐2 in patients with multiple myeloma. Lancet Haematol. 2021;8(6):e389‐e392. 10.1016/s2352-3026(21)00110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao Y, Wei J, Zou L, Jiang T, Wang G, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID‐19): a multicenter, single‐blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137‐146.e133. 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. La Rosée F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID‐19 with severe systemic hyperinflammation. Leukemia. 2020;34:1805‐1815. 10.1038/s41375-020-0891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. NIH COVID‐19 Treatment Guidelines . https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/convalescent-plasma/. Accessed April 20, 2021.
- 26. Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS‐CoV‐2 infection in health care workers. N Engl J Med. 2021;384:533‐540. 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallais F, Velay A, Nazon C, Wendling MJ, et al. Intrafamilial exposure to SARS‐CoV‐2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis. 2021;27:113‐121. 10.3201/eid2701.203611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahin U, Muik A, Derhovanessian E, Vogler I, et al. COVID‐19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature. 2020;586:594‐599. 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 29. Ng OW, Chia A, Tan AT, et al. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post‐infection. Vaccine. 2016;34:2008‐2014. 10.1016/j.vaccine.2016.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parry H, Bruton R, Stephens C, Brown K, Amirthalingam G, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. medRxiv. 2021. 10.1101/2021.05.15.21257017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borobia AM, Carcas AJ, Pérez Olmeda MT, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1‐S‐primed participants (CombiVacS): a multicentre, open‐label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


