Abstract
Background
Critically ill patients with coronavirus disease 2019 (COVID‐19) are prone to developing macrothrombosis and microthrombosis. COVID‐19 has been reported to be rarely associated with thrombotic microangiopathies. A disintegrin and metalloprotease with thrombospondin type I repeats, member 13 (ADAMTS13) severe deficiency, the hallmark of thrombotic thrombocytopenic purpura (TTP), induces the formation of platelet, unusually large von Willebrand factor (VWF) multimer microthrombi. In immune‐mediated TTP, ADAMTS13 adopts specifically an open conformation. The VWF/ADAMTS13 couple may contribute to the microthrombi formation in pulmonary alveolar capillaries in COVID‐19.
Objective
To investigate clinical features, hemostatic laboratory parameters, VWF/ADAMTS13 axis, and ADAMTS13 conformation in critically ill COVID‐19 patients at admission.
Methods
Fifty three critically ill COVID‐19 patients were enrolled between March 18 and May 9 2020 in a monocentric hospital.
Results
The median age was 59 years and the male‐to‐female ratio was 2.8/1. We reported seven pulmonary embolisms and 15 deaths. Biological investigations showed increased fibrinogen and factor V levels, and strongly increased D‐dimers correlated with mortality. No patient presented severe thrombocytopenia nor microangiopathic hemolytic anemia. An imbalance between high VWF antigen levels and normal or slightly decreased ADAMTS13 activity levels (strongly elevated VWF/ADAMTS13 ratio) was correlated with mortality. Three patients had a partial quantitative deficiency in ADAMTS13. We also reported a closed conformation of ADAMTS13 in all patients, reinforcing the specificity of an open conformation of ADAMTS13 as a hallmark of TTP.
Conclusion
We suggest that slightly decreased or normal ADAMTS13 activity and highly elevated VWF are rather biomarkers reflecting both the strong inflammation and the endothelial damage rather than drivers of the thrombotic process of COVID‐19.
Keywords: ADAMTS13, ADAMTS13 conformation, COVID‐19, thrombotic microangiopathy, von Willebrand factor
Essentials
-
•
High frequency of macrovascular and microvascular thromboembolic events is reported in COVID‐19.
-
•
von Willebrand factor/ADAMTS13 ratio is strongly elevated and correlated to mortality in COVID19.
-
•
ADAMTS13 conformation is closed in severe COVID‐19 in contrast to immune‐mediated TTP.
-
•
VWF and ADAMTS13 are biomarkers of endotheliopathy in COVID‐19.
Alt-text: Unlabelled Box
1. INTRODUCTION
ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type I repeats, member 13) is the specific cleaving protease for von Willebrand factor (VWF), a multimeric glycoprotein released in blood from endothelial cells and that mediates platelet adhesion and aggregation.1 Under physiological conditions, ADAMTS13 regulates the size of VWF multimers to prevent platelet‐rich thrombi formation in the blood microvessels and it circulates in a closed conformation through a CUB‐spacer (complement component Clr/Cls, Uegf, and bone morphogenic protein 1) domains interaction, which is temporarily disrupted upon binding to VWF.2 The pathologic development of autoantibodies to ADAMTS13 induces a severe ADAMTS13 deficiency (activity <10 IU/dl), leading to the accumulation of ultralarge VWF multimers and subsequent systemic microvascular thrombosis that causes a specific thrombotic microangiopathy (TMA) named immune‐mediated thrombotic thrombocytopenic purpura (iTTP). Moreover, specifically in acute iTTP, ADAMTS13 adopts a sustained open conformation, likely mediated by ADAMTS13 autoantibodies.2., 3. In addition, bacterial and viral infections are triggers of TTP but their role on ADAMTS13 conformation is not known yet.
Interestingly, a high frequency of both macrovascular and microvascular thrombosis affecting lungs, kidneys, heart, and potentially complicated by multiorgan failure has been reported in prospective cohort studies led in critically ill patients with coronavirus disease 2019 (COVID‐19).4., 5., 6. This novel coronavirus, termed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), binds to angiotensin‐converting enzyme 2, highly expressed in lung alveolar cells but also in the vascular endothelium.6 The aggression of the endothelial host cells by SARS‐CoV‐2 explains both the endotheliopathy and the thromboinflammation processes supporting thrombotic microvascular events.6
Since 2020, to further understand the pathophysiology for SARS‐CoV‐2‐related microthrombosis, several studies have focused on the VWF/ADAMTS13 axis in COVID‐19 patients admitted on general wards and/or intensive care units (ICUs).7., 8., 9., 10., 11., 12. In summary, most results converge to an imbalance between VWF and ADAMTS13 showing markedly increased VWF antigen levels and VWF collagen‐binding capacity but normal or slightly decreased ADAMTS13 activity,7., 8., 9., 10., 11., 12., 13., 14. resulting in the formation of large VWF multimers,15., 16., 17., 18. with the latter biomarkers sometimes correlating with mortality.9., 10., 11., 12., 15., 17. This VWF/ADAMTS13 imbalance may support the concept of a secondary TMA‐like syndrome potentially present in some critically ill COVID‐19 patients and contributing to the microthrombi formation in pulmonary alveolar capillaries.8., 11., 15., 19. Surprisingly, however, ~20 TTP cases likely triggered by a SARS‐CoV‐2 infection were recently reported.20., 21., 22., 23., 24., 25. Also, the link between a TMA process and the COVID‐19‐associated microthrombosis may be supported by the clinical improvement and the reduction of circulating thromboinflammatory markers (i.e., the VWF/ADAMTS13 ratio) induced by plasma exchange undertaken in some critically ill COVID‐19 patients.15., 26. One ex vivo study suggested that purified recombinant ADAMTS13 should be considered as a potential therapy for COVID‐19 patients.16 To date however, no study has investigated ADAMTS13 conformation in COVID‐19 patients, although infectious triggers are candidates to explain the initial disruption of the interaction between self‐domains of ADAMTS13 leading to its switch from a folded to an open conformation.
In the current study, our major aim was thus to investigate hemostatic laboratory parameters and plasmatic ADAMTS13 features including ADAMTS13 conformation in critically ill COVID‐19 patients at ICU admission.
2. PATIENTS AND METHODS
2.1. COVID‐19 patients
The protocol was approved by the local institutional ethical committee. All consecutive patients admitted for acute respiratory distress syndrome (ARDS) by SARS‐CoV‐2 in the ICU of Saint‐Louis hospital (AP‐HP, Paris, France) between March 18 and May 9, 2020, were enrolled.27 Real‐time quantitative PCR assay for SARS‐CoV‐2 RNA on nasopharyngeal swab specimens was positive in all patients. Clinical data and outcome were analyzed from medical records.
2.2. Biological investigations
The following biological parameters were measured at admission: hemoglobin, platelet count, lactate dehydrogenase (LDH), fibrinogen, D‐dimers, and factor V in the context of care.
Measurement of VWF antigen (VWF:Ag) and ADAMTS13 investigation were also performed on citrated plasma samples collected at admission and stored at −80°C until use: VWF:Ag was measured with an ELISA (Asserachrom VWF:Ag), ADAMTS13 activity with FRETS‐VWF73 assay (Institute Inc), anti‐ADAMTS13 IgG titration with ELISA (TECHNOZYM ADAMTS‐13 INH, Technoclone), ADAMTS13 antigen with a homemade 3H9‐ELISA,2., 3. and ADAMTS13 conformation with a homemade 1C4‐ELISA, before and after incubation with the anti‐CUB1 antibody 17G2 inducing an open conformation in ADAMTS13 (defined by a conformation index >0.5).2., 3.
2.3. Statistical analysis
Quantitative variables were expressed as medians [25th, 75th percentiles] (minimum‐maximum) and categorical variables as numbers and percentages. Comparisons were performed using Mann‐Whitney and Fisher's exact tests, as appropriate, using GraphPad Prism v8.4.2 software (GraphPad Software). Any p values <0.05 were considered significant.
3. RESULTS AND DISCUSSION
Characteristics and outcome of the 53 patients enrolled are shown in Table 1 . The median age at admission was 59 years [53, 66] and the male‐to‐female ratio was 39/14 (73.6% of men). Forty‐three patients (81.1%) had at least one risk factor for cardiovascular disease such as overweight (body mass index: 25–29; n = 18, 34.0%) and obesity (body mass index ≥ 30, n = 16, 30.2%), dyslipidemia (n = 11, 20.7%), diabetes (n = 13, 24.5%), and hypertension (n = 24, 45.3%). The overall in‐hospital mortality rate was 28.3% (n = 15) and was higher in males (n = 13/39, 33%). Pulmonary embolism (PE) was diagnosed by computed tomography pulmonary angiography in seven patients (13.2%) with no history of thromboembolic event, a male‐to‐female ratio of 4/3, and associated with death in four cases (57% of patients with PE).
TABLE 1.
Characteristics of 53 critically ill COVID‐19 patients at ICU admission
| Parameters | Methodology | Reference Values | COVID‐19 Patients (n = 53) | Survivors (n = 38, 71.7%) |
Nonsurvivors (n = 15, 28.3%) |
p |
|---|---|---|---|---|---|---|
| Age (y) | Medical records | 59 [53, 66] (29–76) |
60 [50, 64] (29–76) |
58 [56, 67] (49–70) |
0.77 | |
| Gender | Medical records | 14F / 39 M | 12F / 26 M | 2F / 13 M | 0.18 | |
| BMI (kg/m2) | Medical records | <25 | 28 [24, 31] (20–47) |
28 [25, 32] (20–47) |
28 [25, 29] (20–37) |
0.66 |
| ≥2 cardiovascular risk factors | Medical records |
n = 22 (42%) |
n = 15 (39%) |
n = 7 (47%) |
0.63 | |
| Pulmonary embolism | CTPA |
n = 7 (13%) |
n = 3 (8%) |
n = 4 (27%) |
0.07 | |
| Time of hospitalization (in days) | Medical records | 196 [189, 202] (158–210) |
197 [190, 202] (158–208) |
193 [183, 203] (174–210) |
0.56 | |
| Hemoglobin (g/dl) | CBC (XN10, Sysmex) |
♂13–17 ♀12–16 |
11.9 [10.2, 13.3] (5.9–16.3) |
12.4 [10.8, 13.3] (5.9–16.3) |
11.6 [10.0, 12.4] (6.1–14.6) |
0.28 |
| Platelet count (×109/L) | CBC (XN10, Sysmex) |
150–450 | 207 [168, 275] (85–635) |
214 [179, 275] (85–461) |
183 [153, 286] (94–635) |
0.26 |
| LDH (U/L) | Enzymatic (Roche Cobas) |
135–225 | 844 [699, 939] (380–1597) |
806 [660, 870] (519–1597) |
909 [853, 1000] (380–1420) |
0.03 (p*) |
| IL‐6 (pg/ml) | ECLIA (Roche Cobas) |
<7 | 83.5 [40.5, 150.0] (3.3–2470.0) |
81.0 [38.5, 109.0] (3.3–319) |
113.0 [79.8, 299.5] (14.5–2470.0) |
0.03 (p*) |
| Fibrinogen (g/L) | Chronometric (STA‐FIB Liquid, Stago) |
2–4 | 6.68 [5.87, 7.60] (3.92–10,47) |
6.68 [5.96, 7.42] (3.92–9.47) |
6.70 [5.74, 7.98] (4.91–10.47) |
0.72 |
| D‐dimers (ng/ml) | Immunoturbidimetry (STA‐Liatest D‐Di Plus, Stago) |
<500 | 1120 [770, 2840] (270–20000) |
985 [675, 2185] (270–20000) |
2310 [1125, 3655] (730–20000) |
0.02 (p*) |
| Factor V (%) | Chronometric (STA‐Deficient V, Stago) |
70–120 | 140 [118, 154] (56–200) |
141 [123, 158] (56–200) |
127 [115 145] (92–195) |
0.21 |
| VWF antigen (IU/dl) | ELISA (Asserachrom VWF:Ag, Stago) |
50–150 | 354 [285, 429] (144–704) |
326 [284, 378] (144–704) |
416 [355, 554] (145–596) |
0.05 |
Note
Quantitative variables are expressed as medians [25th, 75th percentiles], (min‐max) and categorical variables as numbers and percentages.
Comparisons were performed using Mann‐Whitney and Fisher's exact tests, as appropriate. p‐values <0.05 were considered significant.
Abbreviations: BMI, body mass index; CBC, complete blood count; CTPA, computed tomography pulmonary angiography; ICU, intensive care unit; VWF, von Willebrand factor.
At ICU admission, three patients had a moderate thrombocytopenia over 80 × 109/L and 10 patients had an anemia with a hemoglobin level <10 g/dl without hemolysis. No patient had clinical nor biological features of TMA syndrome (platelet counts: 207 × 109/L [168, 275]; hemoglobin levels: 11.9 g/dl [10.2, 13.3]). All patients presented elevated LDH levels (844 U/L [699, 939]) and a marked increase of fibrinogen (6.68 g/L [5.87, 7.60]; >8 g/L in 9 patients [17%]), factor V (140% [118, 154]; >120% in 31 patients [59%]) and D‐dimers (1120 ng/ml [770, 2840]; >3000 ng/ml in 13 patients [25%]) (Table 1).28 Interestingly, nonsurvivor patients had significantly higher LDH, IL‐6, and D‐dimer concentrations when compared with survivor patients (LDH: 909 U/L [853, 1000] vs. 806 U/L [660, 870], p= 0.026; IL‐6: 113 pg/ml [79.8, 299.5] vs. 81 pg/ml [38.5, 109.0], p= 0.031; D‐dimers: 2310 ng/ml [1125, 3655] vs. 985 ng/ml [675, 2185], p= 0.018) (Table 1). These results are the most consistent biological features with intense inflammatory response and hypercoagulability in COVID‐19. However, no difference was reported when comparing PE with non‐PE COVID‐19 patients (data not shown). Our results, especially highly elevated D‐dimer levels predictive of mortality, are in line with those previously reported in critically ill COVID‐19 patients at admission.29
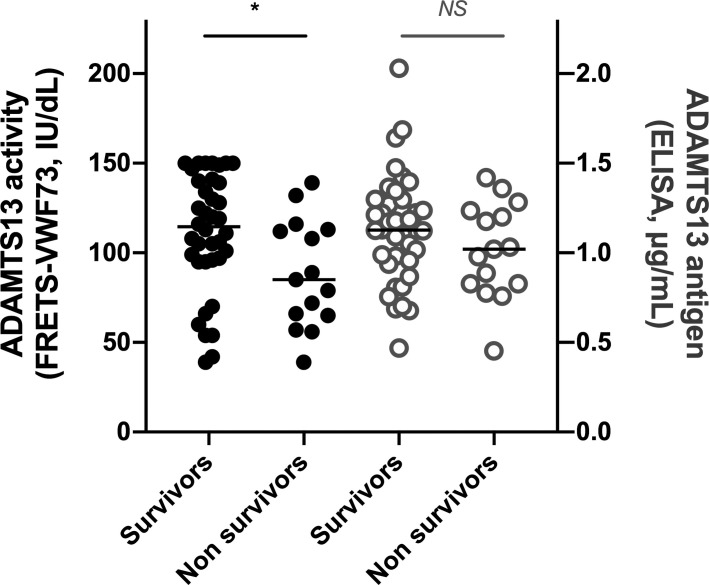
VWF:Ag levels were markedly increased in both survivor and nonsurvivor patients (326 [284, 378], vs. 416 IU/dl [355, 554], respectively) compatible with severe endothelial damage, but with borderline significant difference (p= 0.05) (Table 1). In our 53 critically ill COVID‐19 patients, ADAMTS13 activity ranged from 39 to 150 IU/dl (108 IU/dl [79, 132]), without detectable anti‐ADAMTS13 IgG (<15 U/ml in all patients), ADAMTS13 antigen ranged from 0.453 to 2.030 µg/ml (1.125 µg/ml [0.887, 1.269]), and only three patients (6%) had a partial quantitative deficiency in ADAMTS13 (both ADAMTS13 activity <50 IU/dl [42, 39, and 39 IU/dl] and ADAMTS13 antigen <0.50 µg/ml), ruling out TTP diagnosis (Figure 1 ). No significant difference of either VWF or ADAMTS13 was identified between patients with PE and those with no PE (data not shown). In contrast, interestingly, median ADAMTS13 activity was significantly lower in nonsurvivor patients when compared with survivor patients (85 vs. 115 IU/dl, p= 0.026) (Figure 1). As a consequence, median VWF:Ag/ADAMTS13 activity ratio was strongly elevated in nonsurvivors (4.94 [3.13, 7.21] vs. 3.01 [2.47, 3.69], p= 0.025) and was overall increased in the 53 patients (3.18 [2.59, 4.88], normal range 0.5–2). In contrast, ADAMTS13 antigen levels were not significantly different between survivors and nonsurvivors (1.127 vs. 1.020 µg/ml, p= 0.283) (Figure 1). Our results suggested a consumption of the metalloprotease ADAMTS13 by its massively increased substrate, VWF, in line with strong inflammation and endothelial damage and/or a partial catalytic inhibition of ADAMTS13 by IL‐6 and other cytokines released during the cytokine storm in severe COVID‐19.30
FIGURE 1.
ADAMTS13 activity and antigen in 53 critically ill COVID‐19 patients at ICU admission. ADAMTS13 activity (black circle) and antigen (gray circle) were measured in 53 COVID‐19 patients at admission, including 38 survivors and 15 nonsurvivors to their hospitalization. Comparisons were performed using Mann‐Whitney. p values <0.05 were considered significant. Median [25th, 75th percentiles] ADAMTS13 activity levels were 108 [79, 132] IU/dl in the total patients, 115 [96, 140] IU/dl in the survivors, and 85 [66, 113] IU/dl in the nonsurvivors, respectively. Median [25th, 75th percentiles] ADAMTS13 antigen levels were 1.125 [0.887, 1.269] μg/mL in the total patients, 1.127 [0.963, 1.289] μg/ml in the survivors, and 1.020 [0.828, 1.218] μg/ml in the nonsurvivors, respectively. ADAMTS13 activity was significantly lower in nonsurvivor patients when compared with survivor patients (p= 0.026), although no significant difference was observed for ADAMTS13 antigen between both groups
Interestingly, in all 53 COVID‐19 patients, ADAMTS13 was not captured by the monoclonal antispacer ADAMTS13 antibody 1C4, defining a closed conformation of ADAMTS13 (conformation index <0.5). As expected, in a control experiment, addition of the monoclonal anti‐CUB1 ADAMTS13 antibody 17G2 was able to induce an open conformation of ADAMTS13 in vitro in all 53 patients (Figure 2 ).
FIGURE 2.
ADAMTS13 conformation in 53 critically ill COVID‐19 patients at ICU admission. Plasma samples of 53 COVID‐19 patients at admission were added to wells coated with the anti‐ADAMTS13 antibody 1C4 before and after incubation with the anti‐CUB1 antibody 17G2 inducing an open conformation in ADAMTS13. If ADAMTS13 was not captured by 1C4, the conformation of ADAMTS13 is closed (conformation index ≤0.5). If ADAMTS13 was captured by 1C4, the conformation of ADAMTS13 is open (conformation index >0.5). All patients exhibited a closed ADAMTS13 conformation, which was induced open after addition of the anti‐CUB1 antibody 17G2
In this monocentric retrospective study, we confirm that SARS‐CoV‐2 infection leads to severe inflammatory response and hypercoagulability with markedly increased fibrinogen, factor V, D‐dimers, and IL‐6 levels in critically ill COVID‐19 patients at ICU admission, with both latter biologic parameters correlating with mortality.9., 10., 11., 12. Endotheliitis lesions were highly suggested by increased VWF levels. Previous studies supported a potential link between severe COVID‐19‐related microthrombosis and secondary TMA‐like syndrome enhanced by partial consumption and/or inhibition of ADAMTS13 by inflammatory cytokines as IL‐6, in line with a process similar to that observed in sepsis.1., 6. In COVID‐19, a phenomenon of thrombotic pulmonary capillaritis supporting ARDS could be partially explained by the unbalance between VWF and ADAMTS13 and play a crucial role in short‐term prognosis.6., 7., 8., 9., 10., 11., 12. In our group of 53 critically ill patients with COVID‐19, both ADAMTS13 activity and the VWF:Ag/ADAMTS13:activity ratio were correlated to mortality, in agreement with other studies.7., 8., 9., 10., 11., 12., 15., 17. However, this result needs to be nuanced because ADAMTS13 activity remained in the normal or subnormal range, so it cannot support a strong thrombotic tendency in the pathogenesis of ARDS. Also, none of our patients exhibited diagnostic hallmarks of classic TMA because there is no severe thrombocytopenia or microangiopathic hemolytic anemia. Of course, our study may be limited by its monocentric and its ~8‐week prospective enrollment. However, when considering our data combined with those of the recent literature, it seems more reasonable to think that both VWF and ADAMTS13 are biomarkers reflecting the severity of the endothelial disease caused by SARS‐CoV‐2 infection (and collateral prognosis biomarkers) rather than strong pathophysiologic actors of the microthrombotic process of COVID‐19‐associated ARDS.31 This interpretation is further supported by our main result showing that SARS‐CoV‐2 is not an infectious agent able to induce an open conformation of ADAMTS13, a biological feature that could be one of the triggers for the preliminary step to immune‐mediated ADAMTS13 deficiency.2., 3. Altogether, our results also reinforce the specificity of an open conformation of ADAMTS13 as a hallmark of iTTP.2., 3.
In conclusion, COVID‐19‐associated TMA is likely mostly restricted to the pulmonary microcirculation. This study suggests that normal or slightly low ADAMTS13 activity and highly elevated VWF are rather biomarkers reflecting strong inflammation and endothelial damage rather than drivers of the thrombotic process reported in COVID‐19. Further studies are definitely needed to consider the VWF/ADAMTS13 axis as a rational therapeutic option in severe COVID‐19.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Bérangère S. Joly performed and supervised the experiments, analyzed the data, and wrote the manuscript. Bérangère S. Joly and Michael Darmon performed the statistical analysis. Charlotte Dekimpe was highly involved in the ADAMTS13 conformation experiments, analyzed data, and critically reviewed the manuscript. Elie Azoulay, Michael Darmon, Thibault Dupont, Guillaume Dumas, and Elise Yvin enrolled the patients, provided the clinical data, and critically reviewed the manuscript. Nicolas Beranger performed some experiments and critically reviewed the manuscript. Karen Vanhoorelbeke, Elie Azoulay, and Agnès Veyradier supervised the study and critically reviewed the manuscript. All authors accepted the final version of the manuscript.
ACKNOWLEDGMENTS
This work was partly funded by KU Leuven grant COVID19‐Thrombosis in COVID19. We thank Sandrine Benghezal, Sophie Capdenat, Adeline Delton, and Sylvaine Savigny for expert technical assistance.
KU Leuven
Footnotes
Manuscript handled by: X. Long Zheng.
Final decision: Jill Johnsen and 01‐Jul‐2021.
REFERENCES
- 1.Roose E., Joly B.S. Current and future perspectives on ADAMTS13 and thrombotic thrombocytopenic purpura. Hamostaseologie. 2020;40:322–336. doi: 10.1055/a-1171-0473. [DOI] [PubMed] [Google Scholar]
- 2.Roose E., Schelpe A.S., Joly B.S., et al. An open conformation of ADAMTS‐13 is a hallmark of acute acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2018;16:378–388. doi: 10.1111/jth.13922. [DOI] [PubMed] [Google Scholar]
- 3.Roose E., Schelpe A.‐.S., Tellier E., et al. Open ADAMTS13, induced by antibodies, is a biomarker for subclinical immune‐mediated thrombotic thrombocytopenic purpura. Blood. 2020;136:353–361. doi: 10.1182/blood.2019004221. [DOI] [PubMed] [Google Scholar]
- 4.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients in severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joly B.S., Siguret V., Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID‐19. Intensive Care Med. 2020;46:1603–1606. doi: 10.1007/s00134-020-06088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rovas A., Osiaevi I., Buscher K., et al. Microvascular dysfunction in COVID‐19: the MYSTIC study. Angiogenesis. 2021;24:145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escher R., Breakey N., Lämmle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D‐dimers in COVID‐19 inpatients. Thromb Res. 2020;192:174–175. doi: 10.1016/j.thromres.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinelli N., Montagnana M., Pizzolo F., et al. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb Res. 2020;193:170–172. doi: 10.1016/j.thromres.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morici N., Bottiroli M., Fumagalli R., Marini C., Cattaneo M. Role of von Willebrand factor and ADAMTS‐13 in the pathogenesis of thrombi in SARS‐CoV‐2 infection: time to rethink. Thromb Haemost. 2020;120:1339–1342. doi: 10.1055/s-0040-1713400. [DOI] [PubMed] [Google Scholar]
- 10.Mancini I., Baronciani L., Artoni A., et al. The ADAMTS13‐von Willebrand factor axis in COVID‐19 patients. J Thromb Haemost. 2021;19:513–521. doi: 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry B.M., Benoit S.W., Oliveira M.H.S., Lippi G., Favaloro E.J., Benoit J.L. ADAMTS13 activity to von Willebrand factor antigen ratio predicts acute kidney injury in patients with COVID‐19: evidence of SARS‐CoV‐2 induced secondary thrombotic microangiopathy. International Journal of Laboratory Hematology. 2020;10 doi: 10.1111/ijlh.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philippe A., Chocron R., Gendron N., et al. Circulating von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID‐19 in‐hospital mortality. Angiogenesis. 2021:1–13. doi: 10.1007/s10456-020-09762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philippe A., Gendron N., Bory O., et al. Von Willebrand factor collagen‐binding capacity predicts in‐hospital mortality in COVID‐19 patients: insight from VWF/ADAMTS13 ratio imbalance. Angiogenesis. 2021:1–5. doi: 10.1007/s10456-021-09789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delrue M., Siguret V., Neuwirth M., et al. von Willebrand factor / ADAMTS13 axis and venous thromboembolism in moderate‐to‐severe COVID‐19 patients. Br J Haematol. 2021;192:1097–1100. doi: 10.1111/bjh.17216. [DOI] [PubMed] [Google Scholar]
- 15.Doevelaar A.A.N., Bachmann M., Hölzer B., et al. von Willebrand factor multimer formation contributes to immunothrombosis in coronavirus disease 2019. Crit Care Med. 2021;49:e512–e520. doi: 10.1097/CCM.0000000000004918. [DOI] [PubMed] [Google Scholar]
- 16.Turecek P.L., Peck R.C., Rangarajan S., et al. Recombinant ADAMTS13 reduces abnormally up‐regulated von Willebrand factor in plasma from patients with severe COVID‐19. Thromb Res. 2021;201:100–112. doi: 10.1016/j.thromres.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweeney J.M., Barouqa M., Krause G.J., Gonzalez‐Lugo J.D., Rahman S., Gil M.R. Low ADAMTS13 activity correlates with increased mortality in COVID‐19 patients. TH Open. 2021;5:e89–e103. doi: 10.1055/s-0041-1723784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascreau T., Zia‐Chahabi S., Zuber B., Tcherakian C., Farfour E., Vasse M. ADAMTS 13 deficiency is associated with abnormal distribution of von Willebrand factor multimers in patients with COVID‐19. Thromb Res. 2021:138–140. doi: 10.1016/j.thromres.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capecchi M., Mocellin C., Abbruzzese C., Mancini I., Prati D., Peyvandi F. Dramatic presentation of acquired thrombotic thrombocytopenic purpura associated with COVID‐19. Haematologica. 2020;105:e540. doi: 10.3324/haematol.2020.262345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaulieu M.‐.C., Mettelus D.S., Rioux‐Massé B., Mahone M. Thrombotic thrombocytopenic purpura as the initial presentation of COVID‐19. J Thromb Haemost. 2021;19:1132–1134. doi: 10.1111/jth.15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albiol N., Awol R., Martino R. Autoimmune thrombotic thrombocytopenic purpura (TTP) associated with COVID‐19. Ann Hematol. 2020;99:1673–1674. doi: 10.1007/s00277-020-04097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hindilerden F., Yonal‐Hindilerden I., Akar E., Kart‐Yasar K. Covid‐19 associated autoimmune thrombotic thrombocytopenic purpura: report of a case. Thromb Res. 2020;195:136–138. doi: 10.1016/j.thromres.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhingra G., Maji M., Mandal S., Vaniyath S., Negi G., Nath U.K. COVID 19 infection associated with thrombotic thrombocytopenic purpura. J Thromb Thrombolysis. 2021:1–4. doi: 10.1007/s11239-021-02452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tehrani H.A., Darnahal M., Vaezi M., Haghighi S. COVID‐19 associated thrombotic thrombocytopenic purpura (TTP); a case series and mini‐review. Int Immunopharmacol. 2021;93:107397. doi: 10.1016/j.intimp.2021.107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arulkumaran N., Thomas M., Brealey D., et al. Plasma exchange for COVID‐19 thrombo‐inflammatory disease. eJHaem. 2021;2(1):26–32. doi: 10.1002/jha2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azoulay E., Zafrani L., Mirouse A., Lengliné E., Darmon M., Chevret S. Clinical phenotypes of critically ill COVID‐19 patients. Intensive Care Med. 2020;46:1651–1652. doi: 10.1007/s00134-020-06120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susen S., Tacquard C.A., Godon A., et al. Prevention of thrombotic risk in hospitalized patients with COVID‐19 and hemostasis monitoring. Crit Care. 2020;24:364. doi: 10.1186/s13054-020-03000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L., Yan X., Fan Q., et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peigne V., Azoulay E., Coquet I., et al. The prognostic value of ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13) deficiency in septic shock patients involves interleukin‐6 and is not dependent on disseminated intravascular coagulation. Crit Care. 2013;17(6):R273. doi: 10.1186/cc13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falter T., Rossmann H., Menge P., et al. No evidence for classic thrombotic microangiopathy in COVID‐19. J Clin Med. 2021;10:671. doi: 10.3390/jcm10040671. [DOI] [PMC free article] [PubMed] [Google Scholar]