Abstract
Objective
The inflammatory/anti‐inflammatory balance has an important role in the clinical course of SARS‐CoV‐2 infection (COVID‐19), which has affected over 100 million people since it first appeared in China in December 2019. The aim of this study was to investigate the relationship between triggering receptor expressed on myeloid cells (TREM)‐1/TREM‐2 ratio and COVID‐19 severity.
Methods
A total of 171 individuals were included in the study: 121 patients who were admitted to the chest diseases department and intensive care unit of our hospital and diagnosed with COVID‐19 by real‐time PCR of nasopharyngeal swab samples from December 2020 to March 2021 and a control group consisting of 50 asymptomatic health workers in our hospital who had negative real‐time PCR results during routine COVID‐19 screening.
Results
TREM‐1 level was significantly higher in patients with severe disease compared with the moderate and control groups (P = .003, P = .001). TREM‐2 levels did not differ significantly in moderate and severe patients (P = .36) but were significantly higher in both patient groups compared with the control group (P = .001 for both). TREM‐1/TREM‐2 ratio was significantly higher in the severe patient group than in the moderate and control groups (P = .001 for both). In receiver operating characteristic curve analysis of TREM‐1/TREM‐2 ratio in patients with moderate and severe COVID‐19, the area under the curve was 0.723. Using a cut‐off value of 0.125 for TREM‐1/TREM‐2 ratio in the Youden index calculation, the sensitivity was 60% and specificity was 71%.
Conclusion
Experience with the positive effects of medical treatments to restore inflammatory balance in the course of COVID‐19 is steadily increasing. TREM‐1 and TREM‐2 have an important role in inflammation and may serve as biomarkers and therapeutic targets in the early treatment and follow‐up of COVID‐19.
What’s known
The triggering receptor expressed on myeloid cells (TREM) family of immune receptors can be synthesised from many tissues and cells, especially monocytes, macrophages, dendritic cells, and neutrophils.
TREM‐1 is a member of this family and induces the synthesis of tumour necrosis factor alpha (TNF‐α), interleukin 1 (IL‐1), monocyte chemoattractant protein‐1 and IL‐6, particularly in inflammatory diseases.
TREM‐2, another member of the TREM family, is also synthesised by macrophages and together with its receptor, DNAX‐activating protein of 12 kDa (DAP12), functions to suppress the proinflammatory response.
Initial studies with TREM‐2 in microglia cells showed that it plays an important role in suppressing TNF‐α and IL‐6 levels.
What’s new
Anti‐inflammatory treatments have yielded positive results and increased survival in COVID‐19.
Therefore, medical treatments that suppress TREM‐1 synthesis or increase TREM‐2 expression may have an important role in preventing disease progression.
In addition, detecting a high TREM‐1/TREM‐2 ratio early in the disease may provide early information about progression.
1. INTRODUCTION
SARS‐CoV‐2 is a non‐segmented, positive‐sense, single‐stranded RNA virus from the Coronaviridae family. Coronavirus infection is generally mild in humans; however, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) are serious respiratory tract diseases caused by coronaviruses that appeared in 2002 and 2012, respectively. 1
Respiratory failure in COVID‐19, the disease caused by SARS‐CoV‐2, is a serious manifestation that can lead to multiple organ involvement and death. At present, the immune pathogenesis of the disease has not been fully clarified, but the available data suggest that many possible mechanisms may be involved, such as antibody‐dependent enhancement, systemic inflammatory response, T‐cell overactivation and angiotensin‐converting enzyme 2 (ACE 2) receptor downregulation, and antibody cross‐reactivity to pneumocytes. 2 , 3 Overproduction of proinflammatory cytokines can cause endothelial dysfunction in many vital organs, especially the lungs. Proinflammatory cytokines synthesised by T helper cells and macrophages play an important role in this phenomenon, which is referred to as a cytokine storm. 4
The triggering receptor expressed on myeloid cells (TREM) family of immune receptors can be synthesised from many tissues and cells, especially monocytes, macrophages, dendritic cells and neutrophils. TREM‐1 is a member of this family and induces the synthesis of tumour necrosis factor alpha (TNF‐α), interleukin 1 (IL‐1), monocyte chemoattractant protein‐1 and IL‐6, particularly in inflammatory diseases. 5 Similar to COVID‐19, disruption of the inflammatory/anti‐inflammatory balance is important in sepsis. Mouse sepsis studies have shown that suppression of high TREM‐1 production with recombinant TREM‐1/Fc fusion protein or antagonistic peptides may prevent endotoxemia. However, a moderate concentration of TREM‐1 silencer RNA (siRNA) reduced mortality in sepsis while a higher concentration prevented respiratory burst in neutrophils. 6
TREM‐2, another member of the TREM family, is also synthesised by macrophages and together with its receptor, DNAX‐activating protein of 12 kDa (DAP12), functions to suppress the proinflammatory response. Initial studies with TREM‐2 in microglia cells showed that it plays an important role in suppressing TNF‐α and IL‐6 levels. 7 , 8 , 9 Subsequent studies indicated that TREM‐2 is involved in many neurodegenerative diseases, especially Alzheimer's disease. A substantial portion of the studies investigating TREM‐2 pertain to neurological diseases, but there is a paucity of data in the literature on its role in lung parenchymal diseases.
In this study, we aimed to investigate the relationship between serum TREM‐1, TREM‐2 and TREM‐1/TREM‐2 ratio and clinical course in COVID‐19, in which the inflammatory/anti‐inflammatory balance is an important factor.
2. MATERIALS AND METHODS
2.1. Study design
The study included 121 patients over 18 years of age who were diagnosed as having COVID‐19 using real‐time polymerase chain reaction (PCR) analysis of a nasopharyngeal swab sample and 50 health workers over 18 years of age who had no symptoms and a negative SARS‐CoV‐2 PCR result from December 2020 to March 2021.
High‐resolution computed tomography (HRCT) was performed routinely in all patients at high risk of COVID‐19. Predominantly peripheral bilateral ground‐glass opacities, subsegmental consolidation or linear opacities, crazy‐paving pattern and reverse halo sign were considered typical HRCT findings. Patients with these findings and patients with radiologically atypical findings but consistent clinical symptoms were hospitalised. Haematological and biochemical parameters including liver and kidney function tests, coagulation parameters, ferritin, D‐dimer, troponin‐I and C‐reactive protein (CRP) were analysed at admission and daily thereafter.
2.2. Study group
The 171 individuals in the study were divided into three groups. The first group included asymptomatic, PCR‐negative health workers who volunteered for the study (n = 50). The second group included patients with moderate illness, defined as clinical signs of pneumonia but no signs of severe pneumonia (respiratory rate ≥30 breaths/min, SpO2 ≤ 92%, and/or lung infiltration rate >50%) (n = 68). The third group included patients with severe illness, defined as having signs of severe pneumonia and developing macrophage activation syndrome (MAS) during follow‐up (n = 53).
2.3. Exclusion criteria
Exclusion criteria were the presence of chronic or clinically significant infectious or inflammatory conditions within the last month, asthma, chronic obstructive pulmonary disease (COPD), malignancy, invasive surgical procedures within the last month, uncontrolled hypertension, high fasting blood glucose, diabetes, cerebrovascular disease, kidney disease and coronary artery disease. Medical history and laboratory parameters obtained at admission were used to evaluate the patients for exclusion criteria. The cardiology, chest diseases and internal medicine departments were consulted regarding the presence of coronary artery disease, asthma, COPD and diabetes.
2.4. Definitions and diagnosis
Fever was defined as an axillary temperature of 37.3°C or higher. In patients with a high fever while under treatment for COVID‐19, blood, urine and sputum cultures were obtained to test for possible bacterial and fungal superinfections and empiric antibiotherapies were revised according to the culture results. Diagnosis and grading of acute respiratory distress were done using the Berlin 2015 diagnostic criteria. 10 Patients with elevated daily cardiac‐specific troponin levels underwent echocardiographic evaluation for nascent cardiac pathologies. Coagulopathy was defined as prothrombin and partial thromboplastin times 3 and 5 seconds longer than normal, respectively. The treatment strategy for each patient was determined based on their clinical severity and the Turkish Ministry of Health COVID‐19 adult diagnosis and treatment guidelines. Patients with findings such as refractory fever, persistently high or increasing CRP and ferritin levels, elevated D‐dimer level, cytopenia (lymphopenia or thrombocytopenia), abnormal liver function tests, hypofibrinogenemia or elevated triglyceride levels despite treatment were monitored for MAS. If serial measures demonstrated further deterioration in these parameters that could not be explained by secondary bacterial infection, the patients were treated for MAS with >250 mg/d methylprednisolone if they had no contraindication. The patients were followed up for 72 hours and those who did not show a clinical response were treated with 400 mg tocilizumab. After 24 hours, patients who still did not exhibit clinical and laboratory response received a second dose of tocilizumab.
2.5. Measurement of biochemical markers
After 15 minutes of semi‐supine rest, blood samples were obtained from an antecubital vein into tubes containing ethylenediaminetetraacetic acid to prevent coagulation. Troponin I concentrations were measured by chemiluminescent immunoassay using an Immulite 2500 (Siemens Medical Solutions, Erlangen, Germany). TREM‐1 and TREM‐2 concentrations were measured by enzyme‐linked immunosorbent assay (Elabscience human ELISA kit, UK).
2.6. Statistical analysis
The data were analysed using IBM SPSS Statistics for Windows version 20.0 (IBM Corp., Armonk, NY). Pearson's chi‐square test and Mann–Whitney U test were used for intergroup comparisons of parametric data and non‐normally distributed numerical data, respectively. Independent‐samples t test was used to compare demographic data and laboratory parameters between the groups. Wilcoxon analysis was used for intragroup comparisons of laboratory values during follow‐up. Receiver operating characteristic (ROC) curve analysis was used to determine whether the TREM‐1/TREM‐2 ratio had diagnostic value and an optimal cut‐off value was determined using Youden index. Pearson correlation analysis was used to evaluate relationships between TREM‐1/TREM‐2 levels and age, white blood cell (WBC) count, lactose dehydrogenase (LDH), ferritin, and ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2). A p‐value less than 0.05 was considered statistically significant.
3. RESULTS
The mean age of the COVID‐19 patients was 55.5 ± 14.3 years and that of the control group was 53.4 ± 16.1 years. There was no statistically significant difference in age between the study and control groups (P = .43). The study group included 73 men and 48 women. Thirty‐seven of the 68 patients with moderate COVID‐19 and 36 of the 53 patients with severe COVID‐19 were men. There was no statistically significant difference in sex between patients with moderate and severe COVID‐19 (P = .09).
A comparison of the patients’ laboratory data is shown in Table 1. Compared with patients with moderate COVID‐19, patients with severe illness had significantly lower lymphocyte count and PaO2/FiO2 levels (P = .001, P = .001) and significantly higher neutrophil/lymphocyte ratio (NLR), LDH, CRP, D‐dimer, ferritin and fibrinogen levels (P = .001, P = .001, P = .001, P = .006, P = .001, P = .001, respectively).
TABLE 1.
Comparison of age and laboratory data at admission in patients with moderate and severe COVID‐19
|
Moderate Illness (n = 68) Mean ± SD |
Severe Illness (n = 53) Mean ± SD |
P | |
|---|---|---|---|
| Age (y) | 56.3 ± 15.2 | 54.5 ± 13.2 | .495 |
| WBC (/µL) | 9550.9 ± 4067.6 | 13 756.1 ± 5546.4 | .001 |
| Lymphocytes (/µL) | 934.4 ± 481.7 | 540.9 ± 325.4 | .001 |
| Neutrophils (/µL) | 7689.3 ± 3952.6 | 9289.3 ± 4291.6 | .03 |
| NLR | 10.2 ± 7.8 | 28.4 ± 32.9 | .001 |
| AST (U/L) | 58.2 ± 40.2 | 58.4 ± 38.1 | .985 |
| ALT (U/L) | 71.5 ± 67.1 | 73.9 ± 71.2 | .853 |
| LDH (U/L) | 433.1 ± 134.4 | 630.2 ± 184.8 | .001 |
| GGT (U/L) | 73.6 ± 39.6 | 116.8 ± 226.9 | .126 |
| ALP (U/L) | 95.8 ± 49.4 | 97.9 ± 54.1 | .822 |
| Creatine (mg/dL) | 1.0 ± 0.8 | 0.9 ± 1 | .73 |
| Prothrombin time (s) | 13.1 ± 1.3 | 14.6 ± 4.3 | .07 |
| CRP (mg/dL) | 57.5 ± 64.5 | 165.5 ± 86.7 | .001 |
| Troponin‐I (ng/dL) | 26.4 ± 60.5 | 39.2 ± 46.8 | .127 |
| PaO2/FiO2 | 277.6 ± 72.2 | 174.3 ± 26.6 | .001 |
| D‐Dimer (ng/mL) | 1230.7 ± 1731.4 | 2935.4 ± 4669.8 | .006 |
| Ferritin (ng/mL) | 619.4 ± 373.7 | 1404.9 ± 300.5 | .001 |
| Fibrinogen (ng/mL) | 406.1 ± 141.3 | 502.4 ± 155.8 | .001 |
Statistically significant results are indicated in bold.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CRP, C‐reactive protein; GGT, gamma‐glutamyl transferase; LDH, lactose dehydrogenase; NLR, neutrophil to lymphocyte ratio; PaO2/FiO2, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen; SD, standard deviation; WBC, white blood cells.
The comparison of TREM‐1 and TREM‐2 levels between the control and patient groups is shown in Table 2. TREM‐1 level was significantly higher in patients with severe illness compared with patients with moderate illness and controls (P = .003, P = .001). TREM‐2 levels did not differ significantly between patients with moderate and severe illness (P = .36) but was significantly higher in both patient groups compared with the control group (P = .001 for both). TREM‐1/TREM‐2 ratio was significantly higher in the severe patient group than in the moderate and control groups (P = .001 for both).
TABLE 2.
Comparison of TREM‐1, TREM‐2, and TREM‐1/TREM‐2 ratio between COVID‐19 patients and healthy controls
| COVID‐19 Clinical Severity | Controls (mean ± SD) (n = 50) | P */P ** | ||
|---|---|---|---|---|
| Moderate (mean ± SD) (n = 68) | Severe (mean ± SD) (n = 53) | |||
| TREM‐1 (ng/mL) | 0.24 ± 0.08 | 0.29 ± 0.07 | 0.11 ± 0.02 | .003/.001 |
| TREM‐2 (ng/mL) | 4.2 ± 4.8 | 3.4 ± 4.2 | 1.8 ± 2.1 | .36/.001 |
| TREM‐1/TREM‐2 | 0.1 ± 0.05 | 0.14 ± 0.08 | 0.05 ± 0.01 | .001/.001 |
Statistically significant results are indicated in bold.
Abbreviations: TREM, triggering receptor expressed on myeloid cells, SD, standard deviation.
P *: Moderate vs severe COVID‐19 patients, P **: COVID‐19 patients vs controls.
Five patients with severe COVID‐19 died, while there was no mortality in the patients with moderate COVID‐19. Comparison of TREM‐1, TREM‐2, and TREM‐1/TREM‐2 ratio between the surviving and non‐surviving severe COVID‐19 patients showed that TREM‐1 level and TREM‐1/TREM‐2 ratio were statistically higher in the non‐surviving patients, while TREM‐2 level was higher in the survivors (P = .001, 0.001 and 0.02, respectively) (Table 3).
TABLE 3.
Comparison of TREM‐1, TREM‐2, and TREM‐1/TREM‐2 ratio between surviving and non‐surviving severe COVID‐19 patients
| Severe COVID‐19 (n = 53) | P | ||
|---|---|---|---|
| Survival (mean ±SD) (n = 48) | Mortality (mean ± SD) (n = 5) | ||
| TREM‐1 (ng/mL) | 0.27 ± 0.04 | 0.3 ± 0.06 | .001 |
| TREM‐2 (ng/mL) | 3.7 ± 2.9 | 3.1 ± 3.6 | .02 |
| TREM‐1/TREM‐2 | 0.15 ± 0.06 | 0.18 ± 0.04 | .001 |
Statistically significant results are indicated in bold.
Abbreviations: SD, standard deviation; TREM, triggering receptor expressed on myeloid cells.
TREM‐1/TREM‐2 ratio was positively correlated with age, WBC count and ferritin levels (r = .206, P = .05; r = .354, P = .01; r = .262, P = .01) and negatively correlated with PaO2/FiO2 (r = −.358, P = .01) (Figure 1).
FIGURE 1.
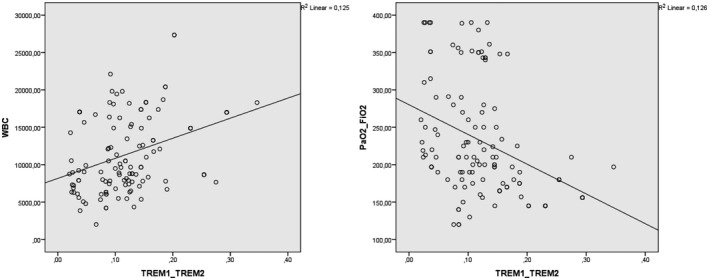
Correlation analysis between TREM‐1/TREM‐2 ratio and white blood cell count (WBC) and PaO2/FiO2 ratio
In the ROC curve analysis of TREM‐1/TREM‐2 ratio to differentiate between patients with moderate and severe COVID‐19 patients, the area under the curve was 0.723. Using the cut‐off value of 0.125 according to Youden index yielded a sensitivity of 60% and specificity of 71% (Figure 2).
FIGURE 2.
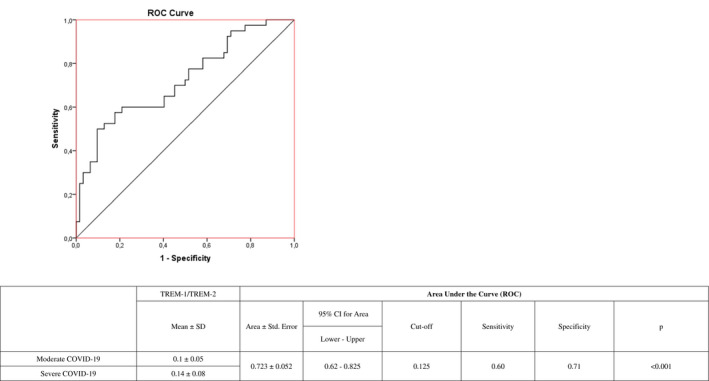
Receiver operating characteristic (ROC) curve analysis of TREM‐1/TREM‐2 ratio in the differentiation of patients with moderate and severe COVID‐19
4. DISCUSSION
The inflammatory/anti‐inflammatory balance plays an important role in the clinical course of COVID‐19. Our findings indicate that levels of TREM‐1, which has a pro‐inflammatory function, increased more in patients with severe illness, whereas levels of TREM‐2, which has an anti‐inflammatory role, did not show a similar increase to balance the increase in TREM‐1. This resulted in higher TREM‐1/TREM‐2 ratio in patients with severe COVID‐19 compared with those with moderate illness.
The World Health Organization (WHO) declared COVID‐19 a pandemic on 11 March 2020 and as of 24 April 2021, more than 146 million patients had been confirmed worldwide. Large‐scale studies on COVID‐19 have documented extensive lung damage both during and after infection. 11 A recent report stated that patients who had COVID‐19 pneumonia exhibited persistent abnormalities in lung CT scans after discharge and that ground‐glass opacities are the most common pulmonary sequelae. Data on COVID‐19 suggests that the disease affects the respiratory system more than other systems, in line with other diseases caused by coronaviruses such as SARS and MERS. 12
Many proinflammatory cytokines are released in COVID‐19, primarily TNF‐α, IL‐1, ‐2, ‐6 and ‐18, and nitric oxide. These cytokines may increase vascular permeability, leading to impaired tissue perfusion, endothelial damage and microthrombus formation. Increased vascular permeability causes fluid accumulation in the lung tissue and interstitial spaces, which clinically manifest as acute respiratory failure. 13 , 14 Suppression of these pro‐inflammatory cytokines has been shown to be therapeutically beneficial in many inflammatory conditions, including viral infections. 15 , 16 Failure to adequately suppress increased cytokine levels in the lungs with timely treatment leads to alveolar epithelial damage, alveolar septal fibrous proliferation, and hyaline membrane formation. This causes hypoxic respiratory failure or associated complications in a significant proportion of patients, resulting in higher mortality. Surviving patients may require long‐term oxygen therapy because of reduced lung compliance. 17 Therefore, restoring the inflammatory/anti‐inflammatory balance early and adequately helps prevent mortality and morbidity in COVID‐19. 18
The immune system is continuously being stimulated by infectious and non‐infectious inflammatory triggers. These molecules are initially recognised by pattern recognition receptors (PRRs), which are located intracellularly and on the surface of immune and non‐immune cells and recognise damage‐associated molecular patterns and microbial‐associated molecular patterns. Among these PRRs, the TREM family has attracted considerable attention in the last decade. TREM‐1 is activated by lipopolysaccharides, is primarily expressed on the surface of monocytes and neutrophils, and plays a role in acute inflammation. 19 TREM‐1 is also expressed on the surface of non‐immune cells such as hepatic endothelial cells and gastric epithelial cells during the inflammatory process. 20 , 21 TREM‐1 is activated during the inflammatory process and has been observed to increase the synthesis of proinflammatory cytokines such as IL‐1β, ‐6 and ‐18 via nucleotide‐binding oligomerisation domain‐containing protein 2 (NOD2) and nuclear factor kappa beta (NF‐κB). 22 , 23 Studies have shown that methylprednisolone therapy significantly reduces TREM‐1, IL‐6 and TNF‐α synthesis via NF‐κB in patients with acute respiratory distress syndrome. 24 Likewise, pravastatin and azithromycin were also found to significantly decrease proinflammatory cytokine levels, especially TREM‐1, when tested in mouse models of atherosclerosis and sepsis, respectively. 25 , 26 It was observed that TREM‐1‐deficient mice had markedly reduced morbidity secondary to viral and parasitic infections, while pathogen clearance was not affected. This led to the conclusion that TREM‐1 can be safely targeted as a therapeutic agent. 19 TREM‐2 is another member of the TREM family. Unlike TREM‐1, TREM‐2 is not synthesised on the monocyte surface but can be synthesised by macrophages, dendritic cells, osteoclasts, and microglial cells. TREM‐2 and its receptor DAP12 function as key regulators of the inflammatory response. 7 , 8 , 27 , 28 , 29 , 30 TREM‐2‐deficient macrophages and bone marrow‐derived dendritic cells have been shown to cause an increase in inflammatory cytokines via Toll‐like receptor. 7 , 27 , 28 Conversely, the expression of a chimeric receptor consisting of the extracellular domain of TREM‐2 and the intracellular domain of DAP12 in DAP‐deficient macrophages inhibited Toll‐like receptor‐induced cytokine production. 29 TREM‐2 also binds a wide variety of bacteria and acts as a phagocytic receptor for bacteria. 31 , 32 , 33 The multifunctional activities of TREM‐2 indicate that it may play a crucial role in infectious and inflammatory diseases such as sepsis. In a study conducted in a mouse sepsis model, abnormal TNF‐α, IL‐1β and IL‐6 levels were observed in TREM‐2‐ and DAP12‐deficient animals compared with the control group. 34 , 35
In our study, we observed that laboratory parameters, such as NLR, LDH, CRP, D‐dimer fibrinogen, and ferritin, which have been extensively investigated and found to be associated with morbidity and mortality in previous COVID‐19 studies, were increased in patients with severe COVID‐19 compared with those with moderate illness. When evaluated with previous studies, these parameters may be related to disease severity. The present study focused on TREM‐1 and TREM‐2 levels and showed that both of these parameters were increased in COVID‐19 patients compared with the control group, but the increase in TREM‐1 in correlation with disease severity was not observed in TREM‐2. As a result, TREM‐1/TREM‐2 ratio was higher in the severe COVID‐19 group than in the moderate COVID‐19 group. The inability of TREM‐2, which plays a role in anti‐inflammatory balance, to adequately balance TREM‐1, which plays an important role in proinflammatory cytokine discharge, may have contributed to the more severe disease progression. The inadequate TREM‐2 response in patients with severe COVID‐19 may be caused either by impaired expression of TREM‐2 itself or its receptor DAP12. This is consistent with our findings that TREM‐1 level and TREM‐1/TREM‐2 ratio were higher while TREM‐2 level was lower in the severe COVID‐19 patients who did not survive compared with the survivors. However, more extensive studies are needed to be able to verify these assumptions. The correlation between age and TREM‐1/TREM‐2 ratio may be evaluated as associated with the fact that COVID‐19 follows a more severe course in older patients. The positive correlation between TREM‐1/TREM‐2 ratio and WBC count may be because of an increase in the monocytes and neutrophils that synthesise TREM‐1 in patients with severe COVID‐19, while the negative correlation with PaO2/FiO2 ratio may be because of impaired oxygenation in these patients. In the ROC analysis of TREM‐1/TREM‐2 ratio in severe and moderate COVID‐19 patients, we found that at the optimal cut‐off value of 0.125 determined by Youden index, specificity was higher than sensitivity (71% vs 60%). This suggests that TREM‐1/TREM‐2 ratio may be more efficient in identifying patients with severe course.
The most important limitation of our study was the inability to investigate whether genetic factors may be responsible for the effects on TREM‐1, TREM‐2 or DAP12 synthesis. Obtaining these data may also facilitate our ability to maintain the anti‐inflammatory balance in COVID‐19 and prevent disease progression. Another limitation is that the small patient sample and the few patients with severe illness who died precluded a stronger statistical analysis. However, this can be primarily attributed to reaching patients relatively earlier after disease onset and to the widespread use of systemic steroids in intensive care patients.
In conclusion, anti‐inflammatory treatments have yielded positive results and increased survival in COVID‐19. Therefore, medical treatments that suppress TREM‐1 synthesis or increase TREM‐2 expression may have an important role in preventing disease progression. In addition, detecting a high TREM‐1/TREM‐2 ratio early in the disease may provide early information about progression.
5. DISCLSOURE
All authors disclosure no Conflict of Interest.
Kerget F, Kerget B, İba Yılmaz S, Kızıltunç A. Evaluation of the relationship between TREM‐1/TREM‐2 ratio and clinical course in COVID‐19 pneumonia. Int J Clin Pract. 2021;75:e14697. 10.1111/ijcp.14697
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wu D, Wu T, Liu Q, Yang Z. The SARS‐CoV‐2 outbreak: what we know. Int J Infect Dis. 2020;94:44‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yuki K, Fujiogi M, Koutsogiannaki S. COVID‐19 pathophysiology: A review. Clin Immunol. 2020:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID‐19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020;215:87‐93. [DOI] [PubMed] [Google Scholar]
- 4. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID‐19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ford JW, McVicar DW. TREM and TREM‐like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gibot S, Massin F, Marcou M, et al. TREM‐1 promotes survival during septic shock in mice. Eur J Immunol. 2007;37:456‐466. [DOI] [PubMed] [Google Scholar]
- 7. Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)‐2 and DAP12. J Immunol. 2006;177:2051‐2055. [DOI] [PubMed] [Google Scholar]
- 8. Turnbull IR, Gilfillan S, Cella M, et al. Cutting edge: TREM‐2 attenuates macrophage activation. J Immunol. 2006;177:3520‐3524. [DOI] [PubMed] [Google Scholar]
- 9. Raha AA, Henderson JW, Stott SR, et al. Neuroprotective effect of TREM‐2 in aging and Alzheimer’s disease model. J Alzheimers Dis. 2017;55:199‐217. [DOI] [PubMed] [Google Scholar]
- 10. Sjoding MW, Hofer TP, Co I, Courey A, Cooke CR, Iwashyna TJ. Interobserver reliability of the Berlin ARDS definition and strategies to improve the reliability of ARDS diagnosis. Chest. 2018;153:361‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Velavan TP, Meyer CG. The COVID‐19 epidemic. Tropical Med Int Health. 2020;25:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kerget B, Aksakal A, Kerget F. Evaluation of the relationship between laboratory parameters and pulmonary function tests in COVID‐19 patients. Int J Clin Pract. 2021:e14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kerget B, Aksakal A, Afşin DE, Araz O, Ucar EY, Akgün M. Acute respiratory distress syndrome after the use of gadolinium contrast agent. Respir. Med. Case Rep. 2018;25:336‐338. 10.1016/j.rmcr.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerget B, Araz O, Ucar EY, Akgun M, Sağlam L. Acute respiratory distress syndrome; A rare complication caused by usage of ruxolitinib. Respir. Med. Case Rep. 2017;22:243‐245. 10.1016/j.rmcr.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kerget B, Kerget F, Koçak AO, et al. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID‐19? Lung. 2020;198:777‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerget B, Kerget F, Aksakal A, Aşkın S, Sağlam L, Akgün M. Evaluation of alpha defensin, IL‐1 receptor antagonist, and IL‐18 levels in COVID‐19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J Med Virol. 2020;93(4):2090‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID‐19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stebbing J, Phelan A, Griffin I, et al. COVID‐19: combining antiviral and anti‐inflammatory treatments. Lancet Infect Dis. 2020;20:400‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dantas PHdS, Matos ADO, da Silva FE, Silva‐Sales M, Sales‐Campos H. Triggering receptor expressed on myeloid cells‐1 (TREM‐1) as a therapeutic target in infectious and noninfectious disease: a critical review. Int Rev Immunol. 2020;39:188‐202. [DOI] [PubMed] [Google Scholar]
- 20. Chen LC, Laskin JD, Gordon MK, Laskin DL. Regulation of TREM expression in hepatic macrophages and endothelial cells during acute endotoxemia. Exp Mol Pathol. 2008;84:145‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM‐1 and its potential ligands in non‐infectious diseases: from biology to clinical perspectives. Pharmacol Ther. 2017;177:81‐95. [DOI] [PubMed] [Google Scholar]
- 22. Netea MG, Azam T, Ferwerda G, Girardin SE, Kim SH, Dinarello CA. Triggering receptor expressed on myeloid cells‐1 (TREM‐1) amplifies the signals induced by the NACHT‐LRR (NLR) pattern recognition receptors. J Leukoc Biol. 2006;80:1454‐1461. [DOI] [PubMed] [Google Scholar]
- 23. Tejera A, Santolaria F, Diez M‐L, et al. Prognosis of community acquired pneumonia (CAP): value of triggering receptor expressed on myeloid cells‐1 (TREM‐1) and other mediators of the inflammatory response. Cytokine. 2007;38:117‐123. [DOI] [PubMed] [Google Scholar]
- 24. Jeon K, Chung CR, Yang JH, Park C‐M, Suh GY. Predictors of response to corticosteroid treatment in patients with early acute respiratory distress syndrome: results of a pilot study. Yonsei Med J. 2015;56:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tong J, Liu Z‐C, Wang D‐X. Azithromycin acts as an immunomodulatory agent to suppress the expression of TREM‐1 in Bacillus pyocyaneus‐induced sepsis. Immunol Lett. 2011;138:137‐143. [DOI] [PubMed] [Google Scholar]
- 26. Dai M, Chen Y, Mei X. Pravastatin sodium attenuated TREM‐1‐mediated inflammation in human peripheral blood mononuclear cells. Biochem Biophys Res Comm. 2019;508:225‐229. [DOI] [PubMed] [Google Scholar]
- 27. Gao X, Dong Y, Liu Z, Niu B. Silencing of triggering receptor expressed on myeloid cells‐2 enhances the inflammatory responses of alveolar macrophages to lipopolysaccharide. Mol Med Rep. 2013;7:921‐926. [DOI] [PubMed] [Google Scholar]
- 28. Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll‐like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ito H, Hamerman JA. TREM‐2, triggering receptor expressed on myeloid cell‐2, negatively regulates TLR responses in dendritic cells. Eur J Immunol. 2012;42:176‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Piccio L, Buonsanti C, Mariani M, et al. Blockade of TREM‐2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37:1290‐1301. [DOI] [PubMed] [Google Scholar]
- 31. Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM‐2: binding of anionic ligands. J Immunol. 2003;171:594‐599. [DOI] [PubMed] [Google Scholar]
- 32. N'Diaye E‐N, Branda CS, Branda SS, et al. TREM‐2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184:215‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quan DN, Cooper MD, Potter JL, Roberts MH, Cheng H, Jarvis GA. TREM‐2 binds to lipooligosaccharides of Neisseria gonorrhoeae and is expressed on reproductive tract epithelial cells. Mucosal Immunol. 2008;1:229‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharif O, Knapp S. From expression to signaling: roles of TREM‐1 and TREM‐2 in innate immunity and bacterial infection. Immunobiology. 2008;213:701‐713. [DOI] [PubMed] [Google Scholar]
- 35. Chen Q, Zhang K, Jin Y, et al. Triggering receptor expressed on myeloid cells‐2 protects against polymicrobial sepsis by enhancing bacterial clearance. Am J Respir Crit Care Med. 2013;188:201‐212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


