Abstract
Background
A systematic analysis of concomitant arterial hypertension in COVID‐19 patients and the impact of angiotensin‐converting‐enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs) have not been studied in a large multicentre cohort yet. We conducted a subanalysis from the international HOPE Registry (https://hopeprojectmd.com, NCT04334291) comparing COVID‐19 in presence and absence of arterial hypertension.
Materials and Methods
Out of 5837 COVID‐19 patients, 2850 (48.8%) patients had the diagnosis arterial hypertension. 1978/2813 (70.3%) patients were already treated with ACEI or ARBs. The clinical outcome of the present subanalysis included all‐cause mortality over 40 days of follow‐up.
Results
Patients with arterial hypertension suffered significantly more from different complications including respiratory insufficiency (60.8% vs 39.5%), heart failure (9.9% vs 3.1%), acute kidney injury (25.3% vs 7.3%), pneumonia (90.6% vs 86%), sepsis (14.7% vs 7.5%), and bleeding events (3.6% vs 1.6%). The mortality rate was 29.6% in patients with concomitant arterial hypertension and 11.3% without arterial hypertension (P < .001). Invasive and non‐invasive respiratory supports were significantly more required in presence of arterial hypertension as compared without it.
In the multivariate cox regression analysis, while age≥65, benzodiazepine, antidepressant at admission, elevated LDH or creatinine, respiratory insufficiency and sepsis might be a positive independent predictors of mortality, antiviral drugs, interferon treatment, ACEI or ARBs at discharge or oral anticoagulation at discharge might be an independent negative predictor of the mortality.
Conclusions
The mortality rate and in‐hospital complications might be increased in COVID‐19 patients with a concomitant history of arterial hypertension. The history of ACEI or ARBs treatments does not seem to impact the outcome of these patients.
Keywords: COVID, hypertension
1. INTRODUCTION
Since spreading the designated coronavirus disease 2019 (COVID‐19) in December 2019 and leading to a pandemic, the disease raises a real concern in all countries. Of note, at the 7 January 2020, this new coronavirus has been isolated by Chinese scientists from patients with severe acute respiratory syndrome (SARS‐CoV‐2) and patients with virus‐infected pneumonia. 1
Up to date, the highest infection rate and mortality rate linked to COVID‐19 are reported in United States, Italy, Spain, China and France. 2 Different models have been extracted to describe the severity of the disease, which has caused until now nearly 2.4 million deaths and more than 60 000 000 infections. 3 , 4 , 5 , 6 Of note, patients may present with symptoms such as fever, myalgia or fatigue, dry cough and diarrhoea. 7 But nevertheless, asymptomatic patients are not rare. A bevy of co‐factors have been debated as a risk of declined outcome and/or increased mortality including arterial hypertension, diabetes mellitus, heart diseases, chronic kidney diseases, chronic pulmonary diseases and elderly patients. 7 Despite the advantages in the description of patients with COVID‐19, the majority of illustrated data giving the spectrum of this disease are sparse and came from Wuhan and/or Lombardy. 8
A debate has been raised on the evidence that the majority of patients with the above‐mentioned co‐factors of COVID‐19 are suffering from arterial hypertension and treated with blockers of renin‐angiotensin system (RAS), which leads to the hypothesis that ACE2 (angiotensin‐converting enzyme 2) is the receptor allowing the entry of COVID‐19 into the cells and its expression might be increased by blockers of RAS.
Although no randomized trial evidence or systematic analysis is yet available on the effects of RAS blockers in treating COVID‐19, this issue prompted European Society of Cardiology (ESC) and The American College of Cardiology (ACC) to give a recommendation and suggested that physicians, who are already using RAS blockers, should continue treatment since the low evidence of harms. Nevertheless, it has been also recommended that an individualized treatment decision should be made according to each patient's haemodynamic status and clinical presentation. 9 These different reports, which based on lack of data, led to a huge debate among the lay press, physicians and patients.
In a recently published data registry, it seems that the history of use of RAS blockers might not decline the outcome of COVID‐19 patients. 10 , 11 , 12 , 13 These reports have included patients from individual healthcare systems with quite different patient characteristics, different backgrounds and different outcome points. Data from an international registry including different European countries have not been reported yet. This promoted us to analyse the large cohort of patients with a history of arterial hypertension with a focus on patients from the international multicentre HOPE Registry (Health Outcome Predictive Evaluation for COVID‐19).
2. METHODS
2.1. Study design and population
This multicentre international HOPE Registry (https://hopeprojectmd.com), Registry NCT04334291 on ClinicalTrials.gov included consecutive patients in an international manner.
The HOPE Registry was established through a consortium of physicians from Italy, Spain, Ecuador and Germany. Detailed information about participating countries and hospitals is reported on website of the Registry. All patients were diagnosed with COVID‐19 according to WHO interim guidance. In this interim analysis, hospital data and patients were included from the beginning of March until 2 June 2020. All patients discharged (deceased or alive) from any hospital centre were included in the HOPE Registry. Of at least 5837 patients, 48.8% of patients have had a history of arterial hypertension. The local ethics committee approved this study, and it was consistent with the guidelines of Helsinki. All local principal investigators reviewed the draft and checked for the accuracy and veracity of data. A list of participating hospitals, investigators, collaborators and the protocol are available on the website.
2.2. Data extraction
Epidemiological, clinical and outcome data were extracted from electronic medical records. The cut‐off for performing the data analysis corresponded to the HOPE COVID‐19 Registry predefined interim analysis until June 2020.
The individual components of all definitions of clinical outcomes were recorded separately and checked by at least two persons in each hospital. Patient's data were confidentiality protected by assigning all the data in anonym form, and the electronic data were stored and/or filled in a locked, password‐protected computer/website.
Throat swab samples were obtained from all patients at admission and tested using real‐time reverse transcriptase‐polymerase chain reaction assays according to the WHO recommendation. Additionally, patient data including blood test, coagulation and biochemical tests and chest x‐rays or computed tomography (CT) were extracted. Comorbidities were evaluated at admission including hypertension, dyslipidaemia, diabetes mellitus, obesity, current smoking, renal insufficiency, lung disease, cardiac disease, cerebrovascular disease, connective tissue disease, liver disease, cancer disease, Parkinson and dementia. In addition, different factors including the history of intake of immunosuppression, prior tuberculosis, human immunodeficiency virus (HIV) infection were evaluated. All drugs at admission were asked and are presented in supplementary Table S1 and S2.
2.3. Outcomes
The evaluated outcomes of the present analysis included the mortality rate in presence and absence of arterial hypertension. The admission to the intensive care unit (ICU) and type of respiratory support (nasal cannula, non‐invasive mechanical ventilation, invasive mechanical ventilation, or invasive mechanical support) was not included in the outcome analysis related to the fact that there might be a selection bias due to different capacity. In addition, different in‐hospital complications are presented.
2.4. Statistical analysis
Data are presented as means ± standard deviation for continuous variables with a normal distribution, median (interquartile range) for continuous variables with a non‐normal distribution, and as frequency (%) for categorical variables. The Kolmogorov‐Smirnov test was used to assess normal distribution. Student's t test and the Mann‐Whitney U test were used to compare continuous variables with normal and non‐normal distributions, respectively. The chi‐squared‐test or Fisher's exact test was used to compare categorical variables. Factors with P < .05 on univariate analysis were entered into the Cox multivariate regression analysis to define independent risk factors for the outcome. Statistical analysis was performed with SPSS statistics 23.0 in all analyses. All tests were 2‐sided, and a P value less than .05 was considered statistically significant.
Reporting of the study conforms to broad EQUATOR guidelines. 14
3. RESULTS
3.1. Demographics of COVID‐19 patients in presence and absence of arterial hypertension
Twenty‐five Percent of patients in presence of arterial hypertension were <65 years as compared to 67.6% without arterial hypertension (P < .001) and 75% ≥65 years in presence of arterial hypertension as compared to 32.4% without arterial hypertension (P < .001) with a predominance of male gender (60% vs 57.2%; P = .02), Table 1 and Table S1 and S2. Cardiovascular risk factors were significantly more presented in the cohort with arterial hypertension, dyslipidaemia (51.6% vs 16.8%), diabetes mellitus (29.8% vs 7.8%), obesity (30.1% vs 14.6%), current smoking (6.8% vs 5.1%) and cardiac diseases (36.5% vs 9.8%) including arrhythmias, coronary artery disease, cardiomyopathy and/or other cardiac diseases. On the other hand, pulmonary diseases were presented in 24% of patients with arterial hypertension as compared to 13.6% in patients without arterial hypertension. In 79.9% of patients in presence of arterial hypertension, oxygen therapy was required at admission as compared to 62.6% without arterial hypertension (P < .001). A detailed description of type of pulmonary disease, cardiac disease and cancer types is presented in Table S1 and S2.
TABLE 1.
Patients with arterial hypertension as compared to patients without aHt; baseline characteristics, laboratory and radiographic findings, complications and clinical outcomes
| Characteristic | Patients with hypertension N = 2850 | Patients without hypertension N = 2960 | P value* |
|---|---|---|---|
| Age—no. (%) | |||
| <65 | 713/2850 (25) | 2001/2958 (67.6) | <.001 |
| ≥65 | 2137/2850 (75) | 957/2958 (32.4) | <.001 |
| Male—no. (%) | 1710/2850 (60) | 1692/2960 (57.2) | .02 |
| ICU admission—no. (%) | 296/2850 (10.4) | 222/2960 (7.5) | <.001 |
| Chronic conditions—no. (%) | |||
| Dyslipidaemia | 1456/2822 (51.6) | 494/2947 (16.8) | <.001 |
| Diabetes mellitus | 848/2850 (29.8) | 232/2960 (7.8) | <.001 |
| Obesity | 680/2221 (30.1) | 349/2393 (14.6) | <.001 |
| Current smoking | 170/2514 (6.8) | 135/2673 (5.1) | .009 |
| Renal insufficiency a | 329/2850 (11.5) | 51/2960 (1.7) | <.001 |
| Lung disease | 684/2850 (24) | 404/2960 (13.6) | <.001 |
| Heart disease | 1035/2839 (36.5) | 288/2950 (9.8) | <.001 |
| Atrial fibrillation | 160/2850 (5.6) | 41/2960 (1.4) | <.001 |
| Cerebrovascular disease | 348/2779 (12.5) | 101/1908 (3.5) | <.001 |
| Connective tissue disease | 86/2780 (3) | 75/2912 (2.6) | <.001 |
| Liver disease | 135/2766 (4.8) | 77/2907 (2.6) | <.001 |
| Cancer disease | 496/2791 (17.8) | 284/2917 (9.7) | <.001 |
| Immunosuppression—no. (%) b | 238/2610 (9.1) | 176/2773 (6.3) | <.001 |
| Prior tuberculosis—no. (%) | 10/2850 (0.4) | 5/2960 (0.2) | <.001 |
| Human Immunodeficiency virus—no. (%) | 7/2850 (0.2) | 14/2960 (0.5) | .15 |
| Home Oxygen Therapy—no. (%) | 122/2819 (4.3) | 53/2937 (1.8) | <.001 |
| Premedication—no. (%) | |||
| ASA d | 672/2788 8 (24.1) | 191/2926 (6.5) | <.001 |
| Antiplatelet drug | 167/2741 (6.1) | 39/2899 (1.3) | <.001 |
| Oral Anticoagulation | 467/2778 (16.8) | 125/2921 (4.3) | <.001 |
| ACEi/ARB | 1978/2813 (70.3) | 78/2929 (2.7) | <.001 |
| Beta‐blockers | 781/2795 (27.9) | 145/2923 (5) | <.001 |
| Beta Agonist Inhalation Therapy | 359/2780 (12.9) | 212/2923 (7.3) | <.001 |
| Glucocorticoids Inhalation Therapy | 331/2793 (11.9) | 178/2928 (6.1) | <.001 |
| Vitamin D3 | 431/2777 (15.5) | 164/2915 (5.6) | <.001 |
| Benzodiazepine | 572/2800 (20.4) | 291/2929 (9.9) | <.001 |
| Antidepressant | 478/2792 (17.1) | 265/2923 (9.1) | <.001 |
| Symptomatic—no. (%) | |||
| Asymptomatic | 137/2803 (4.9) | 159/2926 (5.4) | .35 |
| Dyspnoea | 1706/2792 (61.1) | 1576/2910 (54.2) | <.001 |
| Tachypnoea >22 breaths per minute | 879/2727 (32.2) | 630/2847 (22.1) | <.001 |
| Haemoptysis | 59/2771 (2.1) | 37/2867 (1.3) | .01 |
| Fatigue | 1302/2726 (47.8) | 1273/2858 (44.5) | .01 |
| Anosmia/Hyposmia | 150/2618 (5.7) | 222/2744 (8.1) | .001 |
| Dysgeusia | 174/2615 (6.7) | 227/2740 (8.3) | .02 |
| Sore throat | 286/2659 (10.8) | 380/2776 (13.7) | .001 |
| Fever | 2150/2870 (74.9) | 2417/2930 (82.5) | <.001 |
| Cough | 1861/2791 (66.7) | 2040/2920 (69.9) | .01 |
| Vomiting | 198/2719 (7.3) | 222/2836 (7.8) | .44 |
| Diarrhoea | 534/2727 (19.6) | 553/2842 (19.5) | .90 |
| Erythromelalgia | 756/2703 (28) | 1041/2854 (36.5) | <.001 |
| Clinical parameters—no. (%) | |||
| Peripheral Oxygen Saturation <92% | 1205/2761 (43.6) | 779/2862 (27.2) | <.001 |
| Reduced Blood Pressure e | 254/2551 (10) | 153/2683 (5.7) | <.001 |
| GCS f < 15—no. (%) | 259/2311 (11.2) | 101/2468 (4.1) | <.001 |
| Laboratory parameters—no. (%) or median (IQR) | |||
| Elevated Di‐Dimer | 1703/2411 (70.6) | 1470/2506 (58.7) | <.001 |
| Elevated Procalcitonin | 543/2029 (26.8) | 356/2092 (17) | <.001 |
| Elevated CRP g | 2549/2766 (92.2) | 2445/2842 (86) | <.001 |
| Elevated TnI h | 292/1357 (21.5) | 112/1458 (7.7) | <.001 |
| Elevated Transaminases i | 1042/2599 (40.1) | 1103/2683 (41.1) | .45 |
| Elevated Ferritin | 881/1453 (60.6) | 898/1541 (58.3) | .18 |
| Elevated Triglyceride | 266/1238 (21.5) | 256/1329 (19.3) | .16 |
| Elevated LDHº | 1930/2543 (75.9) | 1771/2595 (68.2) | <.001 |
| Elevated Creatinine (>1.5 mg/dL) | 614/2755 (22.3) | 191/2837 (6.7) | <.001 |
| Leukocytopenia (<4000 10E9/l) | 357/2778 (12.9) | 476/2866 (16.6) | <.001 |
| Lymphocytopenia (<1500 10E9/I) | 2176/2720 (80) | 2095/2815 (74.4) | <.001 |
| Anaemia haemoglobin (<12 g/dL) | 902/2760 (32.7) | 556/2848 (19.5) | <.001 |
| Thrombocytopenia (<150 000 10E9/l) | 770/2768 (27.8) | 653/2855 (22.9) | <.001 |
| Moderate Hyponatremia | 149/2045 (7.3) | 82/2311 (3.5) | <.001 |
| Severe Hyponatremia | 44/1940 (2.3) | 14/2243 (0.6) | <.001 |
| In‐Hospital complication | |||
| Respiratory Insufficiency | 1709/2813 (60.8) | 1149/2908 (39.5) | <.001 |
| Heart Failure | 276/2782 (9.9) | 90/2893 (3.1) | <.001 |
| Acute kidney Injury | 708/2796 (25.3) | 211/2895 (7.3) | <.001 |
| Upper Respiratory Tract Infection | 354/2716 (13) | 367/2853 (12.9) | .85 |
| Pneumonia | 2537/2800 (90.6) | 2491/2898 (86) | <.001 |
| Sepsis | 407/2769 (14.7) | 216/2879 (7.5) | <.001 |
| Any relevant bleeding j | 98/2743 (3.6) | 47/2857 (1.6) | <.001 |
| Embolic event | 68/2773 (2.5) | 52/2866 (1.8) | .09 |
| Oxygen Therapy | |||
| O2 at the admission | 2225/2784 (79.9) | 1813/2895 (62.6) | <.001 |
| High‐flow Nasal Cannula | 631/2772 (22.8) | 478/2856 (16.7) | <.001 |
| Non‐Invasive Mechanical Ventilation | 442/2783 (15.9) | 329/2883 (11.4) | <.001 |
| Invasive Mechanical Ventilation | 227/2761 (8.2) | 182/2863 (6.4) | .007 |
| Another Medication or Intervention Procedures during the Admission | |||
| Prone Position | 324/2751 (11.8) | 241/2854 (8.4) | <.001 |
| ECMO k | 148/2754 (5.4) | 103/2858 (3.6) | .001 |
| Use of Glucocorticoids | 888/2766 (32.1) | 651/2867 (22.7) | <.001 |
| Use of Hydroxychloroquine | 2369/2803 (84.5) | 2447/2906 (84.2) | .74 |
| Use of Antiviral Drugs l | 1592/2792 (57) | 1842/2901 (63.5) | <.001 |
| Use of Interferon | 381/2745 (13.9) | 367/2861 (12.8) | .24 |
| Use of Tocilizumab | 230/2751 (8.4) | 238/2873 (8.3) | .91 |
| Use of Antibiotics | 2122/2669 (79.5) | 1980/2758 (71.7) | <.001 |
| ACEi/ARB | 1005/2667 (37.7) | 82/2826 (2.9) | <.001 |
| Anticoagulation | 1313/1635 (80) | 1159/1689 (68.6) | <.001 |
| Discharge | |||
| ACEi/ARB | 947/2850 (33.2) | 53/2959 (1.8) | <.001 |
| Antiplatelet Drug | 304/2160 (14.1) | 103/2582 (4) | <.001 |
| Anticoagulation Drug | 609/2781 (21.9) | 492/2892 (17) | <.001 |
| Death† | 845/2850 (29.6) | 335/2960 (11.3) | <.001 |
CrCL <30.
Immunosuppressive therapy for psoriasis arthritis, lung transplantation, kidney transplantation or systemic lupus erythematosus; oncological disease such as mamma‐ca, prostate‐ca, myelodysplastic syndrome or gammopathy; glucocorticoid therapy caused by COPD; dialysis; HIV or hepatitis.
Angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker
Acetylsalicylic acid.
Systolic blood pressure <90 mm Hg or diastolic blood pressure < 60 mm Hg.
Glasgow coma scale.
C‐reactive Protein.
High sensitive Troponin I (cardiac injury; troponin >99th percentile upper reference limit).
ALAT and ASAT.
Rectorrhagia, haematuria, epistaxis, and popliteal aneurysm bleeding with relevant decreased haemoglobin >2 mg/L.
Extracorporeal membrane oxygenation.
Lopinavir or/and Ritonavir.
3.2. Symptoms and drugs at admission in presence and absence of arterial hypertension
Dyspnoea, tachypnea, haemoptysis and fatigue were more presented in presence of arterial hypertension as compared without arterial hypertension. Other symptoms such as anosmia/hyposmia, dysgeusia, sore throat, fever, cough and erythromelalgia were less common in presence of arterial hypertension as compared without it. In presence of a history of arterial hypertension, a reduced peripheral oxygen saturation at admission was presented in 43.6% as compared to 27.2% (P < .001) and 10% of patients had a systolic blood pressure <90 mm Hg or diastolic blood pressure <60 mm Hg at admission as compared to 5.7% (P < .001).
Of patients with a history of arterial hypertension at admission, 24.1%% of patients were already treated with aspirin as compared to 6.5% without arterial hypertension (P < .001), 16.8% with therapeutic oral anticoagulation as compared to 4.3%, 70.3% in presence of arterial hypertension were treated with ACEI or ARBs as compared to 2.7% without arterial hypertension (P < .001) and 27.9% with beta‐blockers as compared to 5% (P < .001). A detailed description of other drug history is illustrated in Table 1.
3.3. Laboratory parameters in presence and absence of arterial hypertension
At admission, different laboratory parameters were elevated including D‐dimer, C‐reactive protein (CRP), LDH, creatinine, procalcitonin, TNI, transaminases and ferritin. 32.7% of patients suffering from arterial hypertension showed anaemia<12 g/dL as compared to 19.5% without arterial hypertension and 80% lymphocytopenia (<1500 10E9/I) as compared to 74.4% without arterial hypertension, Table 1.
3.4. Outcome data in presence and absence of arterial hypertension
Patients with arterial hypertension suffered significantly more from different complications including respiratory insufficiency (60.8% vs 39.5), heart failure (9.9% vs 3.1%), acute kidney injury (25.3% vs 7.3%), pneumonia (90.6% vs 86%), sepsis (14.7% vs 7.5%) and bleeding events (3.6% vs 1.6%). The mortality rate was 29.6% in patients with concomitant arterial hypertension and 11.3% without arterial hypertension (P < .001).
3.5. Treatment approaches in presence and absence of arterial hypertension
Invasive and non‐invasive respiratory support was significantly more required in presence of arterial hypertension as compared without, respectively. High‐flow nasal cannula was used in 22.8% in presence of arterial hypertension as compared to 16.7% in absence of arterial hypertension, non‐invasive mechanical ventilation in 15.9% in presence of arterial hypertension as compared to 11.4% without arterial hypertension, invasive mechanical ventilation in 8.2% as compared to 6.4% without arterial hypertension, ECMO in 5.4% in presence of arterial hypertension as compared to 3.6% without arterial hypertension and prone position 11.8% in presence of arterial hypertension as compared to 8.4% without arterial hypertension. Pneumonia was documented using chest x‐ray or CT in 90.6% in presence of arterial hypertension as compared to 86% without arterial hypertension. In addition to antibiotics, different drugs including glucocorticoids, chloroquine or hydrochloroquine, antiviral drugs Lopinavir or/and Ritonavir and therapeutic anticoagulation drugs were more used in patients with arterial hypertension.
3.6. Outcome and Predictors
We compared the mortality rate over 40 days of follow‐up and found that COVID‐19 patients with a concomitant arterial hypertension suffered more significantly from a higher mortality rate as compared without a history of arterial hypertension (Figure 1). A history of treatment with ACEI or ARB’s in presence of arterial hypertension showed a more declined outcome comparing to without a history of ACEI or ARB’s (Figure 2). To find out predictors for the combined outcome, we adjusted different factors together, Table 2. The variables were selected according to the recently published studies of COVID‐19 and our estimation of their impact. Approximately all variables including demographics, symptoms at admission, blood parameters, premedication and other relevant complications during the hospital stay and use of drugs during the hospital stay were evaluated. In the multivariate Cox regression analysis, whereas age≥65 (HR 2.26, 95% CI: 1.20‐4.24, P =.01), benzodiazepine (HR 2.20, 95% CI: 1.19‐4.05, P =.01), antidepressant at admission (HR 2.94, 95% CI: 1.60‐5.43, P =.001), elevated LDH (HR 2.59, 95% CI: 1.29‐5.20, P =.008), elevated creatinine (>1.5 mg/dl) (HR 3.94, 95% CI: 2.02‐7.67, P <.001) respiratory insufficiency (HR 6.27, 95% CI: 2.26‐17.45, P <.001) and sepsis (HR 2.24, 95% CI: 1.24‐4.07, P =.008) might be a positive independent predictors of mortality, and antiviral drugs (HR 0.46, 95% CI: 0.26‐0.81, P =.007), interferon treatment (HR 0.22, 95% CI: 0.08‐0.57, P =.002), ACEI/ARBs at discharge (HR 0.03, 95% CI: 0.00‐0.24, P =.001) and oral anticoagulation at discharge (HR 0.03, 95% CI: 0.01‐0.13, p«.001) might be independent negative predictor of the outcome.
FIGURE 1.
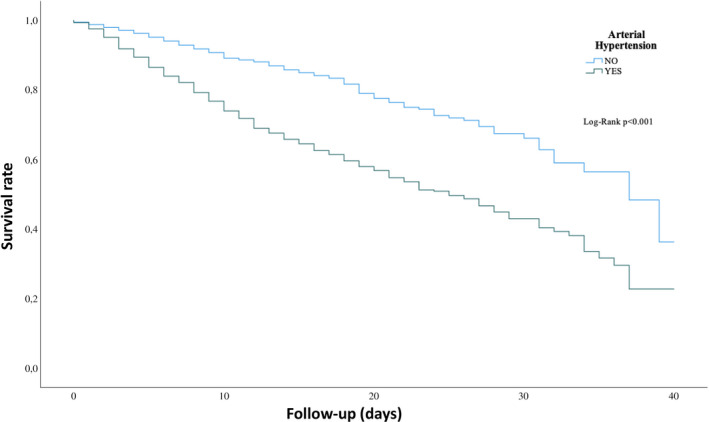
Kaplan‐Meier curve of survival over 40 days of follow‐up in presence and absence of arterial hypertension
FIGURE 2.
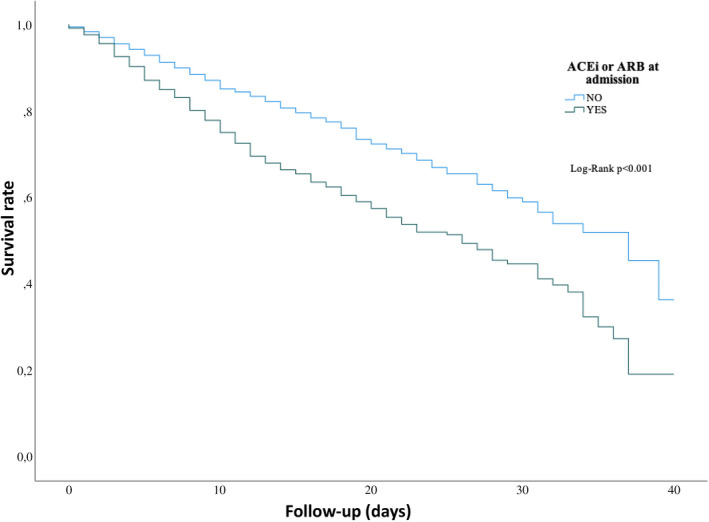
Kaplan‐Meier curve of survival in patients with arterial hypertension and concomitant treatment with ACEI or ARBs as compared to arterial hypertension without ACEI or ARB treatment
TABLE 2.
Predictors of Mortality, multivariate analysis
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Patients characteristics | ||||||
| Age ≥65 | 4.39 | 3.74‐5.16 | <.001 | 2.26 | 1.20‐4.24 | .01 |
| Male | 1.31 | 1.51‐1.49 | <.001 | |||
| ICU admission | 1.57 | 1.33‐1.85 | <.001 | |||
| Premedication | ||||||
| ASA a | 2.01 | 1.74‐2.31 | <.001 | |||
| Oral Anticoagulation | 2.39 | 2.39‐3.21 | <.001 | |||
| ACEi/ARB | 1.85 | 1.63‐2.09 | <.001 | |||
| Beta‐blockers | 1.67 | 1.44‐1.92 | <.001 | |||
| Benzodiazepine | 1.27 | 1.07‐1.51 | .005 | 2.20 | 1.19‐4.05 | .01 |
| Antidepressant | 1.64 | 1.38‐1.95 | <.001 | 2.94 | 1.60‐5.43 | .001 |
| Laboratory parameters—no. (%) or median (IQR) | ||||||
| Elevated Di‐Dimer | 2.53 | 2.11‐3.02 | <.001 | |||
| Elevated Procalcitonin | 2.48 | 2.13‐2.89 | <.001 | |||
| Elevated CRP | 3.60 | 2.56‐5.07 | <.001 | |||
| Elevated TnI | 2.56 | 2.10‐3.12 | <.001 | |||
| Elevated LDH | 3.08 | 2.52‐3.76 | <.001 | 2.59 | 1.29‐5.20 | .008 |
| Elevated Creatinine (>1.5 mg/dL) | 2.57 | 2.24‐2.94 | <.001 | 3.94 | 2.02‐7.67 | <.001 |
| Lymphocytopenia (<1500 10E9/I) | 1.90 | 1.58‐2.28 | <.001 | |||
| Anaemia haemoglobin (< 12 g/dL) | 1.59 | 1.40‐1.80 | <.001 | 0.51 | 0.30‐0.87 | .01 |
| Thrombocytopenia (<150 000 10E9/l) | 1.38 | 1.21‐1.57 | <.001 | |||
| Moderate Hyponatremia | 1.35 | 1.03‐1.77 | .02 | |||
| In‐Hospital complication | ||||||
| Respiratory insufficiency | 10.69 | 8.45‐13.53 | <.001 | 6.27 | 2.26‐17.45 | <.001 |
| Heart failure | 2.86 | 2.42‐3.37 | <.001 | |||
| Acute kidney injury | 4.01 | 3.54‐4.54 | <.001 | |||
| Pneumonia | 3.35 | 2.44‐4.60 | <.001 | |||
| Sepsis | 3.31 | 2.89‐3.79 | <.001 | 2.24 | 1.24‐4.07 | .008 |
| Any relevant bleeding b | 1.74 | 1.35‐2.25 | <.001 | |||
| Embolic events | 1.55 | 1.15‐2.07 | .003 | |||
| Oxygen therapy | ||||||
| O2 at the admission | 7.46 | 5.39‐10.32 | <.001 | |||
| High‐flow Nasal Cannula | 2.24 | 1.97‐2.55 | <.001 | |||
| Non‐Invasive Ventilation | 1.50 | 1.29‐1.75 | <.001 | |||
| Invasive mechanical ventilation | 2.14 | 1.82‐2.53 | <.001 | |||
| Therapeutic procedures in‐hospital | ||||||
| Glucocorticoids | 1.60 | 1.41‐1.81 | <.001 | |||
| Hydroxychloroquine | 0.54 | 0.47‐0.62 | <.001 | |||
| Antiviral Drugs c | 0.51 | 0.45‐0.57 | <.001 | 0.46 | 0.26‐0.81 | .007 |
| Interferon | 0.79 | 0.66‐0.94 | .008 | 0.22 | 0.08‐0.57 | .002 |
| Tocilizumab | 1.17 | 0.97‐1.40 | .09 | |||
| Antibiotics | 1.81 | 1.50‐2.17 | <.001 | |||
| Anticoagulation | 1.41 | 1.28‐1.56 | <.001 | |||
| Drugs at discharge | ||||||
| ACEI/ARB | 0.08 | 0.05‐0.12 | <.001 | 0.03 | 0.00‐0.24 | .001 |
| Anticoagulation | 0.12 | 0.09‐0.16 | <.001 | 0.03 | 0.01‐0.13 | <.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Acetylsalicylic acid.
Rectorrhagia, haematuria, epistaxis, and popliteal aneurysm bleeding with relevant decreased haemoglobin >2 mg/L.
Lopinavir or/and Ritonavir.
In addition, we compared the outcome and in‐hospital course of patients with continued treatment of ACEI ond ARBs vs stopped treatment. Different complications including respiratory insufficiency (RI), heart failure (HF), acute kidney injury (AKI), upper respiratory tract infection (URTI), pneumonia, sepsis, O2 requirement at admission, invasive respiratory support (MV) or use of high‐flow nasal cannula (HFNC) are significantly decreased in the group with continued use of ACEI or ARBs compared to the group, who stopped the treatment with ACEI or ARBs, Figure 3.
FIGURE 3.
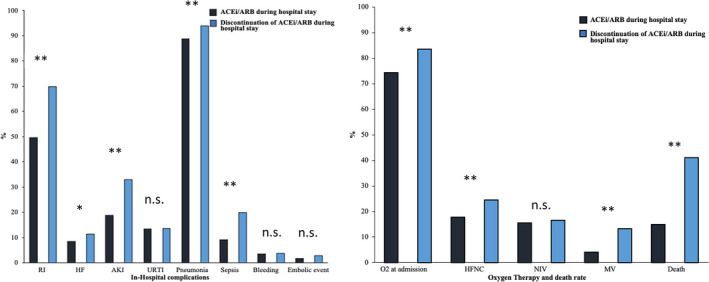
The outcome and in‐hospital course of patients with continued treatment of ACEI ond ARBs vs stopped treatment is presented. Different complications including respiratory insufficiency (RI), heart failure (HF), acute kidney injury (AKI), upper respiratory tract infection (URTI), pneumonia, sepsis, O2 requirement at admission, invasive respiratory support (MV) or use of high‐flow nasal cannula (HFNC) are compared (*P < .05, **P < .001)
4. DISCUSSION
We analysed data of 5837 patients COVID‐19 patients with approximately 48.8% presence of concomitant arterial hypertension and found the following: (i) the mortality rate of COVID‐19 patients with concomitant arterial hypertension is higher than without; (ii) in‐hospital complications are higher presented in presence of arterial hypertension; (iii) while age≥65, benzodiazepine, antidepressant at admission, elevated LDH and creatinine level, respiratory insufficiency and sepsis might be a positive independent predictors of mortality, antiviral drugs and interferon treatment, ACEI/ARBs or oral anticoagulation at discharge might be an independent negative predictor of the mortality; and (iii) continuing the ACEI/ARBs treatment in‐hospital may reduce the mortality rate and the in‐hospital complications of COVID‐19.
The attributable risk participation of arterial hypertension on cardiovascular disease is estimated up to 40%. 9 This risk could be reduced by ACEI and ARBs. Until now, without any evidence, it was controversial whether ACEI and ARBs could be harmful in COVID‐19 patients. The high debate in conventional, social media and scientific journals forced the major international societies to issue position statements with different intensity and different opinions. Fang et al 15 discussed a possible declined impact of ACEI and ARBs in COVID‐19 patients, which led to spread alarms among physicians and patients as well. This comment was taken into consideration and complicated by the evidence that arterial hypertension and diabetes mellitus were the highest comorbidity of COVID‐19 patients. In our non‐Asian cohort, the presence of arterial hypertension has been documented in approximately the half of patients. Of one of the first reported studies from Wuhan were cerebrovascular diseases (22%) and diabetes mellitus (22%) highly presented in the non‐survivors of the ICU. 16 Guan et al 17 also reported about hypertension and diabetes mellitus in (23.7%) and (16.2%) as well. Weeks later and after spreading the disease in Lombardy in Italy, one report about patient characteristics of the ICU showed that 49% of the whole cohort had a family history of arterial hypertension. 8 Notably, the most frequent comorbidities reported in small studies are often treated with ACEI and ARB’s. However, data about the impact of arterial hypertension or ACEI and ARB’s on the mortality rate are controversial.
Since the majority of our cohort is Caucasian from European countries, our data might to be compared with data from Lombardy. In their arterial hypertension patients, who were treated in the ICU, they reported about 38% of mortality, which was higher than patients without arterial hypertension (22%). In our cohort including all patients, we recorded a mortality rate of 29.6%, which is lower than the study from Lombardy. Of note, we decided not to use the ICU or respiratory support as one outcome parameter because of the differed capacity in hospitals. It has been reported that hypertension may delay virus clearance and could exacerbate inflammation in the airway. 18
A hypothesis suggested that angiotensin‐converting enzyme 2 (ACE2) is a functional receptor of the SARS‐CoV virus. 19 More in detail two years later, it has been reported that virus‐ACE2 receptor binding has a crucial role for cell invasion and for the manifestation of the disease. 20 After spreading the COVID‐19 and the raised evidence of a real pandemic, it has been documented that the SARS‐CoV2 receptor‐binding domain interacts with ACE2. 21 It has been thought that the virus may down‐regulate the ACE2 receptor without influence the ACE.
On the other hand, the ancestor of ARBs such as losartan might prevent the inflammation of the lung as has been shown in animal models 15 years ago. Based on these data, reviews speculated that ARB’s could be a possible treatment tool in COVID‐19 patients. 20 , 22
In the present large multicentre cohort, although the mortality rate of patients suffering from arterial hypertension is higher than without suffering from arterial hypertension, the multivariate analysis did not confirm an impact of prior treatment with ACEI and ARBs on the outcome of COVID‐19 patients. Moreover, it seems that continuing the treatment with ACEI or ARBs may reduce the mortality rate or complications including respiratory insufficiency, heart failure, acute kidney injury, upper respiratory tract infection, pneumonia, sepsis, O2 requirement at admission, invasive respiratory support or use of high‐flow nasal cannula. In a Danish cohort of COVID‐19 patients, data have shown that prior use of ACEI or ARBs was not significantly associated with mortality or severe disease among patients diagnosed as having COVID‐19. 10 However, the inpatient use of ACEI or ARBs in COVID‐19 patients with concomitant arterial hypertension improved the outcome and in‐hospital course of COVID‐19 patients. 23
We dissected the disseminated speculations about a hypothetical risk association between declined outcome and medications, which opened up the deleterious possibility of potentially dangerous recommendations to our patients with a high cardiovascular mortality risk. The present analysis presents data in a very large cohort and also may confirm the importance of continuing this treatment as the scientific societies have recommended.
4.1. Limitations
This study has several limitations. This subanalysis included data on a Registry basis. Data were acquired via online system from participating centres. The multicentre character of this cohort might be biased by heterogeneous therapy strategies due to differences of capacity in the ICUs, differences of capacity in hospitals and health resources as well. Furthermore, although the reported data are highly relevant, the follow‐up of the included cases might be too short. Finally, we do not report and compare the different patients according different groups of ACEI or ARBs. No information was provided regarding drug change of beta‐blockers and antidepressants.
5. CONCLUSIONS
Although a history of arterial hypertension or treatment with ACEI or ARBs is associated with a higher mortality rate at a follow‐up of 40 days, a multivariate analysis does not show an impact of ACEI or ARB’s on the mortality rate. These drugs should be continued in COVID‐19 patients, and these results have to be confirmed in randomized trials.
CONFLICT OF INTEREST
None.
Supporting information
Table S1‐S2
ACKNOWLEDGEMENTS
Cardiovascular Excellence SL, for their essential support in the database and HOPE webpage. All HOPE Researcher.
El‐Battrawy I, Núñez Gil IJ, Abumayyaleh M, et al. COVID‐19 and the impact of arterial hypertension—An analysis of the international HOPE COVID‐19 Registry (Italy‐Spain‐Germany). Eur J Clin Invest. 2021;51:e13582. 10.1111/eci.13582
Nuñez‐Gil and Ibrahim Akin equally contributed do this work.
NCT04334291 on ClinicalTrials.govMilan,
Funding information
Non‐conditioned grant (FUNDACIÓN INTERHOSPITALARIA PARA LA INVESTIGACIÓN CARDIOVASCULAR, FIC. Madrid, Spain). This non‐profit institution had no role in the study design; in the collection, analysis, interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication
Contributor Information
Ibrahim El‐Battrawy, Email: Ibrahim.elbattrawy2006@gmail.com.
Ivan J. Nuñez‐Gil, Email: ibnsky@yahoo.es, Email: Ibrahim.elbattrawy2006@gmail.com.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019‐nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS‐CoV‐2). Science. 2020;368(6490):489‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tian H, Liu Y, Li Y, et al. An investigation of transmission control measures during the first 50 days of the COVID‐19 epidemic in China. Science. 2020;368(6491):638‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan A, Liu L, Wang C, et al. Association of Public Health Interventions with the epidemiology of the COVID‐19 outbreak in Wuhan, China. JAMA. 2020;323(19):1915‐1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aronson JK, Ferner RE. Drugs and the renin‐angiotensin system in covid‐19. BMJ. 2020;369:m1313. [DOI] [PubMed] [Google Scholar]
- 10. Fosbol EL, Butt JH, Ostergaard L, et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324(2):168‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382(25):2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(9):1020‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 15. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trump S, Lukassen S, Anker MS, et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID‐19. Nat Biotechnol. 2020;39(6):705‐716. [DOI] [PubMed] [Google Scholar]
- 19. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11(8):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019‐nCoV. Biochem Biophys Res Commun. 2020;525(1):135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized With COVID‐19. Circ Res. 2020;126(12):1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


