Abstract
Background
There is still a lack of consensus on the efficacy of convalescent plasma (CP) treatment in COVID‐19 patients. We performed a systematic review and meta‐analysis to investigate the efficacy of CP vs standard treatment/non‐CP on clinical outcomes in COVID‐19 patients.
Methods
Cochrane Library, PubMed, EMBASE and ClinicalTrials.gov were searched from December 2019 to 16 July 2021, for data from clinical trials and observational studies. The primary outcome was all‐cause mortality. Risk estimates were pooled using a random‐effect model. Risk of bias was assessed by Cochrane Risk of Bias tool for clinical trials and Newcastle‐Ottawa Scale for observational studies.
Results
In total, 18 peer‐reviewed clinical trials, 3 preprints and 26 observational studies met the inclusion criteria. In the meta‐analysis of 18 peer‐reviewed trials, CP use had a 31% reduced risk of all‐cause mortality compared with standard treatment use (pooled risk ratio [RR] = 0.69, 95% confidence interval [CI]: 0.56‐0.86, P = .001, I 2 = 50.1%). Based on severity and region, CP treatment significantly reduced risk of all‐cause mortality in patients with severe and critical disease and studies conducted in Asia, pooled RR = 0.61, 95% CI: 0.47‐0.81, P = .001, I 2 = 0.0%; pooled RR = 0.67, 95% CI: 0.49‐0.92, P = .013, I 2 = 0.0%; and pooled RR = 0.62, 95% CI: 0.48‐0.80, P < .001, I 2 = 20.3%, respectively. The meta‐analysis of observational studies showed the similar results to the clinical trials.
Conclusions
Convalescent plasma use was associated with reduced risk of all‐cause mortality in severe or critical COVID‐19 patients. However, the findings were limited with a moderate degree of heterogeneity. Further studies with well‐designed and larger sample size are needed.
Keywords: convalescent plasma, COVID‐19, donors, emerging diseases, meta‐analysis, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)
1. INTRODUCTION
The coronavirus disease‐19 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has become an enormous health problem worldwide since December 2019. 1 As of 4 August 2021, there have been 199,466,211 confirmed cases of COVID‐19, including 4,244,541 deaths, which were reported by the World Health Organization (WHO). 2 The current management is mostly limited to general supportive care and symptomatic treatment using antivirals remdesivir and favipiravir, antimalarials chloroquine and hydroxychloroquine, and the antibiotic azithromycin. However, no specific drug or treatment has yet proven to be effective. So, clinical trials are ongoing in search for the suitable therapy. Immunotherapy with convalescent plasma (CP), the plasma collected from patients who have recovered from an infection, is one such therapeutic option.
Convalescent plasma has been advocated to treat outbreaks of novel infectious diseases those affecting the respiratory system including severe acute respiratory syndrome‐1 (SARS‐1), Middle East respiratory syndrome (MERS) and Ebola virus disease. 3 , 4 , 5 The antibodies primarily target the trimeric spike (S) surface glycoproteins, which are used by the virus to enter the host cells. This results in the reduction in the ability of the SARS‐CoV‐ACE2 to enter the host cells. Additionally, the antibody is long‐lasting after the onset of infection. 6 CP is currently being explored as one of the treatment opportunities for patients suffering from COVID‐19, which may contain antibodies to SARS‐CoV‐2 and may help suppress the virus as well as amending the inflammatory response. Therefore, in March 2020, the US Food and Drug Administration (US‐FDA) approved the use of CP therapy as an emergency investigational new drug to treat patients with serious or immediately life‐threatening COVID‐19 infections. Additionally, in February 2021, the FDA limited the use of high‐titre COVID‐19 CP only for the treatment of hospitalized patients with COVID‐19 who have impaired humoral immunity and cannot produce an adequate antibody response. 7 The results of the use of plasma are variable, reporting efficacy if its use is in the early stage of illness, which was associated with an improvement in the first days after treatment and lower requirements for ventilatory support. On the other hand, transfusion of COVID‐19 CP in hospitalized patients late in the course of illness has not been associated with clinical benefit. 8 However, evidence for therapeutic COVID‐19 CP efficacy still requires definitive support from large randomized clinical trials (RCT) and observational studies.
As the situation is evolving and newer studies are being reported across the globe, there is still a lack of consensus on the efficacy of CP usage in COVID‐19 patients. We therefore carried out the systematic review and meta‐analysis to evaluate the currently available data and provide evidence on the efficacy of CP for COVID‐19 patients’ treatment to provide an outline of the potential benefits of CP therapy in COVID‐19 patients.
2. MATERIALS AND METHODS
This study was conducted in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement. 9 A predefined study protocol was established but not registered. The study did not require any ethics committee approval as the research was done without patient involvement. Reporting of the study conforms to broad EQUATOR guidelines. 10
2.1. Data sources and search strategy
We searched the Cochrane Library, PubMed, EMBASE and ClinicalTrials.gov from December 2019 to 16 July 2021. The search terms included the following: COVID‐19, SARS‐CoV‐2 and convalescent plasma. The full search strategies for each database are available in Tables S1‐S4. The reference lists of the included studies, prior systematic reviews, and introduction and discussion sections of retrieved studies were also reviewed to identify additional relevant studies.
2.2. Study selection and eligibility criteria
We included clinical trials and observational studies that investigated the efficacy of CP treatment comparing to placebo/usual care/standard treatment in patients with COVID‐19 regardless of severity, level of antibody titre and healthcare settings. We included studies with a specific aim to treat COVID‐19 because the passive antibody administration may be an effective therapy for those patients who have yet to develop their own antibody response rather than the prevention. Studies with no comparator arm, case reports/case series, conference abstracts and systematic reviews were excluded. For overlapping participants, the studies with the longest follow‐up and the most detailed information were chosen. The primary outcome of interest was all‐cause mortality at any time point. The secondary outcomes were all‐cause mortality at 28 days, length of hospital stay, clinical improvement at 28 days and discharge rate at 28 days. The summary of the PICOS criteria used to identify the relevant studies is as follows: population (P)—patients with suspected or confirmed SARS‐CoV2 infection; intervention (I)—the use of CP to treat SARS‐CoV2 infection; comparator (C)—standard treatment or placebo or non‐CP use; outcome (O)—all‐cause mortality, all‐cause mortality at 28 days, length of hospital stay, clinical improvement at 28 days and discharge rate at 28 days; study design (S)—clinical trials or observational studies.
Two investigators (MS and JS) were independently screened titles and abstracts of all studies identified by the search to determine eligibility. Full texts were independently assessed in EndNote by two investigators (MS and JS) if they met the criteria for inclusion. Disagreement between investigators was resolved by consensus, if consensus could not be obtained, by consulting a third reviewer (CK or PM) who made the final decision.
2.3. Data extraction and quality assessment
Data were collected and tabulated by two reviewers (M.S and J.S) using Microsoft Excel. The included data were checked for accuracy by C.K and PM. A standardized data sheet was used to collect information on study characteristics. Data extraction variables included study design, country of study, setting, COVID‐19 severity, antibody titre, sample size, study sample characteristics, CP dose/volume and type of control. Mild, moderate, severe and critical diseases were defined using World Health Organization criteria. 11 Disagreement was resolved by consensus. The risk of bias was evaluated by two investigators (M.S and J.S). Clinical trials were appraised by the Cochrane risk of bias tool. 12 This tool includes seven domains for methodological evaluation: (a) sequence generation; (b) allocation concealment; (c) blinding of participants, personnel and outcome assessors; (d) incomplete outcome data; (e) selective outcome reporting; and (f) other sources of bias. The RCT was classified as low risk of bias (low risk of bias for all domains), high risk (high risk of bias for one or more domains) or unclear risk (unclear risk of bias for one or more key domains). For observational studies, we used the Newcastle‐Ottawa Scale (NOS). 13 Criteria included the following: adequacy selection of cohort, comparability of the study group and the outcome assessment. Studies with a total score of 8 or more were defined as high quality. Disagreement between investigators was resolved by consensus or, if consensus could not be obtained, by consulting a third reviewer (CK or PM), who made the final decision.
2.4. Statistical analysis
We analysed clinical trials and observational studies separately. In terms of clinical trials, meta‐analysis was performed separately for studies published in peer‐reviewed journals (primary analysis) and preprints (secondary analysis). For dichotomous outcomes such as all‐cause mortality, we performed a meta‐analysis using risk ratios (RRs) with 95% confidence intervals (CIs) as the common effect estimates. We recorded the number of events and total number of participants in both CP group and standard treatment group. For continuous outcomes using the same scale such as the length of hospital stay, we conducted analyses using the mean difference with 95% CIs. We recorded mean and standard deviation (SD) in both CP group and standard treatment group. For studies which reported only sample size, median, range and/or interquartile range (IQR), we estimated the sample mean and SD by using Wan et al’s method. 14 We performed meta‐analyses under the DerSimonian‐Laird random‐effects model to pool RR with 95% CIs assuming that the true effect size varied between studies. Homogeneity was assessed using the Cochran Q test, with P < .10. 15 The degree of heterogeneity was estimated by I 2. I2 value <25% indicated low, 25‐75% moderate and >75% high heterogeneity. 15 In order to explore possible sources of heterogeneity, subgroup analyses were carried out for primary outcomes for the following variables: (a) COVID‐19 severity, (b) geographical region, (c) blinding (opened‐label vs. blinded) and (d) randomization. For observational studies, we sub‐grouped based on severity, geographic region and study design (prospective studies versus retrospective studies). Sensitivity analysis was performed by using the ‘leave‐one‐out’ approach. In addition, we included all clinical trials [peer‐reviewed (n = 18) and preprints (n = 3)] and re‐analysed the effect of CP on all‐cause mortality in order to address the robustness of the findings. Given the fact that observational studies were prone to bias and confounding by indication, patients with severe COVID‐19 were more likely to receive CP treatment compared to those with mild or moderate disease. Accordingly, we re‐analysed the primary outcome by including only adjusted effect estimates from individual observational studies. A funnel plot was used to investigate any evidence of publication bias and was statistically assessed by Begg's and Egger's tests only when there were at least 10 studies included in the meta‐analysis. Statistical tests were two‐sided and used a significance threshold of P <.05. All analyses were conducted using STATA, v14.1 (StataCorp, Stata Statistical Software. College Station, TX: StataCorp LP; 2015).
3. RESULTS
3.1. Search results and study characteristics
A total of 4728 records were identified from databases, websites and citation searching. There were 47 studies 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 fulfilled the inclusion criteria and were used for the systematic review and meta‐analysis (Figure 1). Of 47 included studies, 21 were clinical trials 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 60 , 61 , 62 and 26 were observational studies. 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 55 , 56 , 57 , 58 , 59 Among clinical trials, there were 18 studies published in peer‐reviewed journals 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 while the other three were preprints. 60 , 61 , 62 Among clinical trials, there were 14 studies used the randomization process. 17 , 18 , 19 , 20 , 23 , 25 , 26 , 28 , 30 , 31 , 33 , 60 , 61 , 62 Four studies were double‐blind randomized controlled trials (RCTs) 26 , 30 , 31 , 33 whereas the other 17 were open‐label clinical trials. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 32 , 60 , 61 , 62 Three studies were undertaken in India; two in Iran and Argentina; and one each in China, Colombia, Kuwait, Saudi Arabia, the Netherlands, Spain, Iraq, the UK, the USA, Bahrain, Chile, Italy, Austria and the USA & Brazil. Among 21 included clinical trials, there were 7210 patients receiving CP and 7,878 patients receiving placebo/standard treatment with different levels of severity ranging from mild to critical COVID‐19 disease (Table 1). The quality of each clinical study was assessed. Based on Cochrane's risk of bias, 14 out of 21 studies had adequate generation of the allocation sequence. The majority of included clinical trials (n = 16) had high risk of performance bias. All studies provided complete outcome data and were clear from reporting bias (Table S5). For observational studies, there were ten studies conducted in the USA 35 , 42 , 45 , 50 , 52 , 54 , 55 , 56 , 57 , 59 ; three in China 36 , 37 , 43 ; three in Poland 40 , 48 , 58 ; three in India 41 , 47 , 51 ; two in Turkey 34 , 39 ; and one each in United Arab Emirates, 38 Austria, 44 Brazil, 46 Qatar 49 and Argentina. 53 Almost of observational studies included patients with severe or critical COVID‐19 disease (Table 2). Overall risk of bias assessment deemed to be good for cohort and case‐control studies. Sixteen studies 34 , 35 , 38 , 42 , 43 , 44 , 45 , 46 , 49 , 50 , 52 , 54 , 55 , 57 , 58 , 59 had summary scores ranging from 8 to 9 which represented as high quality (Table S6 and Table S7).
FIGURE 1.
PRISMA flow diagram
TABLE 1.
Baseline characteristics of included clinical trials (n = 21 studies)
| Author (Year) | Country | Settings | Study design | Clinical trial identifier | Severity | Sample size | Mean age (SD) | Antibody titre | Duration of COVID‐19 diagnosis until study treatment | CP dose | Type of control | % female | Ethnicity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Open‐label (Y/N) | Randomization (Y/N) | |||||||||||||
| Peer‐reviewed publications (n = 18 studies) | ||||||||||||||
| Abolghasemi et al (2020) 16 | Iran | Hospitals in Iran | Y | N | IRCT20200325046860N1 | Severe | 189 | CP gr = 54.41 (13.71), control gr = 56.83 (14.98) | NR | NR | One unit of CP (500 mL) within four hours and another unit if not improved after 24 hours | Standard care | CP gr = 41.7%, control gr = 50% | NR |
| Agarwal et al (2020) 17 | India | 39 tertiary care hospitals | Y | Y | CTRI/2020/04/024775 | Moderate | 464 | Median (IQR) CP gr = 52 (42‐60), control gr = 52 (41‐60) | Median (IQR) = 1:40 (1:30‐1:80) | NR | Two doses of 200 mL. CP, transfused 24 hours apart | Standard treatment | CP gr = 25%, control gr = 23% | NR |
| Li et al (2020) 18 | China | 7 medical centres in Wuhan | Y | Y | ChiCTR2000029757 | Severe and life‐threatening | 103 | Median (IQR) CP gr = 70 (62‐80), control gr = 69 (63‐76) | At least 1:640 | NR | CP dose: 4 to 13 mL/kg, transfused 10 mL for the first 15 mins, then increased 100 mL/hr | Standard treatment | CP gr = 48.1%, control gr = 35.3% | NR |
| Rasheed et al (2020) 19 | Iraq | Three hospitals | Y | Y | NR | Critical | 49 | CP gr = 55.66 (17.83), control gr = 47.82 (15.36) | NR | NR | CP 400 mL. | Standard treatment | CP gr only = 42.9% | NR |
| Abani et al (2021) 20 | UK | 177 National Health Service (NHS) hospital organizations | Y | Y | ISRCTN 50 189 673, NCT04381936 | Mixed | 11 558 | CP gr = 63.5 (14.7), control gr = 63.4 (14.6) | Neutralizing antibody titre ≥1:100 | NR | CP 2 units (275 mL. [200‐350 mL], the first as soon as possible and the second the following day at least 12 hr apart | Standard treatment | CP gr = 37%, control gr = 34% | CP gr (White 78%, Black/Asian/minority 14%, unknown 8%), control gr (White 77%, Black/Asian/minority 15%, unknown 8%) |
| Acosta‐Ampudia et al. (2021) 21 | Colombia | Clínica del Occidente, Clínica CES, Hospital Universitario Mayor Me'deri | Y | N | NCT04332380 and NCT04332835 | Severe | 18 | CP gr = 47.89 (9.69), control gr = 53.67 (6.71) | Titre IgG ≥1:3200, Titre IgA≥1:800 | NR | One dose of CP 250 mL, transfused two doses within 48 h. | Standard treatment | CP gr = 33.3%, control gr = 55.6% | NR |
| Allahyari et al. (2021) 22 | Iran | Imam Reza hospital | Y | N | IRCT20200409047007N1 | Critical | 64 | CP gr = 58.74 (14.67), control gr = 55.53 (14.10) | NR | NR | One cycle of CP 600 mL. transfused slowly | Standard treatment | CP gr = 43.75%, control gr = 43.75% | NR |
| AlQahtani et al (2021) 23 | Bahrain | Two medical centres | Y | Y | NCT04356534 | Severe | 40 | CP gr = 52.6 (14.9), control gr = 50.7 (12.5) | NR | NR | CP 400 mL, given as 200 mL over 2 hours over 2 successive days | Standard treatment | CP gr = 15%, control gr = 25% | NR |
| Alsharidah et al. (2021) 24 | Kuwait | Four major tertiary hospitals in Kuwait | Y | N | NR | Moderate/Severe | 368 | Median (IQR) CP gr = 54 (48‐60), control gr = 54 (45‐62) | NR | NR | 107 patients received 2 units of CP (each unit of containing 200 mL), 12 hours apart. and 28 received 1 unit of CP (200‐400 mL) | Standard treatment | CP gr = 22.2%, control gr = 15% | NR |
| Balcells et al (2021) 25 | Chile | A single Chilean medical centre | Y | Y | NCT04375098 | Moderate/severe | 58 | Mean (range)CP gr = 64.3 (33‐92), control gr = 67.1 (27‐91) | Anti‐SARS‐CoV‐2 (S1) IgG titres ≥1:400 | ≤ 7 days | 400 mL. of CP, infused as two 200 mL. units, each separated by 24 hours | Deferred plasma group (received CP only if a pre‐specified worsening respiratory function criterion was met) | CP gr = 46.4%, deferred gr = 53.3% | NR |
| Gharbharan et al (2021) 28 | the Netherlands | 14 secondary and academic hospitals | Y | Y | NCT04342182 | Moderate/severe | 86 | Median (IQR) CP gr = 63 (55‐77), control gr = 61 (56‐70) | Neutralizing antibody titres of at least 1:80 | ≤ 96 hours | CP 300 mL | Standard treatment | CP gr = 33%, control gr = 23% | NR |
| Libster et al (2021) 30 | Argentina | Clinical sites and geriatric units | N | Y | NCT04479163 | Mild | 160 | CP gr = 76.4 (8.7), control gr = 77.9 (8.4) | IgG titre greater than 1:1000 | ≤ 72 hours | CP 250 mL, given over period of 1.5 to 2 hours. | Placebo | CP gr = 68%, control gr = 58% | NR |
| O’Donnell et al (2021) 31 | USA and Brazil | Five hospitals in USA and Brazil | N | Y | NCT04359810 | Severe | 223 | Median (IQR) CP gr = 60 (48‐71), control gr = 63 (49‐72) | Titre of ≥1:400 | ≤ 48 hours | A single unit of CP (200‐250 mL) was transfused over 2 hours. | Normal control plasma | CP gr = 36%, control gr = 30% | NR |
| AlShehry et al. (2021) 32 | Saudi Arabia | 22 hospitals | Y | N | NCT04347681 | Critical | 164 | CP gr = 50.25 (14.90), control gr = 52.59 (12.79) | NR | Anytime | CP infused 300 mL (200‐400mL/dose) | Standard treatment | CP gr = 17.5%, control gr = 16.1% | NR |
| Simonovich et al (2021) 33 | Argentina | 12 clinical sites and coordinated by Hospital Italiano de Buenos Aires | N | Y | NCT04383535 | Severe | 333 | Median (IQR) CP gr = 62.5 (53‐72.5), control gr = 62 (49‐71) | Median titre 1:3200 (IQR 1:800 to 1:3200) | NR | CP 500mL (IQR; 415‐ 600 mL) | Placebo and standard treatment | CP gr = 29.4%, control gr = 39% | NR |
| Bennett‐Guerrero E et al (2021) 26 | USA | Hospital in New York. | N | Y | NCT04344535 | Unspecified | 74 | CP gr = 67 (15.8), control gr = 64 (17.4) | NR | NR | A single dose of 2 units of CP (240 mL/unit) over 1‐4 hours. | Standard plasma | CP gr = 39%, control gr = 46.7 | NR |
| Franchini et al (2021) 27 | Italy | the city hospital of Mantua | Y | N | NCT04569188 | Moderate/severe | 755 | Median (IQR) = 87 (82‐90) | Titre of 1:160 or greater | NR | 1‐3 units (300 mL/unit) | Non‐ convalescent plasma | 50% | NR |
| Hoepler et al (2021) 29 | Austria | Hospital setting, single centre | Y | N | The atient had been enrolled in the ACOVACT | Critical | 194 | Median (range)CP gr = 61 (25‐86), non‐CP gr = 63 (20‐87) | >1:100 | Median = 8 days | 200 mL given over 30 mins | Non‐CP | CP gr = 16.4%, non‐CP gr = 28.9% | NR |
| Preprints (n = 3 studies) | ||||||||||||||
| Avendaño‐Solà et al (2020) 60 | Spain | 14 hospitals | Y | Y | NCT04345523 | Severe | 81 | Median age = 59 | Neutralizing antibodies titres >1:80 | ≤12 days | Single unit of CP (250‐300 mL) | Standard treatment | 45.7% | NR |
| Bajpai et al (2020) 61 | India | The Institute of Liver and Biliary Sciences (ILBS) and in collaboration with the Department of Internal Medicine, Lok Nayak Hospital | Y | Y | NCT04346446 | Severe | 29 | CP gr = 48.1 (9.1), control gr = 48.3 (10.8) | Median neutralizing antibody titre ≥80, median S1 RBD IgG antibody titre ≥640 | NR | CP 500 mL in two divided doses on consecutive days | Standard treatment | CP gr = 21.4%, control gr = 26.7 | NR |
| Ray et al (2020) 62 | India | A single centre in Eastern India | Y | Y | CTRI/2020/05/025209 | Critical | 80 | Overall = 64.43 (11.33) | NR | NR | Two consecutive doses of ABO‐matched 200 mL CP on two consecutive days | Standard treatment | 28.75% | NR |
Abbreviations: CP, convalescent plasma; IQR, interquartile range; NR, not reported; SD, standard deviation.
TABLE 2.
Baseline characteristics of included observational studies (n = 26)
| Author (Year) | Country | Settings | Study design | Severity | Sample size | Mean age (SD) | % Female | Antibody titre | Duration of COVID‐19 diagnosis/symptoms until study treatment | CP dose | Type of control | Outcomes for analysis | Method to account for confounders |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altuntas et al (2020) 34 | Turkey | The Republic of Turkey, Ministry of Health database | Retrospective cohort | Severe/critical | 1776 | Median (IQR) CP gr = 60 (19‐96), non‐CP gr = 61 (21‐91) | CP gr = 30.6%, non‐CP gr = 28.6% | NR | NR | 200‐600 mL | Non‐CP | All‐cause mortality, duration of hospital stay | Matching |
| Liu et al (2020) 35 | USA | The Mount Sinai Hospital in New York City | Retrospective cohort | Severe | 195 | CP gr = 55 (13), not defined control group | CP gr = 36% | NR | Median (range) CP gr = 4 (0‐7) | 250 mL. | Non‐CP | All‐cause mortality | Propensity score matching and covariate adjustment |
| Xia et al (2020) 36 | China | Wuhan Huoshenshan Hospital | Retrospective cohort | Severe/critical | 1568 | Median (IQR) CP gr = 65 (57‐73), non‐CP gr = 63 (53‐71) | CP gr = 44.2%, non‐CP = 49.7% | NR | Median (IQR) of symptoms onset to CP therapy) = 45(39‐54) | 200‐1200 mL | Non‐CP | All‐cause mortality, duration of hospital stay | None |
| Zeng et al (2020) 37 | China | The First Affiliated Hospital of Zhengzhou University and The Sixth People's Hospital of Zhengzhou City. | Retrospective cohort | Critical | 21 | Median (IQR) CP gr = 61.5 (31.5‐77.8), non‐CP gr = 73 (60‐79) | CP gr = 16.7%, non‐CP = 26.7% | NR | Median of 21.5 days | Median volume infused was 300mL. | Non‐CP | All‐cause mortality | None |
| Abuzakouk et a. (2021) 38 | United Arab Emirates | Cleveland Clinic Abu Dhabi | Retrospective cohort Study | Critical | 110 | Median (IQR) CP gr = 50 (43‐60), non‐CP gr = 46 (39‐57) | CP gr = 9.4%, non‐CP = 10.3% | ≥1:160 | NR | NR | Non‐CP | All‐cause mortality, duration of hospital stay | Covariate adjustment |
| Aktimur et al (2021) 39 | Turkey | The haematology department, Ministry of Health University, Samsun Training and Research Hospital, Samsun. | Retrospective cohort | Critical | 41 | CP gr = 64.90 (19.12) non‐CP gr = 66.60 (17.49) | CP gr = 38.1% | NR | NR | 200 mL, infused over 1 to 2 hours | Non‐CP | All‐cause mortality, duration of hospital stay | Propensity score matching |
| Biernat et al (2021) 40 | Poland | Wroclaw Medical University | Prospective cohort | Mild/Moderate/Severe | 45 | Median (Range) CP gr = 57 (31‐72), non‐CP gr = 62.5 (20‐80) | CP gr = 39%, non‐CP gr (historical) = 36% | Greater than 1:1000 | 48‐72 h after the diagnosis of infection | At least one plasma dose of 200‐250 mL | Non‐CP | All‐cause mortality | None |
| Budhiraja et al (2021) 41 | India | Tertiary care teaching hospitals in Delhi | Case‐control study | Moderate to critical | 694 | CP gr = 60.1 (12.1), non‐CP gr = 58.9 (13.8) | CP gr = 19.8%, non‐CP gr = 27.7% | Neutralizing antibody titres of >1:640 | NR | 200 mL. | Non‐CP | All‐cause mortality, all‐cause mortality at 28 days | None |
| Cho et al (2021) 42 | USA | Veterans Affairs medical centre | Prospective cohort study | Mild to moderate (non‐severe) | 11 269 | CP gr = 65.0 (11.3), non‐CP gr = 64.1 (12.0) | CP gr = 8%, control gr = 7% | NR | Within 2 days of eligibility. | NR | Non‐CP | All‐cause mortality | Covariate adjustment in sensitivity analysis |
| Dai et al. (2021) 43 | China | Wuhan Huoshenshan Hospital of China | Retrospective cohort | Mild/severe/critical | 367 | Median (range) CP gr = 68 (21‐93), non‐CP gr = 64 (33‐90) | CP gr = 41.03%, control gr = 45.43% | Antibody titre ≥1:160 | NR | 100‐200 mL per unit | Non‐CP | All‐cause mortality | Propensity score matching |
| Hatzl et al (2021) 44 | Austria | Department of Internal Medicine, Medical University of Graz | Prospective cohort | Critical | 120 | Median (IQR) CP gr = 61 (53‐72), non‐CP gr = 69 (55‐76) | CP gr = 25%, control gr = 33% | NR | Median 4 (1‐10) days | 600 mL (400 mL day 1, 200 mL day 2) | Non‐CP | All‐cause mortality | Propensity score weighting |
| Klapholz et al (2021) 45 | USA | Hospital setting | Retrospective cohort | Severe or critical | 94 | CP gr = 58.0 (13.0), non‐CP gr = 57.7 (13.7) | CP gr = 38.3%, control gr = 38.3% | NR | NR | Approximately 200 mL of ABO‐compatible plasma | Non‐CP | All‐cause mortality | Individual‐level matched controls (1:1) |
| Kurtz et al (2021) 46 | Brazil | the Instituto Estadual do Cérebro Paulo Niemeyer (IECPN) | Prospective cohort | Critical | 113 | Median (IQR) CP gr = 58(45‐64), non‐CP gr = 63 (49‐71) | CP gr = 37%, control gr = 40% | titres ≥1:1,080 | 3 days after ICU admission or respiratory failure. | 200 to 250 mL | Non‐CP | All‐cause mortality, all‐cause mortality at 28 days, duration of hospital stay, clinical improvement within 28 days | Propensity score weighting |
| Mahapatra et al (2021) 47 | India | SCB Medical College & Hospital, Cuttack, Odisha, India | Multi‐centric case‐controlled observational prospective | Moderate/severe | 2432 | NR | CP gr = 16.48 | Neutralizing titre more than 1:160 | NR | 200‐250 mL | Non‐CP | All‐cause mortality | None |
| Moniuszko‐Malinowska et al. (2021) 48 | Poland | The SARSTer database, in medical centres Poland | Retrospective cohort | Mixed | 1006 [patients who received CP during the first seven days (55), remdesivir (236), and other drugs (715)] | CP gr = 59.9 (18.2), remdesivir gr = 58.6 (14.4) and other drug gr = 52.5 (21.5) | CP gr = 36.4% and non‐CP gr (remdesivir and other drugs) = 45% | NR | Mean (SD) = 6.6 (9.7) days | 1‐2 dose of CP (one dose = 200‐267 mL.) | Non‐CP | All‐cause mortality, clinical improvement within 28 days | None |
| Omrani et al (2021) 49 | Qatar | Hamad Medical Corporation (HMC) | Retrospective cohort | Severe/critical | 80 | Median (IQR) CP gr = 47.5(39‐60.5), non‐CP gr = 55.5(46.5‐60.5) | CP gr = 15%, non‐CP gr = 12.5% | NR | Within 7 days of admission to ICU | 400 mL. | Non‐CP | All‐cause mortality, all‐cause mortality at 28 days, clinical improvement at 28 days, discharge rate at 28 days | Variable adjustment |
| Rogers et al (2020) 50 | USA | Three hospitals in the Lifespan health system, Rhode Island Hospital and The Miriam Hospital | Retrospective cohort | Severe | 241 | Median (IQR) CP gr = 61(47‐70), non‐CP gr = 61 (50‐75) | CP gr = 42.2%, non‐CP gr = 46.3% | NR | Median of 7 days after symptoms | 1‐2 units | Non‐CP | All‐cause mortality, all‐cause mortality at 28 days, duration of hospital stay, discharge rate at 28 days | Matching, covariate adjustment |
| Sajmi et al (2021) 51 | India | The Institute of Nephrology, Madras Medical College | Prospective cohort | Moderate and severe | 68 | CP gr = 52 (13.6), non‐CP gr = 56.4 (12.3) | CP gr = 19.2%, non‐CP gr = 25.8% | NR | NR | 200 mL. transfused over 4 hours | Non‐CP | All‐cause mortality, duration of hospital stay | None |
| Salazar et al (2021) 52 | USA | Eight Houston Methodist hospitals | Retrospective cohort | Severe/critical | 903 | Overall age within 60 days; median (IQR) alive = 54(44.0‐62.0), deceased = 65(59.0‐76.0) | Overall age within 60 days; alive = 44.6%, deceased = 35.9% | Anti‐RBD IgG titre of ≥1:1350 | NR | 300 mL. | Non‐CP | All‐cause mortality, duration of hospital stay, clinical improvement at 28 days | Propensity score matching |
| Salazar et al (2021) 53 | Argentina | Hospitals in Buenos Aires Province | Retrospective cohort | Severe/critical | 3,529 | CP gr = 56 (13), non‐CP gr = 64 (17) | CP gr = 30.9%, non‐CP gr = 41.9% | ≥1:400 | NR | NR | Non‐CP | All‐cause mortality 28 days | None |
| Shenoy et al (2021) 54 | USA | Hospitals in a single academic health system | Retrospective cohort | Severe/critical | 526 | CP gr = 55.93 (14.01), non‐CP gr = 56.10 (14.0) | CP gr = 36.5%, non‐CP gr = 36.5% | NA | NR | 200‐500 mL, transfused one to two units | Non‐CP | All‐cause mortality, all‐cause mortality at 28 days, duration of hospital stay | Matching |
| Sostin et al. (2021) 55 | USA | Five Nuvance Health Hospitals. | Retrospective cohort | Severe/critical | 96 | Median (IQR) CP gr = 59.8(55.5‐68.3), non‐CP gr = 59.7(48.0‐78.7) | CP gr = 49%, non‐CP gr = 49% | NR | NR | 200‐250 mL, infused over one to two hours | Non‐CP | All‐cause mortality, duration of hospital stay | Matching and adjusted for the important variables |
| Tang et al (2021) 56 | USA | Washington Adventist Medical HealthCare, Maryland | Case‐control | Critical | 16 | 58.9 (10.2) | 0% | NR | Median (IQR) = 16 (9.5‐22.25) | NR | Non‐CP | All‐cause mortality | None |
| Thompson et al (2021) 57 | USA | The COVID‐19 and Cancer Consortium registry | Retrospective cohort | Mixed (mild, moderate, severe) | 966 (143 CP gr and 823 non‐CP gr) | 65 (15) | CP gr = 42.7%, non‐CP gr = 44.5% | NR | NR | NR | Non‐CP | All‐cause mortality | Propensity score matching |
| Tworek et al (2021) 58 | Poland | The Central Clinical Hospital of the Ministry of Internal Affairs in Warsaw | Prospective cohort | Severe | 204 (Propensity score‐matched) | CP gr = 63.04 (15.48), non‐CP gr = 62.74 (20.55) | CP gr = 44.1%, non‐CP gr = 39.2% | NR | Median (range) CP gr = 20.0 (0.0‐63.0), non‐CP gr = 13.0 (0.0‐59.0) | 1‐3 units (200 mL each) | Non‐CP | All‐cause mortality, duration of hospital stay | Propensity score matching and adjusted model |
| Yoon et al (2021) 59 | USA | Mayo Clinic | Retrospective cohort | Severe/critical | 146 | Median (IQR) CP gr = 67(55 −75), non‐CP gr = 66 (56‐77) | CP gr = 43.8%, non‐CP gr = 35.6% | Titre≥1:2430 | 72 hours of admission | 1 unit (200 mL.) | Non‐CP | All‐cause mortality, all‐cause mortality at 28 days, clinical improvement at 28 days | Propensity score matching |
Abbreviations: CP, convalescent plasma; ICU, intensive care unit; IQR, interquartile range; NR, not reported; SD, standard deviation.
3.2. Convalescent plasma and mortality
Across 18 peer‐reviewed clinical trials, 7118 patients received CP and 7780 patients received standard treatment. Patients treated with CP had a lower mortality rate than those treated with the standard treatment [22.3% (1590/7118) vs. 25.8% (2004/7780)]. In the meta‐analysis, CP use had a 31% reduced risk of all‐cause mortality compared with standard treatment use (pooled RR = 0.69, 95% CI: 0.56‐0.86, P = .001, I 2 = 50.1%) (Figure 2). When subgroup analysis based on severity and geographical region, the results showed that CP treatment significantly reduced risk of all‐cause mortality in patients with severe and critical COVID‐19 disease and studies conducted in Asia with low degree of heterogeneity, pooled RR for severe patients = 0.61, 95% CI: 0.47‐0.81, P = .001, I 2 = 0.0%; pooled RR for critical patients = 0.67, 95% CI: 0.49‐0.92, P = .013, I 2 = 0.0%; and pooled RR for Asia region = 0.62, 95% CI: 0.48‐0.80, P <.001, I 2 = 20.3%. When restricted to randomized double‐blind studies, the meta‐analysis showed a trend in reduction in all‐cause mortality among patients receiving CP treatment when compared with standard treatment (pooled RR = 0.70, 95% CI: 0.48‐1.02, P = .066, I 2 = 0.0%) (Table 3). Among three preprint clinical trials, 60 , 61 , 62 the pooled RR for all‐cause mortality with CP treatment was 0.78 (95% CI: 0.22‐2.74, P = .702, I 2 = 38.7%). For observational studies, 5,255 COVID‐19 patients received CP treatment while 21 371 received non‐CP treatment. All‐cause mortality was 25.7% and 16.0% in the CP and non‐CP groups, respectively. The meta‐analysis showed the similar results to the peer‐reviewed clinical trials illustrating that CP use was associated with a significantly reduced risk of all‐cause mortality compared with non‐CP use (pooled RR = 0.82, 95% CI: 0.72‐0.93, P = .002, I 2 = 65.7%) (Figure 3). Further, results from subgroup analysis showed that CP use was associated with a reduced risk of all‐cause mortality in COVID‐19 patients with severe and severe or critical disease, pooled RR = 0.52, 95% CI: 0.34‐0.78, P = .002, I 2 = 5.3% and pooled RR = 0.76, 95% CI: 0.63‐0.92, P = .005, I 2 = 55.3%, respectively. Based on geographical region, CP use was associated with a significantly reduced risk of all‐cause mortality in Asian countries and South American countries, pooled RR = 0.88, 95% CI: 0.78‐0.98, P = .024, I 2 = 24.1% and pooled RR = 0.72, 95% CI: 0.57‐0.91, P = .007, I 2 = 43.8%, respectively (Table S8). In addition, results from peer‐reviewed clinical trials showed a trend towards reduced mortality at day 28 in CP‐treated group compared with standard‐treated group (pooled RR = 0.88, 95% CI: 0.73‐1.05, P = .150, I 2 = 16.1%). However, for observational studies, there was a statistically significant difference between CP treatment and non‐CP treatment regarding all‐cause mortality at 28 days (pooled RR = 0.74, 95% CI: 0.63‐0.88, P < .001, I 2 = 41.9%) (Figure 4).
FIGURE 2.
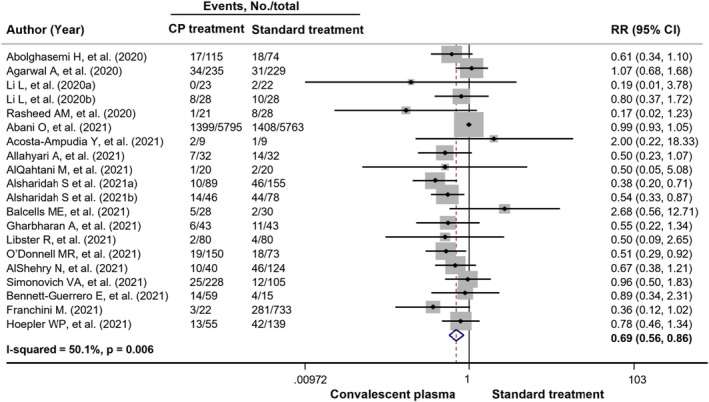
Forest plots showing risk of all‐cause mortality in COVID‐19 patients comparing using convalescent plasma treatment and standard treatment among peer‐reviewed clinical trials. CI, confidence interval; RR, risk ratio
TABLE 3.
Subgroup analysis of peer‐reviewed clinical trials on risk of all‐cause mortality between the convalescent plasma treatment vs the standard treatment
| Outcomes | No. of studies | Pooled RR (95% CI) | P‐value | Heterogeneity test | ||
|---|---|---|---|---|---|---|
| χ 2 | P‐value | I 2‐index | ||||
| Severity | ||||||
| Mild | 1 | 0.50 (0.09‐2.65) | 0.416 | NA | NA | NA |
| Moderate | 2 | 0.65 (0.24‐1.80) | 0.409 | 6.86 | 0.009 | 85.4% |
| Moderate to severe | 3 | 0.69 (0.26‐1.85) | 0.458 | 4.53 | 0.104 | 55.9% |
| Severe | 7 | 0.61 (0.47‐0.81) | 0.001 | 4.17 | 0.653 | 0.0% |
| Critical | 5 | 0.67 (0.49‐0.92) | 0.013 | 2.95 | 0.567 | 0.0% |
| Mixed | 2 | 0.99 (0.93‐1.05) | 0.705 | 0.05 | 0.104 | 55.9% |
| Geographical region | ||||||
| Asia | 8 | 0.62 (0.48‐0.80) | <0.001 | 11.29 | 0.257 | 20.3% |
| South America | 4 | 1.06 (0.61‐1.83) | 0.830 | 2.55 | 0.467 | 0.0% |
| Europe | 4 | 0.78 (0.54‐1.13) | 0.188 | 5.90 | 0.116 | 49.2% |
| North America | 1 | 0.89 (0.34‐2.31) | 0.811 | NA | NA | NA |
| North America and South America | 1 | 0.51 (0.29‐0.92) | 0.025 | NA | NA | NA |
| Randomized vs non‐randomized | ||||||
| Randomized | 11 | 0.87 (0.71‐1.07) | 0.187 | 13.58 | 0.257 | 19.0% |
| Non‐randomized | 7 | 0.57 (0.46‐0.72) | <0.001 | 5.46 | 0.604 | 0.0% |
| Randomized double‐blind vs open label | ||||||
| Randomized double‐blinded | 4 | 0.70 (0.48‐1.02) | 0.066 | 2.40 | 0.494 | 0.0% |
| Open label | 14 | 0.69 (0.54‐0.87) | 0.002 | 33.33 | 0.004 | 55.0% |
Abbreviations: CI, confidence interval; NA, not applicable; RR, risk ratio.
FIGURE 3.
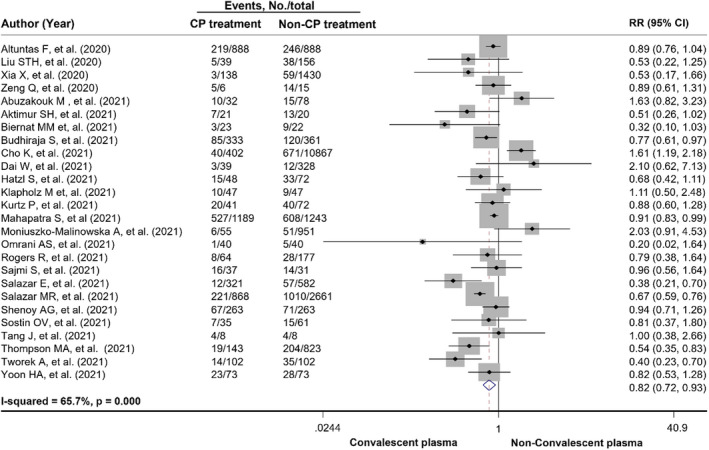
Forest plots showing risk of all‐cause mortality in COVID‐19 patients comparing using convalescent plasma treatment and non‐convalescent plasma treatment among observational studies. CI, confidence interval; CP, convalescent plasma; RR, risk ratio
FIGURE 4.
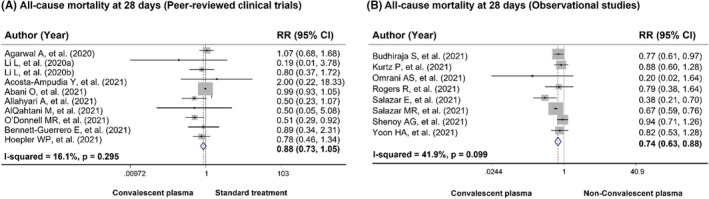
Forest plots showing risk of all‐cause mortality at 28 days in COVID‐19 patients comparing using convalescent plasma treatment and standard treatment/non‐convalescent plasma (A) results from peer‐reviewed clinical trials, (B) results from observational studies. CI, confidence interval; RR, risk ratio
In terms of gender and ethnicity, we found only one study 20 investigated the effect of CP on all‐cause mortality stratified by gender and ethnicity. There was no significant difference in 28‐day mortality between the CP use vs standard treatment across subgroup of sex (RR for male = 1.03, 95% CI: 0.95‐1.13 and RR for female = 0.94, 95% CI: 0.82‐1.07) or ethnicity (RR for White = 0.97, 95% CI: 0.90‐1.06 and RR for Black, Asian or minority ethnic = 1.07, 95% CI: 0.88‐1.31). 20
3.3. Convalescent plasma and length of hospital stay
Ten clinical trials 16 , 17 , 21 , 22 , 23 , 25 , 31 , 32 , 33 , 61 and eleven observational studies 34 , 36 , 38 , 39 , 46 , 50 , 51 , 52 , 54 , 55 , 58 reported the length of hospital stay of CP‐treated patients and standard treatment‐treated patients. The results from meta‐analysis of peer‐reviewed clinical trials (n = 9) demonstrated that there was no significant difference between two groups with respect to the duration of hospital stay (weighted mean difference [WMD] = −1.63, 95% CI: −4.16‐0.90, P = .208, I 2 = 89.2%). The results remained the same after adding the preprint studies (WMD = −1.88, 95% CI: −4.22 to 0.46, P = .116, I 2 = 88.0%). The results from observational studies also showed non‐significant difference in length of hospital stay between two groups with substantial heterogeneity (WMD = 1.44, 95% CI: −0.71 to 3.60, P = .190, I 2 = 91.9%) (Figure S1).
3.4. Convalescent plasma and clinical improvement at 28 days
Seven studies 18 , 38 , 46 , 48 , 49 , 52 , 59 reported clinical improvement at 28 days after receiving treatment. One 18 was randomized controlled trial, and six 38 , 46 , 48 , 49 , 52 , 59 were observational studies. The definition of clinical improvement varied among studies; therefore, the meta‐analysis could not be performed. For the RCT, the finding indicated that for patients with severe disease or life‐threatening disease, there was no significance difference between the CP group vs control group with respect to clinical improvement at 28 days (odds ratio = 1.42, 95% CI: 0.65‐3.09, P = .37). 18
3.5. Convalescent plasma and discharge rate at 28 days
Three clinical trials 18 , 20 , 22 and two observational studies 49 , 50 examined the discharge rate at 28 days between CP treatment and standard treatment. The results from trials showed no significant difference in discharge rate from hospital within 28 days between CP group and standard treatment group. 18 , 20 , 22 For observational studies, no significant differences were found between CP group and non‐CP group in the proportions of patients who were discharged within 28 days. 49 , 50
3.6. Sensitivity analysis
After omitting the individual peer‐reviewed clinical trial and observational studies in leave‐one‐out analysis, the risk of all‐cause mortality among CP‐treated patients and standard‐treated patients appeared to be robust (Table S9 and Table S10). In addition, the meta‐analysis of 18 peer‐reviewed clinical trials and three preprints showed similar results to the primary analysis (Figure S2). Finally, when including only the adjusted estimates from observational studies, the results were identical to the primary analysis demonstrating that CP use was associated with a reduced risk of all‐cause mortality in COVID‐19 patients when compared with non‐CP use (pooled RR = 0.60, 95% CI: 0.39‐0.93, P = .024, I 2 = 80.6%; Figure S3).
3.7. Publication bias
Publication bias was assessed using the data of CP treatment vs standard treatment on the risk of all‐cause mortality. An evidence of asymmetry was observed in the results of Egger's test (P = .002) but not for Begg's test (P = .820). The visually inspected funnel plots of peer‐reviewed clinical trials included are shown in Figure S4. For observational studies, no evidence of small‐study effect was found with Begg's (P = .537) and Egger's tests (P = .575). The funnel plots of observational studies are shown in Figure S5.
4. DISCUSSION
The current systemic review and meta‐analysis aimed to summarize the existing data on the efficacy of CP in COVID‐19 patients, which remains a challenge to explore treatment for SARS‐CoV‐2 pandemic to respond the increasing of the incidence of SARS‐CoV‐2 infection. According to the eligible criteria, 47 studies 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 were included and critically evaluated. Corresponding to the results of our systematic review and meta‐analysis, the CP may be effective in reducing the mortality of CP‐treated COVID‐19 patients compared with non‐CP‐treated COVID‐19 patients, especially in severe or critical patients and in Asia region. The results are supported by the previous study of RCT and matched‐control data demonstrating that COVID‐19 patients transfused with CP had a lower mortality rate compared with patients receiving standard treatments. 63 Additionally, the reduction in mortality associated with CP supports with similar analyses of previous data from CP trials of novel infectious diseases those affecting the respiratory system including severe acute respiratory syndrome‐1 (SARS‐1), Middle East respiratory syndrome (MERS), H1N1 influenza and Ebola virus disease. The results revealed that the pooled odds of mortality were reduced compared with placebo or no therapy (odds ratio, 0.25; 95% confidence interval, 0.14‐0.45) in SARS and influenza. 4
In severe or critical COVID‐19 patients, lung alveoli macrophages or epithelial cells can produce massive proinflammatory cytokines and chemokines, which recruit monocytes and neutrophils to the infection site to eradicate the virus and infected cells, resulting in uncontrolled inflammation. This conducts the additional infiltration of macrophage and subsequently the decline of lung functions. Therefore, the crucial rule of convalescent plasma is antibody‐mediated SARS‐CoV‐2 viral deactivation/neutralization and interference with viral replication. 64
Convalescent plasma, obtained from recovered COVID‐19 patients who had established humoral immunity against the virus, contains a huge quantity of neutralizing antibodies capable of neutralizing SARS‐CoV‐2 and eradicating the pathogen from blood circulation and pulmonary tissues. Potential mechanisms of action of SARS‐CoV‐2 antibodies in COVID‐19 are mediated by the interaction between the SARS‐CoV‐2 spike glycoprotein and the angiotensin‐converting enzyme 2 (ACE2) receptor on the host cell. Antibodies directed against the receptor‐binding domain (RBD) of the spike protein can interfere with its interaction with the ACE2 receptor and prevent viral entry in the host cell. Antibodies directed against epitopes outside the RBD can also exert antiviral functions through other mechanisms. 48 Viral neutralization is then posited to reduce the massive inflammatory response and prevent the immune response progresses to lung damage, interfering of gas exchange and death.
The strength of this study should be mentioned. First, we applied a comprehensive search strategy to ensure that the included studies were representative. Second, the meta‐analysis covered updated evidence including clinical trials and real‐world practice data. Furthermore, our study filled the knowledge gaps from previous studies by investigating the effect of CP in COVID‐19 patients with different severities and different regions. Finally, our study adheres to the standard methodology of systematic review and meta‐analysis required by the Cochrane and PRISMA checklist. 9 However, our study has certain limitations. First, a moderate to high degree of heterogeneity may limit the findings. Yet, we performed subgroup analyses and found that disease severity, geographical region, study design and quality of included studies were potential factors contributing to heterogeneity. In addition, plasma antibody titre, dose of CP used, duration between onset of COVID‐19 diagnosis and transfusion and duration of follow‐up after transfusion varied among studies. This might also be considered as a source of heterogeneity in our study. Second, the results from observational studies are prone to bias and unmeasured confounders. On this point, we performed a sensitivity analysis by including only adjusted values and results remained robust. However, for observational studies, we suggested that the causality of CP use and the reduction in all‐cause mortality cannot be established and the results should be interpreted with caution. Third, methodological quality of included clinical trials in this study was high risk of bias. Generally, high risk of bias was identified in the domain of selection bias, performance bias and detection bias while low risk of bias was detected in the domain of attrition bias and reporting bias using Cochrane’ risk of bias. Even though inadequate random sequence generation and lack of blinding of outcome measurements were observed in some studies, it may not be possible for this type of intervention to blind the participants or investigators in this critical time. However, strong blinding of researchers should be made. Fourth, the included studies yield small sample size and the results might be influenced by small‐study effect, making it difficult to conclude whether CP treatment is effective in the treatment of COVID‐19 patients. However, there are many ongoing randomized clinical trials which currently registered on clinical.gov that assess CP for the treatment of COVID‐19. It is important to note that conclusions regarding CP await the results of large controlled trials such as those emerging from the UK. 20 Further, few studies reported duration of COVID‐19 diagnosis until CP administration as well as the titre of neutralizing antibodies. FDA recommended the use of ‘high‐titre’ convalescent plasma, as defined by a neutralizing antibody titre of ≥250 in the Broad Institute's neutralizing antibody assay or an S/C cut‐off of ≥12 in the Ortho VITROS IgG assay. 65 These factors were considered as an important factor affecting clinical outcomes. Finally, there has been a lack of efficacy information about CP treatment among immunocompromised and vulnerable populations which may due to the limitation of enrolment, for example, transplant recipients 66 and autoimmune disease patients 57 , 67 , 68 who were immunosuppressed by mycophenolate and antimetabolites that impair humoral immunity. Recently, there were accumulated evidences demonstrated that CP administration to these population before pulmonary deterioration is observed, supporting the benefit to alleviate disease severity. However, the potential therapeutic period for immunocompromised patient from CP is exactly unknown due to impaired immune response, comparison with other patients. The well‐designed and well‐conducted randomized clinical trials are necessary to provide more specific, evidence‐based guidance on the role of CP in the treatment of patients with COVID‐19 who have humoral immunodeficiencies. Thus, these issues should be solved to enlighten the knowledge gap. Therefore, we propose that future studies aiming to investigate the efficacy of CP treatment in COVID‐19 patients should include duration of symptom onset until study treatment and investigate the appropriateness of population for CP use, especially in resource‐limited countries which could not access the high‐cost antiviral agents and SARS‐CoV‐2‐specific monoclonal antibodies. The supplemental CP strategy is the valuable treatment option in this situation. In addition, rigorous study design and larger sample size are needed to confirm the effect of CP treatment on clinical outcomes including mortality in patients with COVID‐19.
5. CONCLUSIONS
CP treatment was significantly associated with a decreased risk of all‐cause mortality in severe or critical COVID‐19 patients compared with standard treatment. No significant differences between CP treatment and standard treatment/non‐CP were observed in the length of hospital stay. The results should be interpreted with caution due to the moderate degree of heterogeneity. Future studies with larger sample size and well‐designed are warranted.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
CK and PM conceptualized the study; CK, MS, JS and PM contributed to methodology; PM involved in formal analysis; CK, MS, JS and PM wrote—original draft preparation; CK, PC and PM wrote—review and editing; CK and PM supervised the study. All authors listed have made a substantial, direct and intellectual contribution to the work and approved it in its final format. All authors have read and agreed to the published version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This research project was partially supported by the Thailand Science Research and Innovation Fund and the University of Phayao (Grant No. FF64‐UoE039). The funding source had no role in the study design, collection, analysis and interpretation of data.
Kloypan C, Saesong M, Sangsuemoon J, Chantharit P, Mongkhon P. CONVALESCENT plasma for COVID‐19: A meta‐analysis of clinical trials and real‐world evidence. Eur J Clin Invest. 2021;51:e13663. 10.1111/eci.13663
REFERENCES
- 1. Cucinotta D, Vanelli M. Who declares covid‐19 a pandemic. Acta bio‐medica: Atenei Parmensis. 2020;91:157‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO Coronavirus (COVID‐19) Dashboard; Available from: https://covid19.who.int/. Accessed August 4, 2021.
- 3. Andreano E, Piccini G, Licastro D, et al. Sars‐cov‐2 escape in vitro from a highly neutralizing covid‐19 convalescent plasma. bioRxiv. 2020. 10.1101/2020.12.28.424451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211:80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Griensven J, De Weiggheleire A, Delamou A, et al. The use of ebola convalescent plasma to treat ebola virus disease in resource‐constrained settings: A perspective from the field. Clin Infect Dis. 2016;62:69‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rojas M, Rodríguez Y, Monsalve DM, et al. Convalescent plasma in covid‐19: possible mechanisms of action. Autoimmun Rev. 2020;19: 102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. COVID‐19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines: National Institutes of Health; Available from: https://www.covid19treatmentguidelines.nih.gov/. Accessed August 4, 2021. [PubMed]
- 8. U.S. Food and Drug Administration . Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of COVID‐19 Convalescent Plasma for Treatment of Hospitalized Patients with COVID19: U.S. FDA; Available from: https://www.fda.gov/media/141478/download. Accessed August 4, 2021
- 9. Page MJ, McKenzie JE, Bossuyt PM, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization . Clinical management of covid‐19—interim guidance, 2020.
- 12. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells GSB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa scale (nos) for assessing the quality of nonrandomised studies in meta‐analyses.
- 14. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abolghasemi H, Eshghi P, Cheraghali AM, et al. Clinical efficacy of convalescent plasma for treatment of covid‐19 infections: Results of a multicenter clinical study. Transfus Apher Sci. 2020;59:102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid‐19 in adults in India: Open label phase ii multicentre randomised controlled trial (placid trial). BMJ 2020;371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19. JAMA. 2020;324(5):460‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasheed AM, Fatak DF, Hashim HA, et al. The therapeutic potential of convalescent plasma therapy on treating critically‐ill covid‐19 patients residing in respiratory care units in hospitals in Baghdad. Iraq. Infez Med. 2020;28:357‐366. [PubMed] [Google Scholar]
- 20. Abani O, Abbas A, Abbas F, et al. Convalescent plasma in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised controlled, open‐label, platform trial. Lancet. 2021;397(10289):2049‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Acosta‐Ampudia Y, Monsalve DM, Rojas M, et al. Covid‐19 convalescent plasma composition and immunological effects in severe patients. J Autoimmun. 2021;118: 102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allahyari A, Seddigh‐Shamsi M, Mahmoudi M, et al. Efficacy and safety of convalescent plasma therapy in severe covid‐19 patients with acute respiratory distress syndrome. Int Immunopharmacol. 2021;93:107239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. AlQahtani M, Abdulrahman A, Almadani A, et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe covid‐19 disease. Sci Rep. 2021;11:9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alsharidah S, Ayed M, Ameen RM, et al. Covid‐19 convalescent plasma treatment of moderate and severe cases of sars‐cov‐2 infection: A multicenter interventional study. Int J Infect Dis. 2021;103:439‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balcells ME, Rojas L, Le Corre N, et al. Early versus deferred anti‐sars‐cov‐2 convalescent plasma in patients admitted for covid‐19: A randomized phase ii clinical trial. PLoS Medicine. 2021;18:e1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bennett‐Guerrero E, Romeiser JL, Talbot LR, et al. Severe acute respiratory syndrome coronavirus 2 convalescent plasma versus standard plasma in coronavirus disease 2019 infected hospitalized patients in New York: A double‐blind randomized trial. Crit Care Med. 2021;49(7):1015‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Franchini M, Glingani C, Morandi M, et al. Safety and efficacy of convalescent plasma in elderly COVID‐19 patients: The RESCUE trial. Mayo Clin Proc Innov Qual Outcomes. 2021;5(2):403‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gharbharan A, Jordans CCE, GeurtsvanKessel C, et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS‐CoV‐2 infection. Nat Commun. 2021;12(1):3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoepler WP, Weidner L, Traugott MT, et al. Adjunctive treatment with high‐titre convalescent plasma in severely and critically ill COVID‐19 patients‐a safe but futile intervention. A comparative cohort study. Infect Dis (Lond). 2021;1‐10. 10.1080/23744235.2021.1940271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Libster R, Pérez Marc G, Wappner D, et al. Early high‐titer plasma therapy to prevent severe covid‐19 in older adults. N Engl J Med. 2021;384:610‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Donnell MR, Grinsztejn B, Cummings MJ, et al. A randomized double‐blind controlled trial of convalescent plasma in adults with severe COVID‐19. J Clin Invest. 2021;131(13):e150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. AlShehry N, Zaidi SZA, AlAskar A, et al. Safety and efficacy of convalescent plasma for severe COVID‐19: interim report of a multicenter phase II study from Saudi Arabia. Saudi J Med Med Sci. 2021;9(1):16‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in covid‐19 severe pneumonia. N Engl J Med. 2021;384:619‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Altuntas F, Ata N, Yigenoglu TN, et al. Convalescent plasma therapy in patients with COVID‐19. Transfus Apher Sci. 2021;60(1):102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu STH, Lin H‐M, Baine I, et al. Convalescent plasma treatment of severe covid‐19: a propensity score‐matched control study. Nat Med. 2020;26:1708‐1713. [DOI] [PubMed] [Google Scholar]
- 36. Xia X, Li K, Wu L, et al. Improved clinical symptoms and mortality among patients with severe or critical covid‐19 after convalescent plasma transfusion. Blood. 2020;136:755‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zeng Q‐L, Yu Z‐J, Gou J‐J, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222:38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abuzakouk M, Saleh K, Algora M, et al. Convalescent plasma efficacy in life‐threatening covid‐19 patients admitted to the ICU: A retrospective cohort study. J Clin Med. 2021;10(10):2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aktimur SHOC, Kayabas A. The impact of convalescent (immune) plasma treatment on the clinical course of covid‐19. Int J Clin Exp Med. 2021;14:1226‐1233. [Google Scholar]
- 40. Biernat MM, Kolasińska A, Kwiatkowski J, et al. Early administration of convalescent plasma improves survival in patients with hematological malignancies and COVID‐19. Viruses. 2021;13(3):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Budhiraja S, Dewan A, Aggarwal R, et al. Effectiveness of convalescent plasma in Indian patients with covid‐19. Blood Cells Mol Dis. 2021;88:102548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cho K, Keithly SC, Kurgansky KE, et al. Early convalescent plasma therapy and mortality among US veterans hospitalized with nonsevere COVID‐19: an observational analysis emulating a target trial. J Infect Dis. 2021:jiab330. 10.1093/infdis/jiab330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dai W, Wu J, Li T, et al. Clinical outcomes for COVID‐19 patients with diabetes mellitus treated with convalescent plasma transfusion in Wuhan, China. J Med Virol. 2021;93(4):2321‐2331. [DOI] [PubMed] [Google Scholar]
- 44. Hatzl S, Posch F, Sareban N, et al. Convalescent plasma therapy and mortality in COVID‐19 patients admitted to the ICU: a prospective observational study. Ann Intensive Care. 2021;11(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klapholz M, Pentakota SR, Zertuche JP, et al. Matched cohort study of convalescent COVID‐19 plasma treatment in severely or life threateningly Ill COVID‐19 patients. Open Forum Infect Dis. 2021;8(2):ofab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kurtz P, Righy C, Gadelha M, et al. Effect of convalescent plasma in critically Ill patients With COVID‐19: an observational study. Front Med. 2021;8:630982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mahapatra S, Rattan R, Mohanty CBK. Convalescent Plasma Therapy in the management of COVID‐19 patients‐The newer dimensions. Transfus Clin Biol. 2021;28(3):246‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moniuszko‐Malinowska A, Czupryna P, Zarebska‐Michaluk D, et al. Convalescent plasma transfusion for the treatment of COVID‐19‐experience from Poland: a multicenter study. J Clin Med. 2021;10(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Omrani AS, Zaqout A, Baiou A, et al. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: A preliminary report. J Med Virol. 2021;93(3):1678‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rogers R, Shehadeh F, Mylona EK, et al. Convalescent Plasma for Patients With Severe Coronavirus Disease 2019 (COVID‐19): A Matched Cohort Study. Clin Infect Dis. 2021;73(1):e208‐e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sajmi S, Goutham K, Arumugam V, et al. Efficacy and safety of convalescent plasma therapy in SARS‐CoV2 patients on hemodialysis. Hemodial Int. 2021;1‐8. 10.1111/hdi.12951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Salazar E, Christensen PA, Graviss EA, et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (covid‐19) patients transfused early with convalescent plasma containing high‐titer anti‐severe acute respiratory syndrome coronavirus 2 (sars‐cov‐2) spike protein IgG. Am J Pathol. 2021;191:90‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salazar MR, Gonzalez SE, Regairaz L, et al. Risk factors for COVID‐19 mortality: The effect of convalescent plasma administration. PLoS One. 2021;16(4):e0250386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shenoy AG, Hettinger AZ, Fernandez SJ, Blumenthal J, Baez V. Early mortality benefit with covid‐19 convalescent plasma: A matched control study. Br J Haematol. 2021;192:706‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sostin OV, Rajapakse P, Cruser B, Wakefield D, Cruser D, Petrini J. A matched cohort study of convalescent plasma therapy for COVID‐19. J Clin Apher. 2021;36(4):523‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang J, Grubbs G, Lee Y, Golding H, Khurana S. Impact of convalescent plasma therapy on SARS CoV‐2 antibody profile in COVID‐19 patients. Clin Infect Dis. 2021. 10.1093/cid/ciab317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thompson MA, Henderson JP, Shah PK, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID‐19. JAMA Oncol. 2021. 10.1001/jamaoncol.2021.1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tworek A, Jaroń K, Uszyńska‐Kałuża B, et al. Convalescent plasma treatment is associated with lower mortality and better outcomes in high‐risk covid‐19 patients – propensity‐score matched case‐control study. Int J Infect Dis. 2021;105:209‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoon H, Bartash R, Gendlina I, et al. Treatment of severe COVID‐19 with convalescent plasma in Bronx, NYC. JCI Insight. 2021;6(4):e142270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Avendaño‐Solà C, Ramos‐Martínez A, Muñez‐Rubio E, et al. Convalescent plasma for covid‐19: a multicenter, randomized clinical trial. medRxiv. 10.1101/2020.08.26.20182444 [DOI] [Google Scholar]
- 61. Bajpai M, Kumar S, Maheshwari A, et al. Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill covid‐19 patients: A pilot randomized controlled trial. medRxiv. 10.1101/2020.10.25.20219337 [DOI] [Google Scholar]
- 62. Ray Y, Paul SR, Bandopadhyay P, et al. Clinical and immunological benefits of convalescent plasma therapy in severe covid‐19: Insights from a single center open label randomised control trial. medRxiv. 10.1101/2020.11.25.20237883 [DOI] [Google Scholar]
- 63. Klassen SA, Senefeld JW, Johnson PW, et al. The effect of convalescent plasma therapy on mortality among patients with covid‐19: Systematic review and meta‐analysis. Mayo Clin Proc. 2021;96:1262‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gómez‐Rial J, Rivero‐Calle I, Salas A, Martinón‐Torres F. Role of Monocytes/Macrophages in Covid‐19 Pathogenesis: Implications for Therapy. Infect Drug Resist. 2020;13:2485‐2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. U.S. Food and Drug Administration . Clinical memorandum: COVID‐19 Convalescent Plasma: U.S. FDA. Available from: https://www.fda.gov/media/141480/download Accessed August 4, 2021.
- 66. Kluger MA, Czogalla J, Schmidt‐Lauber C, et al. Convalescent plasma treatment for early post‐kidney transplant acquired COVID‐19. Transpl Infect Dis. 2021;1–3. 10.1111/tid.13685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mira E, Yarce OA, Ortega C, et al. Rapid recovery of a SARS‐CoV‐2‐infected X‐linked agammaglobulinemia patient after infusion of COVID‐19 convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(8):2793‐2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gupta A, Kute VB, Patel HV, et al. Feasibility of convalescent plasma therapy in kidney transplant recipients with severe COVID‐19: a single‐center prospective cohort study. Exp Clin Transplant. 2021;19(4):304‐309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


