Abstract
The effect of COVID‐19 on the male reproductive tract has been sparsely studied. This exploratory study was designed to determine the presence of SARS‐CoV‐2 in the semen of men recovering from COVID‐19. A systematic literature review was also performed as per PRISMA guidelines to gather perspective on this topic. The prospective study included men 21 years and older recovering from COVID‐19 with nasopharyngeal swab negative for SARS‐CoV‐2 or at least two weeks from the last COVID RT‐PCR positivity. After clinical evaluation, freshly ejaculated semen sample by masturbation was collected in a sterile container. Samples were processed for the detection of SARS‐CoV‐2 by RT‐PCR. Twenty‐one patients were contacted for the study, 11 of which consented to provide a semen sample. The mean age of the cohort was 29.72 ± 4.52 years. None of the patients gave a history of epididymo‐orchitis or sexual dysfunction at the time of assessment. None of the semen samples demonstrated SARS‐CoV‐2 on RT‐PCR. Median duration of semen sample collection from the COVID positivity was 44 days (Range 19–59 days). Detailed literature review revealed that SARS‐CoV‐2 is not found in patients recovering from COVID‐19 infection. We conclude that SARS‐CoV‐2 is not found in the semen of patients recovering from COVID‐19.
Keywords: carrier state, COVID‐19, SARS‐CoV‐2, semen, virus
1. INTRODUCTION
COVID‐19 caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) virus is an ongoing pandemic that has affected more than 10.7 million people across India (India, n.d.; Wu & McGoogan, 2020). It is primarily a respiratory virus transmitted via air droplets and surface contact. The primary modality for detecting this virus is the reverse transcriptase‐polymerase chain reaction (RT‐PCR) test on the nasopharyngeal or oropharyngeal swabs of the patients(Li et al., 2003; Wang et al., 2020; Zhou et al., 2020). Apart from these, the SARS‐CoV‐2 has also been found in blood, urine, peritoneal fluid and faeces, suggesting that these may serve as potential sources of transmission (Hoffmann et al., 2020; Li et al., 2003).
The effect of COVID‐19 on the male reproductive tract has been studied sparsely. Interestingly, angiotensin convertase inhibitor‐2 (ACE‐2) receptors, which are abundantly found in the testis and male reproductive system, are utilised by SARS‐CoV‐2 for gaining entry into the cells(Chen et al., 2010; Hoffmann et al., 2020; Li et al., 2003). A recent study documented the presence of SARS‐CoV‐2 in testicular tissue, and there have been reports of loss of testicular architecture in patients with COVID‐19 infection (Achua et al., 2021; Vishvkarma & Rajender, 2020). A recent review (Huang et al., 2021) has suggested cytopathic effects in testes due to virus and indirect damage due to immunopathological reaction as potential reasons for the development of infertility in patients with SARS‐CoV‐2 patients. These studies have also called into question the presence of this virus in semen and its transmissibility via sexual intercourse.
A limited number of studies have explored this intriguing aspect of SARS‐CoV‐2 infection. Song et al. (2020) found no evidence of this virus in the semen of 12 patients recovering from COVID‐19. Another study (Pan et al., 2020) of 34 adults COVID‐19 positive males detected no SARS‐CoV‐2 virus in semen samples at a median duration of 31 days. Contrastingly, Li et al. (2020) enrolled 38 patients aged >15 years and reported SARS‐CoV‐2 in semen samples of 6 patients (15.8%); 4 of them in the acute stage and 2 in the convalescence stage of infection. Similarly, Machado et al. (2021) also found 1/15 active patients having SARS‐CoV‐2 in semen. On the other hand, a recent study failed to demonstrate SARS‐CoV‐2 in the semen of 16 active patients using RT‐PCR (Kayaaslan et al., 2020).
From the current evidence, the presence of SARS‐CoV‐2 in semen and its impact on the reproductive health of men recovering from COVID‐19 remains controversial at best (Achua et al., 2021; Kayaaslan et al., 2020; Li et al., 2020; Ma et al., 2021; Song et al., 2020). Hallak et al. (2021) highlighted the lack of precise methodology on collection and interpretation of RT‐PCR, and the absence of data on viral load poses a significant challenge in the validation of these results by other groups. Hence, we designed this study to determine the presence of SARS‐CoV‐2 in semen in our subpopulation recovering from COVID‐19 to rectify these methodological flaws and shed some more light on this topic. We also performed a systematic literature review and compared all the available literature on this topic.
2. MATERIAL AND METHODS
2.1. Patients and setting
This prospective observational study was conducted at the Departments of Urology Virology and Psychiatry, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh. The institutional ethics committee approval was obtained for the study (NK/6304/Study/095). Men recovering from COVID‐19 and aged 21 years or more, with a nasopharyngeal swab negative for SARS‐CoV‐2 or at least 2 weeks from the last COVID RT‐PCR positivity were included in the study. Patients were classified as mild if they had low‐grade fever, cough, malaise, rhinorrhea and sore throat without shortness of breath. They were classified as moderate if they required admission due to a respiratory rate of >30/min and/or oxygen saturation (SpO2)<93% on room air.
2.2. Data collection
The patients' details were recorded on a proforma, and a history suggestive of epididymo‐orchitis was enquired from all the patients. Additionally, all the patients were evaluated for any sexual dysfunction by a thorough psychosexual history and examination. The external genitalia and testis were examined for any telltale sign of epididymo‐orchitis. Testicular volume was measured using Prader's orchidometer. A freshly ejaculated semen sample was collected in a sterile container by masturbation. The patient was asked to use all precautionary measures to avoid contamination, such as thorough cleaning of hands and genitals before providing the sample.
2.3. Procedures
The samples were transported to the Department of Virology, ensuring a proper cold chain, and were processed to detect SARS‐CoV‐2 using real‐time PCR by taking adequate biosafety precautions. Total RNA was extracted from 140 µl of the sample by using QIAamp Viral RNA mini kit (QIAGEN) according to the manufacturer's instructions. Finally, the RNA was eluted in 30 µl of elution buffer, and extracted RNA was used to detect the SARS‐CoV‐2 by targeting Open Reading Frame 1 (ORF 1 ab gene), nucleoprotein (N gene) and RNase P gene (Internal control) using Q‐line Molecular Coronavirus (COVID‐19) RT‐PCR kit (POCT Services Pvt. Limited) by real‐time PCR. The nasopharyngeal samples of these patients were tested by real‐time PCR/ Antigen detection test as per the availability of ICMR approved kits at that point of time.
2.4. Literature review and Data acquisition
Literature review was performed in consistent with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines using three databases PUBMED/MedLine, Embase and Scopus using the following keywords ‘COVID‐19’ OR ‘SARS‐CoV‐’ AND ‘Semen’ (Figure 1) by two authors (APS & AC). The search results obtained from various databases were transferred to a citation manager, and duplicates were removed. Additional articles were also sought from various review articles from the same topic and hand searched for the references selected for full‐text review. A total of 381 articles were screened, and a final of 13 articles were included for qualitative synthesis.
FIGURE 1.
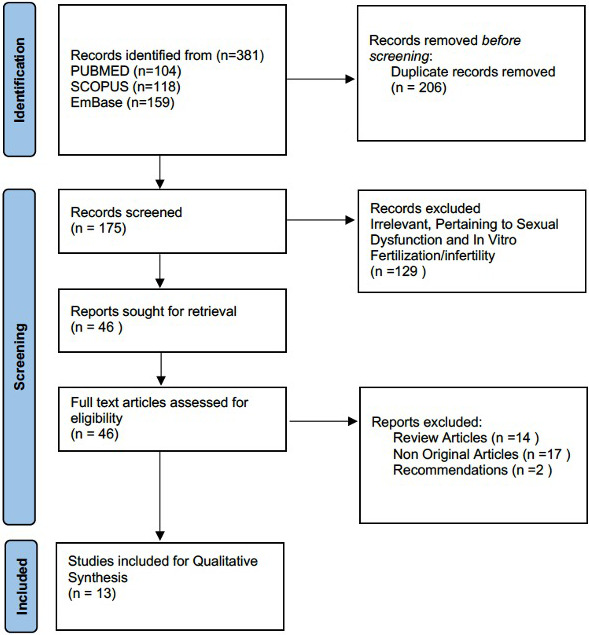
PRISMA Flow diagram for the study
Two study authors (APS and AC) independently extracted data from the included studies on a predetermined format with the following variables Author and year, the number of patients, age of patients, method of detection, the timing between COVID‐19 diagnosis and collection of semen sample in days and summary of results. Discrepancy of data was resolved after arbitration with other study authors (SS & MS).
2.5. Statistical analysis
Statistical analyses were performed using Microsoft Excel 2010 and SPSS v 20. All quantitative variables were estimated using the measures of central location (mean, median) and measures of dispersion (standard deviation and range). Categorical variables are described as frequencies and proportions. A bubble plot was used to depict the data from all the included studies.
3. RESULTS
Twenty‐one patients were contacted for the study from May 2020 to November 2020, out of which 11 patients gave consent, and their semen was tested by RT‐PCR for SARS‐CoV‐2. The clinical details of patients are provided in Table 1. The mean age of the cohort was 29.72 ± 4.52 years. At the time of diagnosis of COVID‐19, 10/11 (90.9%) patients had fever as the presenting complaint, among which 6 (54.6%) patients had associated cough. One patient (9.1%) presented with nasal stuffiness alone. The cycle threshold (Ct) values ranged from 20 to 33 (Table 1). None of the patients gave a history of epididymo‐orchitis. None of the patients reported any sexual dysfunction at the time of assessment in the psychiatric psychosexual evaluation. The genitalia examination for all patients was normal. The median testicular size as measured by the Orchidometer was 15 ml (Range: 15–20 ml). On examination, none of the patients had any telltale sign of epididymo‐orchitis. None of the patients showed SARS‐CoV‐2 positivity in semen on RT‐PCR. Figure 2 shows the duration gap from the nasopharyngeal swab positivity to semen RT‐PCR report. The median duration of semen sample collection from the COVID positivity was 44 days (range 19–59 days). The duration of negativity of nasopharyngeal swab samples has been documented only for the first four patients. Due to a change in Indian guidelines subsequently, the documentation of RT‐PCR negativity became nonmandatory for mild to moderate disease (due to incorporation of home isolation policy); hence, the date of negative report documentation is not available in later cases.
TABLE 1.
Clinical details of patients suffering from COVID‐19 infection in the study
| S. No | Age (Years) | Symptoms | Ct Valuea Of confirmatory gene (Nasopharyngeal samples) | Disease severity | Testicular volume (ml) |
|---|---|---|---|---|---|
| 1 | 25 | Fever, Cough | 28 | Mild | 15 |
| 2 | 30 | Fever | 33 | Mild | 15 |
| 3 | 25 | Nasal stuffiness | 26 | Mild | 15 |
| 4 | 40 | Fever | 26 | Mild | 15 |
| 5 | 31 | Fever, Cough | 25 | Mild | 20 |
| 6 | 24 | Fever, Cough | Antigen Positiveb | Moderate | 15 |
| 7 | 32 | Fever, Cough | 20 | Moderate | 15 |
| 8 | 32 | Fever | 28 | Mild | 15 |
| 9 | 30 | Fever | Antigen Positiveb | Mild | 20 |
| 10 | 31 | Fever, cough | Antigen Positiveb | Mild | 15 |
| 11 | 27 | Fever, cough | 30 | Mild | 15 |
Ct‐ Cycle threshold.
Only tested by SARS CoV‐2 Antigen detection kit and antigen‐positive samples were not processed by real‐time PCR as per National guidelines (https://www.icmr.gov.in/cteststrat.html).
FIGURE 2.
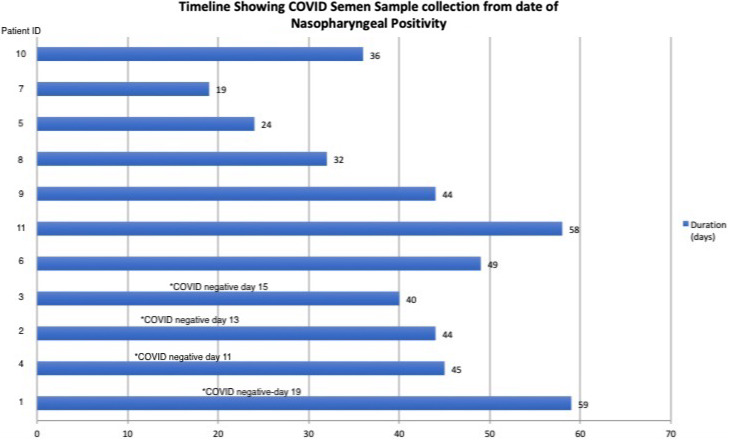
Timeline in days showing the Semen RT‐PCR from the date of nasopharyngeal swab positivity. For 1st four patients, * depicts documented nasopharyngeal RT‐PCR negativity for SARS‐CoV‐2. The vertical axis shows the patient identification (ID) number in accordance with date of recruitment
3.1. Literature review
A total of 13 articles studying the presence of SARS‐CoV‐2 in semen were included for the final analysis (Table 2, Figure 3). Table 2 depicts the characteristics of all the studies on the presence of SARS‐CoV‐2 in semen. The volume of the bubble in the bubble plot (Figure 3) reflects the number of patients in each study, and the Y‐axis shows the number of days after which the semen sample was taken. The orange colour bubbles are the studies, which showed semen positivity for SARS‐CoV‐2 while the blue bubbles are the studies that could not detect SARS‐CoV‐2 in Semen.
TABLE 2.
Studies on presence of SARS‐CoV‐2 in semen using RT‐PCR
| Serial No. | Authors | Number of patients | Age (years) | Method of detection | Time between COVID‐19 diagnosis and collection of semen sample (days) | Results |
|---|---|---|---|---|---|---|
| 1 | Song et al., 2020 |
12 (alive) 1 (deceased) |
22–38 67 (deceased) |
RT‐PCR (semen) RT‐PCR + IgG and IgM for serum |
Median 29.5 (IQR 22.75–32.5) |
No SARS‐CoV‐2 detected in recovering patients No SARS‐CoV‐2 detected in testis of active patient (deceased) |
| 2 | Pan et al., 2020 | 34 |
37 (IQR 31–49) Range (18–55) |
RT‐PCR | Median 31 (IQR 29–36) |
SARS‐CoV‐2 not detected in the semen of men recovering from COVID‐19 SARS‐CoV‐2 unlikely to enter testicular cells through ACE2‐TMPRSS2 pathway in the testicular cells |
| 3 | Li et al., 2020 | 38 | 20s to 50s | RT‐PCR |
Median 10.5 days 6–16 days (data available for patients with positive semen samples only) |
6 /38 (15.8%) positive 4/15 (26.7%) with acute infection 2/23 (8.7%) recovering from COVID‐19 |
| 4 | Rawlings et al., 2020 | 6 | 38 (28–45) | RT‐PCR | 12 (6–17) | No SARS‐CoV‐2 detected in Semen by RT‐PCR |
| 5 | Ma et al., 2021 | 12 | 31 (25–46) | RT‐PCR | 78.5 (56–109) | No SARS‐CoV‐2 detected in Semen by RT‐PCR |
| 6 | Guo et al., 2021 | 46 | 41 (20–62) | RT‐PCR | 32 (27.5–33) | No SARS‐CoV‐2 detected in Semen by RT‐PCR |
| 7 | Ruan et al., 2021 | 70 | 31 (21–49) | RT‐PCR | 80 (IQR−64–93) | No SARS‐CoV‐2 detected in Semen by RT‐PCR |
| 8 | Paoli et al., 2020 | 1 | 31 | RT‐PCR | 27 | SARS‐CoV‐2 not detected in urine or semen |
| 9 | Kayaaslan et al., 2020 | 16 | 33.5 | RT‐PCR | 0–7 days | No SARS‐CoV‐2 detected in Semen by RT‐PCR |
| 10 | Machado et al., 2021 | 15 | 19–43 | RT‐PCR | 4.2 days | 1/15 (6.66%) Positive |
| 11 | Best et al., 2020 | 30 | 18–70 | RT‐PCR | 37 (IQR−23) | No SARS‐CoV‐2 detected in Semen by RT‐PCR |
| 12 | Holtmann et al., 2020 | 18 | 42.7 ± 10.4 | RT‐PCR | 8–54 days | No SARS‐CoV‐2 detected in Semen by RT‐PCR |
| 13 | Temiz et al., 2021 | 20 | 37.21 ± 8.59 | RT‐PCR | NA | No SARS‐CoV‐2 detected in Semen by RT‐PCR |
| 14 | Our Study | 11 | 29.72 ± 4.51 | RT‐PCR | Median 44 days (Range −19–59 days) | No SARS‐CoV‐2 detected in Semen by RT‐PCR |
FIGURE 3.
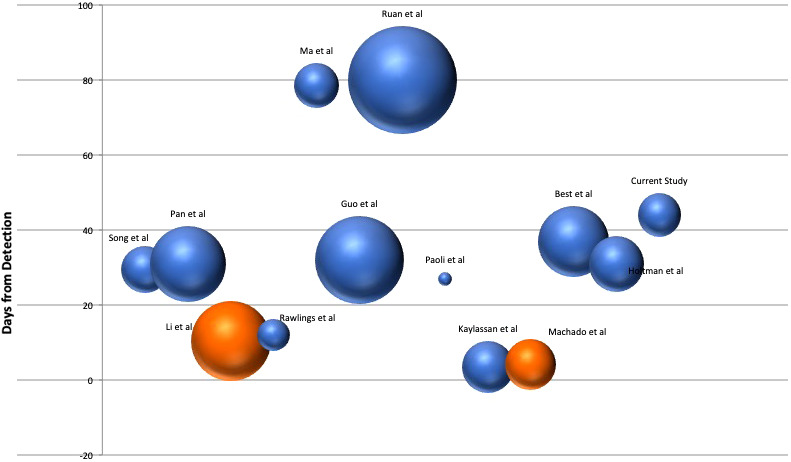
Bubble plot for the studies included in the review. The volume of the bubble reflects the number of patients included in the studies and plotted against the average number of days after which the semen sample was collected in patients diagnosed with COVID‐19 infection. The Orange Colored bubbles are the studies showing presence of SARS‐CoV‐2 in semen
3.2. Studies illustrating the presence of SARS‐CoV‐2 in semen
Only two studies (Li et al., 2020; Machado et al., 2021) have shown the presence of SARS‐CoV‐2 in semen in patients suffering from COVID‐19 (Li et al., 2020) and (Machado et al., 2021) demonstrated detection of SARS‐CoV‐2 in semen of 6/38 (15.8%) and 1/15 (6.6%) patients, respectively. The median number of days from COVID positivity was 10.5 days in the former and 4.2 days in the latter.
3.3. Studies illustrating the absence of SARS‐CoV‐2 in semen
11/13 studies showed that no SARS‐CoV‐2 could be detected in semen (Table 2). The number of patients enrolled in these studies ranged from 1 to 70, and the median duration after which the first sample was taken ranged from 0 days to 80 days.
3.4. Studies stratified as per the duration of semen sample taken
As seen from the bubble plot, four studies have performed RT‐PCR in semen in <21 days. Among them, only 2 studies found a total of 7 patients positive, and the rest all were negative. None of the studies beyond the period of 21 days has shown the presence of SARS‐CoV‐2 in semen.
4. DISCUSSION
Apart from respiratory droplets and contact surfaces, the SARS‐CoV‐2 has been detected in the infected patients' blood, saliva and urine (Wang et al., 2020). Its entry is mediated by ACE2 receptors, which have been expressed in several organs in the human body, including the male reproductive tract(Hoffmann et al., 2020; Wang et al., 2020). Although recent studies have found no evidence of sexual transmission of SARS‐CoV‐2 (Kayaaslan et al., 2020; Ma et al., 2021; Pan et al., 2020; Song et al., 2020), its impact on the male reproductive tract and detection in semen remains controversial with only two studies showing RT‐PCR positivity in semen (Li et al., 2020; Machado et al., 2020).
We conducted this prospective study on patients who had recovered from COVID‐19 infection for further insight in our subpopulation. Out of 21 patients contacted, 13 patients consented to the examination of the external genitalia that was normal in all the patients. The Ct values in Table 1 reflect that all but one patient had high viral load at the time of initial infection. Eleven patients agreed to provide a masturbatory semen sample for RT‐PCR; however, none of them showed the presence of SARS‐CoV‐2 in semen. For the initial four patients, the subsequent nasopharyngeal swab SARS CoV‐2 negative report was also available. It can be seen that in our study (Patient ID number 7), no SARS‐CoV‐2 is seen in semen as early as 19 days after COVID positivity. This is a pertinent finding as it shows that no carrier state is found in COVID‐19 patients in semen. Several viruses (Human immune deficiency virus, hepatitis B Virus, Ebola virus, human papillomavirus and herpes simplex virus) are known to establish a chronic state and are transmitted sexually (Teixeira et al., 2021). On the contrary, shedding of the Zika virus in semen was found almost 70 –132 days after the symptom onset (Huits et al., 2017), yet sexual transmission has not been reported. Whether the mere presence of virus using RT‐PCR in semen translates to sexual transmission again remains debatable. Even the source of the SARS‐CoV‐2 in semen remains a point of discussion. Accessory gland secretions or presence of leucocytes in the seminal fluid have been attributed to its presence (if at all) in the semen (Singh, 2020).
Table 2 depicts 14 studies on RT‐PCR for SARS‐CoV‐2 in semen and their pooled data that was obtained after our systematic search. RT‐PCR on a single semen sample was done in a total of 330 patients. The age of patients ranged from 18 to 55 years. Only 2/13 studies from the review showed the presence of SARS‐CoV‐2 in semen (Figure 3). Sample collection was performed at a median time of 10.5 days from diagnosis by Li and colleagues (Li et al., 2020) and at a median duration of 4.2 days in the study by Machado et al. In 11/13 studies and our study, the SARS‐CoV‐2 could not be detected in semen of patients recovering from COVID‐19. The difference in outcomes could be most probably attributed to a difference in timelines of the studies. The majority of the studies, including ours, have studied the presence of SARS‐CoV‐2 in semen in patients recovering from COVID‐19 infection. The median duration from COVID positivity in 9/13 studies and our study is >21 days (Table 2, Figure 3). Moreover, one study (Kayaaslan et al., 2020) failed to demonstrate SARS‐CoV‐2 in the semen of active patients as well using RT‐PCR. Their timeline in these studies for recruiting the earliest patient ranged between 0 and 8 days from nasopharyngeal positivity. It could also be possible that acute viraemia/viral load was different in these sub‐populations. In our study, the median duration was 44 days with a moderate to high initial viral load (20 to 33) in the nasopharyngeal swab (Table 1). The bubbles plot also shows 4/13 studies testing semen within three weeks of diagnosis of COVID‐19, and all the studies beyond 20 days failed to indicate the presence of SARS‐CoV‐2 in semen.
From our study and analysis of available literature cited in Table 2, it is possible to suggest that SARS‐CoV‐2 RNA may not be detectable in semen well after convalescence (>21 days). Even with evidence in favour of the presence of SARS‐CoV‐2 in semen in the acute phase in two studies (Li et al., 2020), there was no evidence of the persistence of the virus in ejaculate in the convalescence phase of the disease in any of the studies. Based on the available literature, the Italian Society of Andrology in August 2020 had stated that the available data do not support the presence of SARS‐CoV‐2 in semen (Corona et al., 2020). This came as a relief to all the clinicians involved with assisted reproduction as most of the ART programs were initially hit by the fear of transmission of COVID‐19 through semen. Moreover, whether the presence of RNA (using RT‐PCR) in the semen of clinically recovered patients implies transmissibility of infection or detection of noninfectious viral fragments can be answered only by the growth of viable virus particles on viral cultures from semen.
The impact of viral illness on reproductive health is always a matter of concern. Although the presence of ACE‐2 receptors on the testis has been cited as a potential source of infection of the testis by SARS‐CoV‐2, the evidence regarding the COVID‐19 affliction of testis directly is controversial. Presently, the affliction of testis could be attributed to an indirect immune‐mediated reaction, as established by the autopsy studies (Hallak et al., 2021; Teixeira et al., 2021). The COVID‐19 pandemic also hit the assisted reproduction facilities due to uncertainties over the transmission of virus through semen and its effect on embryo and pregnancy. As discussed earlier, researchers have postulated that the chances of presence of virus in the semen are minuscule (Singh, 2020), and thus, the concerns to utilisation of spermatozoa for assisted reproduction should also be alleviated.
Apart from sexual transmission, vertical transmission of SARS‐CoV‐2 to the foetus has also been a matter of concern. The absence of ACE‐2 in vaginal epithelial cells (Qiu et al., 2020) may deter the entry of SARS‐CoV‐2 into the female genital tract. The lack of the viral RNA in vaginal secretions in recent studies(Karimi‐Zarchi et al., 2020; Qiu et al., 2020; Simões e Silva & Leal, 2020) further substantiates this hypothesis, and transmission through heterosexual penetrative intercourse alone seems less likely. However, we still feel intercourse during active illness and early convalescence should be avoided; masturbation is best suited during this period. In conjunction with available literature, our study establishes that a carrier state in COVID‐19 in semen is nonexistent, and the detection of SARS‐CoV‐2 in semen beyond 21 days is not present. Thus, it is safe to say that heterosexual transmission is highly unlikely after 3–4 weeks from the nasopharyngeal positivity with the above considerations. This study also paves the way for utilisation of ejaculated spermatozoa for assisted reproduction in patients recovering from COVID‐19 illness.
To the best of our knowledge, this is the first prospective study on the presence of SARS‐CoV‐2 in semen from India with an exhaustive literature review on the topic. The limitation of this study remains a small sample size and unable to recruit patients with acute infection or early convalescence. Another limitation was the inability to document a negative COVID status on nasopharyngeal swab due to change in national guidelines as mentioned. However, the study does contribute and consolidates the guidelines for safe sex practices in patients recovering from COVID‐19 infection.
5. CONCLUSIONS
SARS‐CoV‐2 is not found in the semen of patients recovering from COVID‐19. Transmission through semen and establishment of carrier state is unlikely after the convalescent phase.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
This work was carried out as a part of routine COVID‐19 diagnostic activity of the Regional Virus Diagnostic Lab (RVDL) under ICMR, New Delhi by the Department of Virology, PGIMER, Chandigarh to identify semen as a possible source of transmission of SARS CoV‐2. We thank the patients who agreed to contribute semen samples for this study.
Sharma AP, Sahoo S, Goyal K, et al. Absence of SARS‐CoV‐2 infection in the semen of men recovering from COVID‐19 infection: An exploratory study and review of literature. Andrologia. 2021;53:e14136. 10.1111/and.14136
Contributor Information
Aditya Prakash Sharma, Email: aditya.p.sharma@gmail.com.
Mini P. Singh, Email: minipsingh@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Achua, J. K., Chu, K. Y., Ibrahim, E., Khodamoradi, K., Delma, K. S., Iakymenko, O. A., Kryvenko, O. N., Arora, H., & Ramasamy, R. (2021). Histopathology and ultrastructural findings of fatal COVID‐19 infections on testis. The World Journal of Men's Health, 39(1), 65. 10.5534/wjmh.200170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best, J. C., Kuchakulla, M., Negris Lima, T. F., Mora, B., Frech, F., Achua, J. K., Arora, H., Ibrahim, E., & Ramasamy, R. (2020). An evaluation of semen parameters in men with confirmed covid‐19 infection. Fertility and Sterility, 114(3), e380–e381. 10.1016/j.fertnstert.2020.08.1121 [DOI] [Google Scholar]
- Chen, Y.‐W., Lee, M.‐S., Lucht, A., Chou, F.‐P., Huang, W., Havighurst, T. C., Kim, K., Wang, J.‐K., Antalis, T. M., Johnson, M. D., & Lin, C.‐Y. (2010). TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. The American Journal of Pathology, 176(6), 2986–2996. 10.2353/ajpath.2010.090665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona, G., Baldi, E., Isidori, A. M., Paoli, D., Pallotti, F., De Santis, L., Francavilla, F., La Vignera, S., Selice, R., Caponecchia, L., Pivonello, R., Ferlin, A., Foresta, C., Jannini, E. A., Lenzi, A., Maggi, M., & Lombardo, F. (2020). SARS‐CoV‐2 infection, male fertility and sperm cryopreservation: A position statement of the Italian Society of Andrology and Sexual Medicine (SIAMS) (Società Italiana di Andrologia e Medicina della Sessualità). Journal of Endocrinological Investigation, 43(8), 1153–1157. 10.1007/s40618-020-01290-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, L., Zhao, S., Li, W., Wang, Y., Li, L., Jiang, S., Ren, W., Yuan, Q., Zhang, F., Kong, F., Lei, J., & Yuan, M. (2021). Absence of SARS‐CoV‐2 in semen of a COVID‐19 patient cohort. Andrology, 9(1), 42–47. 10.1111/andr.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak, J., Teixeira, T. A., Bernardes, F. S., Carneiro, F., Duarte, S. A. S., Pariz, J. R., Esteves, S. C., Kallas, E., & Saldiva, P. H. N. (2021). SARS‐CoV‐2 and its relationship with the genitourinary tract: Implications for male reproductive health in the context of COVID‐19 pandemic. Andrology, 9(1), 73–79. 10.1111/andr.12896 [DOI] [PubMed] [Google Scholar]
- Holtmann, N., Edimiris, P., Andree, M., Doehmen, C., Baston‐Buest, D., Adams, O., Kruessel, J. S., & Bielfeld, A. P. (2020). Assessment of SARS‐CoV‐2 in human semen‐a cohort study. Fertility and Sterility, 114(2), 233–238. 10.1016/j.fertnstert.2020.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M., Kleine‐Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., Schiergens, T. S., Herrler, G., Wu, N.‐H., Nitsche, A., Müller, M. A., Drosten, C., & Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C., Ji, X., Zhou, W., Huang, Z., Peng, X., Fan, L., Lin, G., & Zhu, W. (2021). Coronavirus: A possible cause of reduced male fertility. Andrology, 9(1), 80–87. 10.1111/andr.12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huits, R., De Smet, B., Ariën, K. K., Van Esbroeck, M., Bottieau, E., & Cnops, L. (2017). Zika virus in semen: A prospective cohort study of symptomatic travellers returning to Belgium. Bulletin of the World Health Organization, 95(12), 802–809. 10.2471/BLT.17.181370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- India: WHO Coronavirus Disease (COVID‐19) Dashboard. (n.d.). Retrieved January 28, 2021, from https://covid19.who.int [Google Scholar]
- Karimi‐Zarchi, M., Neamatzadeh, H., Dastgheib, S. A., Abbasi, H., Mirjalili, S. R., Behforouz, A., Ferdosian, F., & Bahrami, R. (2020). Vertical transmission of coronavirus disease 19 (COVID‐19) from infected pregnant mothers to neonates: A review. Fetal and Pediatric Pathology, 39(3), 246–250. 10.1080/15513815.2020.1747120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaaslan, B., Korukluoglu, G., Hasanoglu, I., Kalem, A. K., Eser, F., Akinci, E., & Guner, R. (2020). Investigation of SARS‐CoV‐2 in semen of patients in the acute stage of COVID‐19 infection. Urologia Internationalis, 104(9–10), 678–683. 10.1159/000510531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D., Jin, M., Bao, P., Zhao, W., & Zhang, S. (2020). Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Network Open, (5), e208292. 10.1001/jamanetworkopen.2020.8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Moore, M. J., Vasilieva, N., Sui, J., Wong, S. K., Berne, M. A., Somasundaran, M., Sullivan, J. L., Luzuriaga, K., Greenough, T. C., Choe, H., & Farzan, M. (2003). Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 426(6965), 450–454. 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L., Xie, W., Li, D., Shi, L., Ye, G., Mayo, Y., Xiong, Y., Sun, H., Zheng, F., Chen, Z., Qin, J., Lyu, J., Zhang, Y., & Zhang, M. (2021). Evaluation of sex‐related hormones and semen characteristics in reproductive‐aged male COVID‐19 patients. Journal of medical virology, 93(1), 456–462. 10.1101/2020.03.21.20037267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, B., Barcelos Barra, G., Scherzer, N., Massey, J., Dos Santos Luz, H., Henrique Jacomo, R., Herinques Santa Rita, T., & Davis, R. (2021). Presence of SARS‐CoV‐2 RNA in semen‐cohort study in the United States COVID‐19 positive patients. Infectious Disease Reports, 13(1), 96–101. 10.3390/idr13010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, F., Xiao, X., Guo, J., Song, Y., Li, H., Patel, D. P., Spivak, A. M., Alukal, J. P., Zhang, X., Xiong, C., Li, P. S., & Hotaling, J. M. (2020). No evidence of SARS‐CoV‐2 in semen of males recovering from COVID‐19. Fertility and Sterility, 113(6), 1135–1139. 10.1016/j.fertnstert.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli, D., Pallotti, F., Colangelo, S., Basilico, F., Mazzuti, L., Turriziani, O., Antonelli, G., Lenzi, A., & Lombardo, F. (2020). Study of SARS‐CoV‐2 in semen and urine samples of a volunteer with positive naso‐pharyngeal swab. Journal of Endocrinological Investigation, 43(12), 1819–1822. 10.1007/s40618-020-01261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, L., Liu, X., Xiao, M., Xie, J., Cao, W., Liu, Z., Morse, A., Xie, Y., Li, T., & Zhu, L. (2020). SARS‐CoV‐2 is not detectable in the vaginal fluid of women with severe COVID‐19 infection. Clinical Infectious Diseases, 71(15), 813–817. 10.1093/cid/ciaa375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings, S. A., Ignacio, C., Porrachia, M., Du, P., Smith, D. M., & Chaillon, A. (2020). No evidence of SARS‐CoV‐2 seminal shedding despite SARS‐CoV‐2 persistence in the upper respiratory tract. Open Forum Infectious Diseases, 7(8), ofaa325. 10.1093/ofid/ofaa325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, Y., Hu, B., Liu, Z., Liu, K., Jiang, H., Li, H., Li, R., Luan, Y., Liu, X., Yu, G., Xu, S., Yuan, X., Wang, S., Yang, W., Ye, Z., Liu, J., & Wang, T. (2021). No detection of SARS‐CoV‐2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID‐19 male patients: A perspective and urogenital evaluation. Andrology, 9(1), 99–106. 10.1111/andr.12939 [DOI] [PubMed] [Google Scholar]
- Simões e Silva, A. C., & Leal, C. R. V. (2020). Is SARS‐CoV‐2 vertically transmitted? Frontiers in Pediatrics, 8, 276. 10.3389/fped.2020.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R. (2020). Assisted reproduction in COVID‐19 season: Expend the fear. Fertility and Sterility Dialog. http://www.fertstertdialog.com/posts/assisted‐reproduction‐in‐covid‐19‐season‐expend‐the‐fear [Google Scholar]
- Song, C., Wang, Y., Li, W., Hu, B., Chen, G., Xia, P., Wang, W., Li, C., Diao, F., Hu, Z., Yang, X., Yao, B., & Liu, Y. (2020). Absence of 2019 novel coronavirus in semen and testes of COVID‐19 patients. Biology of Reproduction, 103(1), 4–6. 10.1093/biolre/ioaa050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira, T., Oliveira, Y., Bernardes, F., Kallas, E., Duarte‐Neto, A., Esteves, S., Drevet, J., & Hallak, J. (2021). Viral infections and implications for male reproductive health. Asian Journal of Andrology, 23, 1–13. 10.4103/aja.aja_82_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temiz, M. Z., Dincer, M. M., Hacibey, I., Yazar, R. O., Celik, C., Kucuk, S. H., Alkurt, G., Doganay, L., Yuruk, E., & Muslumanoglu, A. Y. (2021). Investigation of SARS‐CoV‐2 in semen samples and the effects of COVID‐19 on male sexual health by using semen analysis and serum male hormone profile: A cross‐sectional, pilot study. Andrologia, 53(2), e13912. 10.1111/and.13912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvkarma, R., & Rajender, S. (2020). Could SARS‐CoV‐2 affect male fertility? Andrologia, 52(9), e13712. 10.1111/and.13712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Xu, Y., Gao, R., Lu, R., Han, K., Wu, G., & Tan, W. (2020). Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA, 323(18), 1843–1844. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., & McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA, 323(13), 1239. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- Zhou, P., Yang, X.‐L., Wang, X.‐G., Hu, B., Zhang, L., Zhang, W., Si, H.‐R., Zhu, Y., Li, B., Huang, C.‐L., Chen, H.‐D., Chen, J., Luo, Y., Guo, H., Jiang, R.‐D., Liu, M.‐Q., Chen, Y., Shen, X.‐R., Wang, X., … Shi, Z.‐L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


