Abstract
Introduction and objectives
Patients awaiting heart transplantation (HTx) are at increased risk developing severe coronavirus disease 2019 (COVID‐19). Patients supported by a left ventricular assist device (LVAD) face additional risks due to coagulopathies during COVID‐19. Following HTx, elevated risk factors for severe COVID‐19 persist due to chronic immunosuppression and frequent comorbidities. Taken together, COVID‐19 vaccination is of critical importance in all three patient cohorts. Here, we report our experience to deliver COVID‐19 vaccination in a German transplant center.
Methods and results
We screened 211 patients for contraindications and offered the remaining 186 eligible patients COVID‐19 vaccination. Of those, 133 patients (71%) accepted the offer and were vaccinated. Acceptance of vaccination differed between HTx recipients (84 of 113, 74%), patients on the waiting list (34 of 47, 72%), and patients with LVAD support (28 of 50, 56%). The LVAD cohort demonstrated lower acceptance levels for vaccination compared to HTx recipients and patients awaiting HTx (74% vs. 56%; p = 0.028).
Conclusion
We demonstrate for the first time only moderate acceptance levels of COVID‐19 vaccination in HTx recipients and candidates on the waiting list compared to general population, despite perceived high‐risk for severe disease. Additionally, those supported by LVAD have even lower adherence. Efforts may need to be made to increase acceptance in this vulnerable as well as cost‐intensive patient cohort.
Keywords: COVID‐19, heart transplantation, LVAD, vaccination
1. INTRODUCTION
Cardiovascular comorbidities are a major risk factor for adverse outcome of coronavirus disease 2019 (COVID‐19). 1 Thus, patients awaiting heart transplantation (HTx) are perceived at increased risk. Following HTx, elevated risk factors for severe COVID‐19 persist due to chronic immunosuppression and frequent comorbidities. 2 , 3 , 4 , 5 Patients supported by a left ventricular assist device (LVAD) face additional risks due to coagulopathies during COVID‐19. Taken together, delivery of COVID‐19 vaccination is critically important in all three patient cohorts. Here, we report our experience to provide COVID vaccination to these patients in a German transplant center and investigate whether acceptance of vaccination differs between these groups.
2. PATIENTS AND METHODS
We screened patients registered at our center on the waiting list for HTx, who already received a heart transplant or who were supported by LVAD (n = 211). Twenty‐five patients had to be excluded due to contraindications (six with positive COVID‐19 PCR test within the past 6 months, two with active infectious disease, and 17 were hospitalized). A dedicated heart failure nurse, familiar with all patients, contacted the remaining 186 patients and offered COVID‐19 vaccination using the mRNA‐based vaccine Comirnaty (BioNTech). At that time, no side effects concerning potential myocarditis were evident. Statistical analysis was performed using descriptive statistics (mean, standard deviation) and two‐sided Fisher's exact test for testing significance of dichotomous variables, where applicable.
3. RESULTS
Of 186 eligible patients, 133 (71%) received vaccination (Figure 1A). This cohort consisted of 84 HTx recipients, 34 patients on the waiting list (38% supported by LVAD as bridge to transplant) and 15 LVAD patients with destination therapy. Acceptance of vaccination (Figure 1B) differed between HTx recipients (84 of 113, 74%), patients on the waiting list (34 of 47, 72%), and patients with LVAD support (28 of 50; 56%). No difference was observed between patients pre‐ and post‐HTx (72% vs. 74%; p = 0.845). In the LVAD‐cohort, being on the waiting list for HTx did not influence acceptance of adherence (54% vs. 57%; p > 0.99). In the subgroup of patients with LVAD, acceptance for vaccination was significantly reduced in comparison to the HTx‐cohort (74% vs. 56%; p = 0.028).
FIGURE 1.
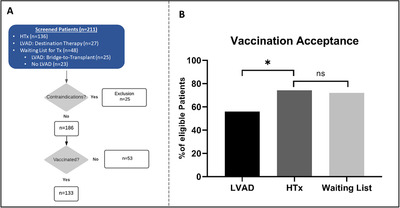
(A) Consort diagram: We screened 211 patients for COVID‐19 vaccination including 136 HTx recipients, 27 LVAD patients with destination therapy, and 48 patients awaiting HTx (including 25 patients with LVAD as bridge‐to‐transplant therapy). After exclusion for contraindications, we offered 186 patients COVID‐19 vaccination. Of those, 133 patients (71%) received vaccination including 84 HTx, 15 patients with LVAD as destination therapy, and 34 patients on the waiting list, including 13 patients with LVAD as bridge‐to‐transplant therapy. (B) Acceptance of vaccination offer in HTx, LVAD and patients on waiting list for HTx as fraction (%) of eligible patients in each subgroup. Acceptance of vaccination was significantly reduced in patients with LVAD (56%) compared to patients HTx (74%, p = 0.028). No difference could be observed between HTx and waiting list patients (72%, p = 0.845). Significance was calculated using two‐sided Fisher's exact test
4. DISCUSSION AND CONCLUSION
The present study reflects our experience to deliver COVID‐19 vaccination in a German transplant center comprising three high‐risk cohorts: patients awaiting HTx, heart transplant recipients, and patients supported by an LVAD. We aimed to investigate whether acceptance of vaccination differs between these groups.
In the general population, Lazarus et al recently reported an acceptance level of vaccination against COVID‐19 of 71%. 6 This is in the range of the acceptance we observed in our patients on the waiting list for Htx and following transplantation. Additionally, a subgroup analysis in our study showed that patients supported by LVAD evidenced conspicuously even lower acceptance for vaccination than HTx‐recipients.
Our main finding is therefore that even if vaccination acceptance in our study is equal compared to a general population, one might expect even higher level of acceptance for a vaccination in a high‐risk cohort of disease, especially when patients are aware of their increased risk. In this regard, it is noteworthy that acceptance levels of COVID‐19 seem geographically heterogeneous as depicted by a nation‐wide survey in Kuwait with acceptance levels around 53%, 7 as well as time‐dependent as indicated by a decline of acceptance over the present year in the USA. 8 Since the referenced surveys only investigated acceptance of a vaccination in theory by interview, while our data reflect the proportion of patients receiving vaccination, one could speculate about a bias to smaller levels of acceptance in our cohort. Of note, when compared to acceptance of vaccinations for diseases as Hepatitis B or pneumococcus by recipients of solid organs in the range of 40%–66%, 9 the levels of our observations seem plausible.
Our report has some limitations: The absolute number of patients was highest in the HTx‐group, which might lead to a bias. However, the effect was statistically significant even with low numbers in the LVAD‐group, which corroborates the difference between those two cohorts.
In conclusion, this report demonstrates for the first time only moderate acceptance levels of COVID‐19 vaccination in HTx recipients and candidates on the waiting list compared to general population, despite perceived high‐risk for severe disease. Additionally, those supported by LVAD have even lower adherence. Efforts may need to be made to increase acceptance in this vulnerable as well as cost‐intensive patient cohort.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
Daniel Oehler wrote the main manuscript text and prepared the figure. All authors reviewed the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Anja Dräger for her commitment to patient care and her substantial contribution to administration and data acquisition.
Oehler D, Bruno RR, Boeken U, Westenfeld R. Moderate acceptance of COVID‐19 vaccination in patients pre‐ and post‐heart transplantation: Experiences from a German Transplant Centre. Transpl Infect Dis. 2021;23:e13681. 10.1111/tid.13681
REFERENCES
- 1. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kates OS, Haydel BM, Florman SS, et al. COVID‐19 in solid organ transplant: a multi‐center cohort study. Clin Infect Dis. 2020. 10.1093/cid/ciaa1097. [DOI] [Google Scholar]
- 4. Nair V, Jandovitz N, Hirsch JS, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID‐19 vaccine. Nat Med. 2021;27(2):225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alqudeimat Y, Alenezi D, AlHajri B, et al. Acceptance of a COVID‐19 vaccine and its related determinants among the general adult population in Kuwait. Med Princ Pract. 2021;30(3):262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ham L, Kirzinger A, Lopes L, Kearney A, Sparks G, Brodie M. KFF Covid‐19 Vaccine Monitor. San Francisco, CA, USA: Kaiser Family Foundation; 2021. [Google Scholar]
- 9. Jandhyala D, Lewis JD. An analysis of adherence to vaccination recommendations in a thoracic organ transplant cohort. Vaccines (Basel). 2020;8(4):622. [DOI] [PMC free article] [PubMed] [Google Scholar]


