Summary
Vitamin D has many protective properties and potential role against acute lung injury. Low serum vitamin D is associated with high risk of pneumonia and development of acute respiratory distress syndrome. This study sought to analyse the efficacy of vitamin D in improving the outcomes of coronavirus disease 2019 (Covid‐19) patients. Using specific keywords, we comprehensively searched the potential articles on PubMed, Europe PMC and ClinicalTrials.gov database until 8th May 2021. All published studies on Covid‐19 and vitamin D were retrieved. Statistical analysis was conducted using Review Manager 5.4 software. A total of 11 studies with 22,265 Covid‐19 patients were included in the meta‐analysis. Our data suggested that vitamin D supplementation was associated with reduction in intensive care unit admission rate (OR 0.27; 95% CI: 0.09–0.76, p = 0.010, I 2 = 70%, random‐effect modelling); reduction of the need for mechanical ventilation (OR 0.34; 95% CI: 0.16–0.72, p = 0.005, I 2 = 61%, random‐effect modelling) and reduction of mortality from Covid‐19 (OR 0.37; 95% CI: 0.21–0.66, p < 0.001, I 2 = 50%, random‐effect modelling). Further analysis showed that the associations were influenced by age (p = 0.020). Our study suggests that vitamin D supplementation may offer beneficial effects on Covid‐19 outcomes. However, more randomized clinical trials are required to confirm this conclusion.
Keywords: coronavirus disease 2019, Covid‐19, vitamin D
Abbreviations
- ACE
angiotensin‐converting enzyme
- ARDS
acute respiratory distress syndrome
- CI
confidence intervals
- Covid‐19
coronavirus disease 2019
- ICU
Intensive Care Unit
- IFN‐γ
interferon‐γ
- IL‐1β
interleukin‐1β
- IL‐6
interleukin 6
- IU
International Unit
- JBI
Joanna Briggs Institute
- MOF
multi‐organ failure
- NF‐kB
nuclear factor‐kappa B
- NK cells
natural killer cells
- NKG2A
NK group 2 member A
- NOS
Newcastle–Ottawa Scale
- OR
odds ratio
- RAS
renin angiotensin system
- RT‐PCR
Reverse Transcriptase‐Polymerase Chain Reaction
- SARS
severe acute respiratory syndrome
- SARS‐CoV‐2
SARS‐coronavirus‐2
- SNP
single nucleotide polymorphism
- Th‐1
T helper‐1
- TNF‐α
tumour necrosis factor‐α
- VDR
vitamin D receptor
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection continues to spread globally causing a pandemic and has become a major medical focus for the last couple of years. The pandemic has involved over 164 million confirmed cases, with more than 3.4 million deaths as of 23 May 2021. 1 While some SARS‐CoV‐2 infections appear as mild upper respiratory symptoms and may be self‐limiting, notable numbers of patients require hospitalizations and intensive treatment following progression into a more severe cases, varying from simple lower respiratory tract infections to acute respiratory distress syndrome (ARDS) and eventually may turn into multi‐organ failure. 2 , 3
Individuals with comorbidities such as obesity, diabetes, chronic respiratory disease, cardiovascular disease and other immunocompromising conditions are facing higher risks in developing the severe form of SARS‐CoV‐2 infections. 4 , 5 , 6 , 7 , 8 , 9 For the past months, medical therapies to treat Covid‐19 have been growing and evolving rapidly, ranging from supportive care, antivirals, anti‐inflammatory agents and possible supplementations such as vitamin D. 10 , 11 , 12 , 13 Several studies have shown that vitamin D has antiviral properties and potential roles against acute lung injury or ARDS, making large interest on vitamin D has rapidly emanated even in the early beginning of pandemic. 14 , 15 Low vitamin D serum levels is associated with an increase in inflammatory cytokine levels and significant increase of risk to develop pneumonia and viral respiratory tract infections, which both contribute to the development of ARDS. 16 , 17 , 18 The goal of this study is to provide evidence whether or not vitamin D supplementation is associated with improved outcomes of Covid‐19 based on the available studies.
2. MATERIALS AND METHODS
2.1. Eligibility criteria
The protocol of this systematic review and meta‐analysis study of the observational and clinical trial studies was registered in PROSPERO (CRD42021256117). Included articles were selected as potentially fulfilling the entry criteria: comply the PICO framework (P: Covid‐19 patients; I: vitamin D supplementation in any form; C: a group of patients who did not receive vitamin D, only receive standard of care therapy or any other medications as control/placebo; O: intensive care unit [ICU] admission, the need for mechanical ventilation and mortality), cohort, case‐control, cross‐sectional and randomized or non‐randomized clinical trial articles were included. All studies other than original research articles (review articles, letter to editor or correspondence), case‐series or case report studies, studies reported other than in English language, studies focussing on populations below 18 years of age and pregnant women were excluded.
2.2. Search strategy and study selection
The papers from three databases (PubMed, Europe PMC and ClinicalTrials.gov) were searched systemically. Search terms used include ‘vitamin D’ OR ‘calcidiol’ OR ‘calciferol’ OR ‘calcifediol’ OR ‘cholecalciferol’ OR ‘calcitriol’ AND ‘SARS‐CoV‐2’, OR ‘coronavirus disease 2019’ OR ‘Covid‐19’ in a time range from 2019 until 8 May 2021 with English‐language restriction. Our searching strategy details are listed in Table 1. Initial screening of titles and abstracts was conducted to identify eligible articles. Searches of potential articles were also done by analysing the list of references of eligible studies. The search strategy was presented in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) diagram.
TABLE 1.
Literature search strategy
| Database | Keyword | Result |
|---|---|---|
| PubMed | (‘vitamin d’[MeSH Terms] OR ‘vitamin d’[All Fields] OR ‘ergocalciferols’[MeSH Terms] OR ‘ergocalciferols’[All Fields]) OR (‘calcifediol’[MeSH Terms] OR ‘calcifediol’[All Fields] OR ‘calcidiol’[All Fields]) OR (‘ergocalciferols’[MeSH Terms] OR ‘ergocalciferols’[All Fields] OR ‘calciferol’[All Fields]) OR (‘calcitriol’[MeSH Terms] OR ‘calcitriol’[All Fields]) AND (‘COVID‐19’[All Fields] OR ‘COVID‐19’[MeSH Terms] OR ‘COVID‐19 Vaccines’[All Fields] OR ‘COVID‐19 Vaccines’[MeSH Terms] OR ‘COVID‐19 serotherapy’[All Fields] OR ‘COVID‐19 Nucleic Acid Testing’[All Fields] OR ‘covid‐19 nucleic acid testing’[MeSH Terms] OR ‘COVID‐19 Serological Testing’[All Fields] OR ‘covid‐19 serological testing’[MeSH Terms] OR ‘COVID‐19 Testing’[All Fields] OR ‘covid‐19 testing’[MeSH Terms] OR ‘SARS‐CoV‐2’[All Fields] OR ‘sars‐cov‐2’[MeSH Terms] OR ‘Severe Acute Respiratory Syndrome Coronavirus 2’[All Fields] OR ‘NCOV’[All Fields] OR ‘2019 NCOV’[All Fields] OR ((‘coronavirus’[MeSH Terms] OR ‘coronavirus’[All Fields] OR ‘COV’[All Fields]) AND 2019/11/01[PubDate]: 3000/12/31[PubDate])) | 612 |
| Europe PMC | ‘vitamin D’ OR ‘calcidiol’ OR ‘calciferol’ OR ‘calcifediol’ OR ‘cholecalciferol’ OR ‘calcitriol’ AND ‘SARS‐CoV‐2’, OR ‘coronavirus disease 2019’ OR ‘Covid‐19’ | 5330 |
| ClinicalTrials.gov | ‘vitamin D’ OR ‘calcidiol’ OR ‘calciferol’ OR ‘calcifediol’ OR ‘cholecalciferol’ OR ‘calcitriol’ AND ‘SARS‐CoV‐2’, OR ‘coronavirus disease 2019’ OR ‘Covid‐19’ | 96 |
2.3. Data extraction and quality assessment
Two authors (TIH and DI) performed the data extraction. An extraction form was developed to list the essential information about the study and its population characteristic (age, gender, hypertension, diabetes and corticosteroids usage/consumption), vitamin D dose, the number of patients receiving vitamin D and the control group, as well as the outcome of Covid‐19 patients.
The outcomes of interest are the rate of ICU admission, the need for mechanical ventilation and the mortality. The ICU admission rate is defined by the number of patients who were subsequently admitted into the ICU during the hospital stay. The need for mechanical ventilation is defined by the number of patients who need assisted ventilation. The total number of patients who were dead during the follow‐up period with positive Covid‐19 status was described as the mortality outcome.
Two authors (JEH and HH) assessed the quality of each study included in this study independently. The quality of clinical trials was assessed using the modified Jadad scale assessment where the random allocation, allocation concealment, blindness and withdrawals and drop‐outs of each study were evaluated. The studies were scored from zero to seven and a study ranked as a high‐quality study if the score was >4. 19 The quality of case‐control and cohort studies were assessed using Newcastle–Ottawa Scale (NOS). The assessment reviews the selection, comparability and outcome of each study, then each study was assigned a total score from zero to nine. A study is graded as good quality if it scores ≥7. Cross‐sectional studies was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Tools for Analytical Cross Sectional Studies.
2.4. Statistical analysis
The meta‐analysis was performed using the Review Manager 5.4 (Cochrane Collaboration) software and Comprehensive Meta‐Analysis version 3. Mantel–Haenszel's formula was employed to calculate odds ratio (OR) and its 95% confidence interval (95%CI) for the ICU admission outcome and the need for mechanical ventilation outcome, while Inverse Variance method was used to obtain the OR and 95% CI for the mortality outcome. The heterogeneity was assessed by using the I 2 statistic with a value of <25%, 26–50% and >50% were considered as low, moderate and high degrees of heterogeneity, respectively. Random effects meta‐regression was performed using a maximum likelihood for pre‐specified variables including age, gender, hypertension, diabetes and the use of corticosteroids. The qualitative risk of publication bias was assessed with funnel plot analysis, while the quantitative risk of publication bias was assessed by using the Begg and Mazumdar rank correlation test. 20
3. RESULTS
3.1. Study selection and characteristics
The searches on the databases yielded 6038 studies. A total of 5128 records remained following the elimination of duplicates. Screening the titles and abstracts and matching the inclusion and exclusion criteria, 5063 studies were removed. Among 65 evaluated full‐text articles for its eligibility, 46 articles were excluded due to unavailable of results (still recruiting or withdrawn), 4 articles had no control or comparison group, 3 articles did not mention the criteria of the outcome of interest and 1 article because the full‐text was not in English. The meta‐analysis included 11 studies 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 with a total of 2265 Covid‐19 patients (Figure 1). Out of 11 studies, one study was double‐blind randomized clinical trial, one study was open‐label randomized clinical trial studies, four were retrospective cohort studies, two were prospective cohort studies and three were cross‐sectional studies. All of the vitamin D doses were administered orally, although the dosage varied between each of the included studies, ranging from 25,000 IU/month up to 200,000 IU/day for two consecutive days. The baseline characteristics and severity between the vitamin D group and the control groups were already controlled in each of the included studies, meaning that there is no significant difference between two groups' characteristics. Table 2 presents the characteristics of the studies.
FIGURE 1.
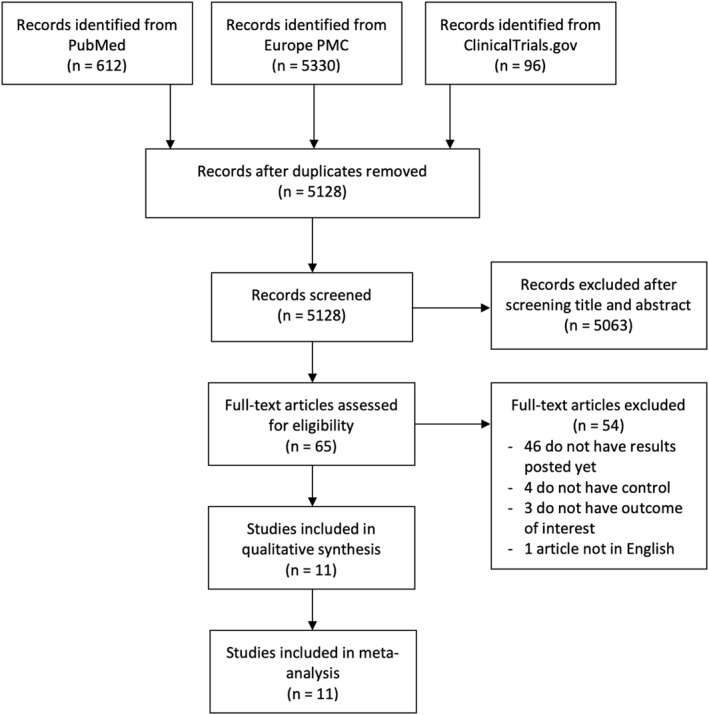
PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta‐analysis
TABLE 2.
Characteristics of included studies
| Study | Sample size | Design | Overall age mean ± SD | Malen (%) | Hypertension | Diabetes | Corticosteroid use | Vitamin D dose | Number of patients receiving Vitamin D versus Control |
|---|---|---|---|---|---|---|---|---|---|
| Annweiler C et al. 21 2020 | 66 | Retrospective cohort | 87.7 ± 9 | 15 (22.7%) | N/A | N/A | 4 (6.1%) | Cholecalciferol oral: 80,000 IU every 2–3 months | 57 (86.3%) versus 9 (13.7%) |
| Annweiler G et al. 22 2020 | 77 | Retrospective cohort | 88.3 ± 5.1 | 39 (50.6%) | 49 (63.6%) | N/A | 13 (16.9%) | Group 1: Cholecalciferol oral 50,000 IU per month, or the doses of 80,000 IU or 100,000 IU every 2–3 months. Group 2: Cholecalciferol oral 80,000 IU/months | 45 (58.4%) versus 32 (41.6%) |
| Cangiano B et al. 23 2020 | 98 | Prospective cohort | 89.6 ± 6.53 | 38 (24.2%) | 48 (48.9%) | 11 (11.2%) | 8 (8.1%) | Cholecalciferol oral: 25,000 IU twice a months | 20 (20.4%) versus 78 (79.6%) |
| Castillo ME et al. 24 2020 | 76 | Open‐label, randomized clinical trial | 53 ± 10 | 45 (59%) | 26 (34.2%) | 8 (10.5%) | N/A | Calcifediol oral: 0.532 mg on Day 1, then 0.266 on Day 3 and 7 | 50 (65.7%) versus 26 (34.3%) |
| Giannini S et al. 25 2021 | 91 | Retrospective cohort | 74 ± 13 | 50 (55%) | N/A | 30 (33%) | 41 (45%) | Cholecalciferol oral: 200,000 IU daily for 2 consecutive days | 36 (39.5%) versus 55 (60.5%) |
| Hernandez JL et al. 26 2020 | 216 | Retrospective case‐control | 59.5 ± 16.6 | 130 (60.1%) | 88 (40.7%) | 34 (15.7%) | 47 (21.7%) | Cholecalciferol oral: 25,000 IU/monthly in 11 patients. Calcifediol oral: 0.266 mg/monthly in eight patients | 19 (8.7%) versus 197 (91.3%) |
| Jevalikar G et al. 27 2021 | 409 | Cross‐sectional | 46.3 ± 15.4 | 134 (68%) | 163 (39.8%) | 188 (45.9%) | N/A | Cholecalciferol oral: 60,000 IU | 197 (48.1%) versus 212 (51.9%) |
| Ling SF et al. 28 2020 | 444 | Cross‐sectional | 73.3 ± 14.8 | 245 (55.2%) | 197 (44.4%) | 129 (29.1%) | N/A | Cholecalciferol oral: 40,000 IU weekly (47.9%), 20,000 IU twice weekly (28.8%), 20,000 IU weekly (11%) for a maximum of 7 weeks | 73 (16.4%) versus 371 (83.6%) |
| Murai IH et al. 29 2021 | 237 | Double‐blind, randomized clinical trial | 56.5 ± 13.8 | 133 (56.1%) | 126 (53.1%) | 84 (35.4%) | 150 (63.2%) | Cholecalciferol oral: 200,000 IU single dose | 119 (50.2%) versus 118 (49.8%) |
| Tan CW et al. 30 2020 | 43 | Prospective cohort | 61.7 ± 7.5 | 26 (60.4%) | 24 (55.8%) | 6 (13.9%) | N/A | Cholecalciferol oral: 1000 IU/day for up to 14 days | 17 (39.5%) versus 26 (60.5%) |
| Vasheghani M et al. 31 2021 | 508 | Cross‐sectional | 56 ± 17 | 264 (52%) | 35 (7%) | 116 (23%) | 27 (5.3%) | Cholecalciferol oral: at least 50,000 IU during the past months | 88 (17%) versus 420 (83%) |
3.2. Quality of study assessment
Jadad scale assessments suggested that one clinical trial study was graded high quality, while another study was graded moderate quality (Table 3). Quality assessment of cohort and case‐control studies using NOS scale and cross‐sectional studies using JBI Critical Appraisal checklist indicated all included studies had a good quality (Table 4 and Table 5). Altogether, all studies were acceptable to be further analysed using meta‐analysis.
TABLE 3.
Quality appraisal of studies included in the meta‐analysis using Jadad scale assessment
| Study | Random allocation | Concealment schemes | Blinding | Withdrawals and drop‐out | Total score | Interpretation |
|---|---|---|---|---|---|---|
| Castillo ME et al. 24 2020 | 2 | 1 | 0 | 1 | 4 | Moderate quality |
| Murai IH et al. 29 2020 | 2 | 2 | 2 | 1 | 7 | High quality |
Note: Points were determined as follows: (1) random allocation: computer‐generated random numbers, 2 points; not described, 1 point; inappropriate method, 0 point; (2) allocation concealment: central randomization, sealed envelopes or similar, 2 points; not described, 1 point; inappropriate or unused, 0 point; (3) blindness: identical placebo tablets or similar, 2 point; inadequate or not described, 1 point; inappropriate or no double blinding, 0 point; and (4) withdrawals and drop‐outs: numbers and reasons are described, 1 point; not described, 0 point. The Jadad scale score ranges from 1 to 7; higher score indicates better RCT quality. If a study had a modified Jadad score >4 points, it was considered to be of high quality; if the score was 3–4 points, it was moderate quality; and if the score was <3 points, it was low quality.
TABLE 4.
Newcastle–Ottawa quality assessment of observational studies
| First author, year | Study design | Selection | Comparability | Outcome | Total score | Result |
|---|---|---|---|---|---|---|
| Annweiler C et al. 21 2020 | Cohort | *** | ** | *** | 8 | Good |
| Annweiler G et al. 22 2020 | Cohort | *** | ** | *** | 8 | Good |
| Cangiano B et al. 23 2020 | Cohort | *** | ** | ** | 7 | Good |
| Giannini S et al. 25 2021 | Cohort | *** | ** | *** | 8 | Good |
| Hernandez JL et al. 26 2020 | Case‐control | *** | ** | ** | 7 | Good |
| Tan CW et al. 30 2020 | Cohort | *** | ** | ** | 7 | Good |
Note: ** means the score is 2, *** means the score is 3. All the scores were sum up to get the final score and to conclude the quality of included studies.
TABLE 5.
Joanna Briggs Institute Critical Appraisal tool for cross‐sectional study
| Jevalikar G et al. 27 2021 | Ling SF et al. 28 2020 | Vasheghani M et al. 31 2021 | |
|---|---|---|---|
| 1. Were the criteria for inclusion in the sample clearly defined? | Yes | Yes | Yes |
| 2. Were the study subjects and the setting described in detail? | Yes | Yes | Yes |
| 3. Was the exposure measured in a valid and reliable way? | Yes | Yes | Yes |
| 4. Were objective, standard criteria used for measurement of the condition? | Yes | Yes | Yes |
| 5. Were confounding factors identified? | Yes | Yes | Yes |
| 6. Were strategies to deal with confounding factors stated? | Yes | Yes | Yes |
| 7. Were the outcomes measured in a valid and reliable way? | Yes | Yes | Yes |
| 8. Was appropriate statistical analysis used? | Yes | Yes | Yes |
| Overall appraisal | Include | Include | Include |
3.3. Vitamin D and ICU admission of Covid‐19 patients
Five studies (n = 772) reported the effect of vitamin D on the ICU admission of Covid‐19 patients. Our pooled analysis showed that vitamin D supplementation was associated with reduction of ICU admission rate (OR 0.27; 95% CI: 0.09–0.76, p = 0.010, I 2 = 70%, random‐effect modelling; Figure 2a).
FIGURE 2.
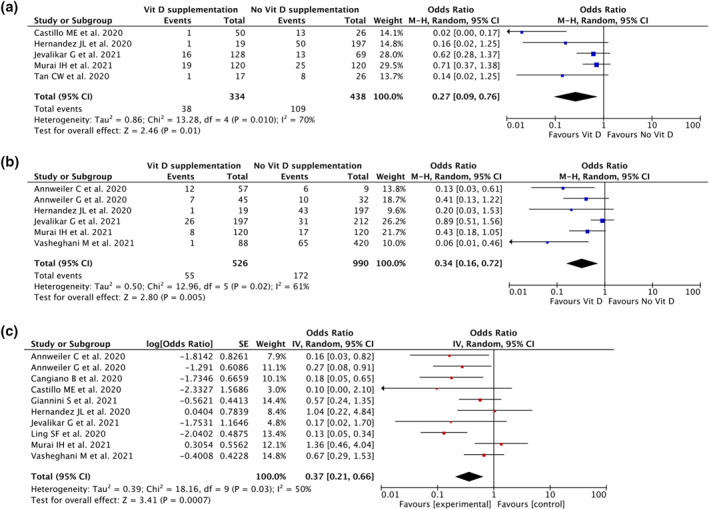
Forest plot that demonstrates the association of vitamin D supplementation with ICU admission rate (a), the need for mechanical ventilation (b) and mortality (c) outcomes
3.4. Vitamin D and the need for mechanical ventilation
Six studies (n = 1516) reported the effect of vitamin D on the need for mechanical ventilation outcome. The pooled analysis suggested that vitamin D supplementation was associated with reduction of the need for mechanical ventilation (OR 0.34; 95% CI: 0.16–0.72, p = 0.005, I 2 = 61%, random‐effect modelling; Figure 2b).
3.5. Vitamin D and mortality of Covid‐19 patients
Ten studies (n = 2223) reported on mortality. The pooled estimate showed that vitamin D supplementation was associated with reduction of mortality from Covid‐19 (OR 0.37; 95% CI: 0.21–0.66, p < 0.001, I 2 = 50%, random‐effect modelling; Figure 2c).
3.6. Meta regression
Our meta‐regression suggested the association between vitamin D supplementation and mortality was affected by age (p = 0.027; Figure 3a), meaning that older people will gain more protective measures towards mortality in comparison with younger people. The association between vitamin D supplementation and mortality was not affected by gender (p = 0.191; Figure 3b), hypertension (p = 0.566; Figure 3c), diabetes (p = 0.608; Figure 3d), nor the use of corticosteroids (p = 0.070; Figure 3e).
FIGURE 3.
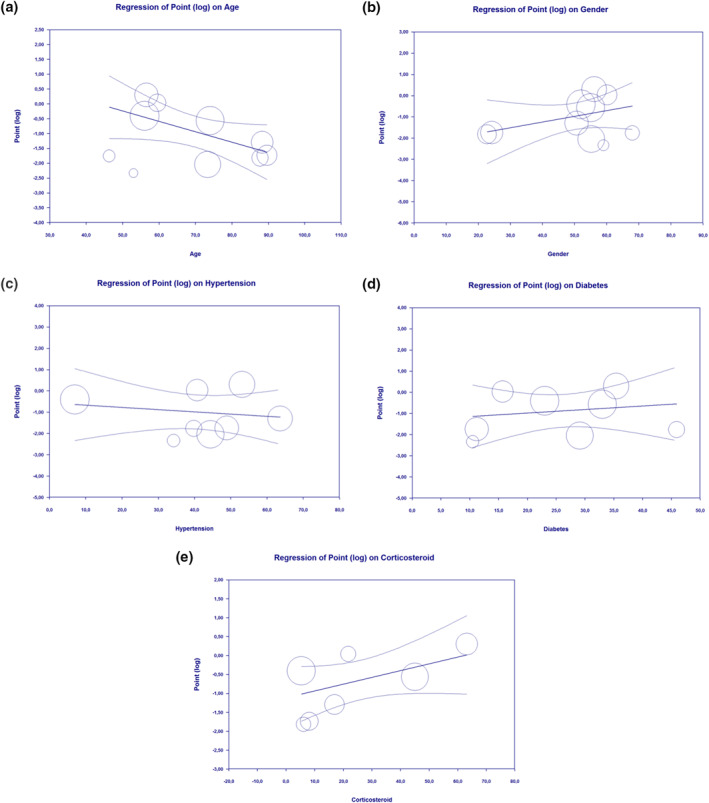
Bubble‐plot for meta‐regression. Meta‐regression analysis showed that the association between vitamin D supplementation and mortality outcome was affected by age (a), but not by gender (b), hypertension (c), diabetes (d) and the use of corticosteroids (e)
3.7. Publication bias
Funnel plot analysis showed an asymmetrical inverted‐plot for the ICU admission outcome (Figure 4a) and the need for mechanical ventilation outcome (Figure 4b), showing some indication of publication bias. Funnel plot analysis showed a relatively symmetrical inverted‐plot for the mortality outcomes (Figure 4c). Begg and Mazumdar rank‐correlation test were not statistically significant for ICU admission (p = 0.086), the need for mechanical ventilation (p = 0.060) and mortality outcome (p = 0.371), showing no indication of publication bias. However, because the number of included studies in the ICU admission and mechanical ventilation outcomes are fewer than 10 studies, the funnel plots and statistical tests for detecting publication bias are not very reliable when compared with larger numbers of included studies in each outcome. 32 , 33
FIGURE 4.
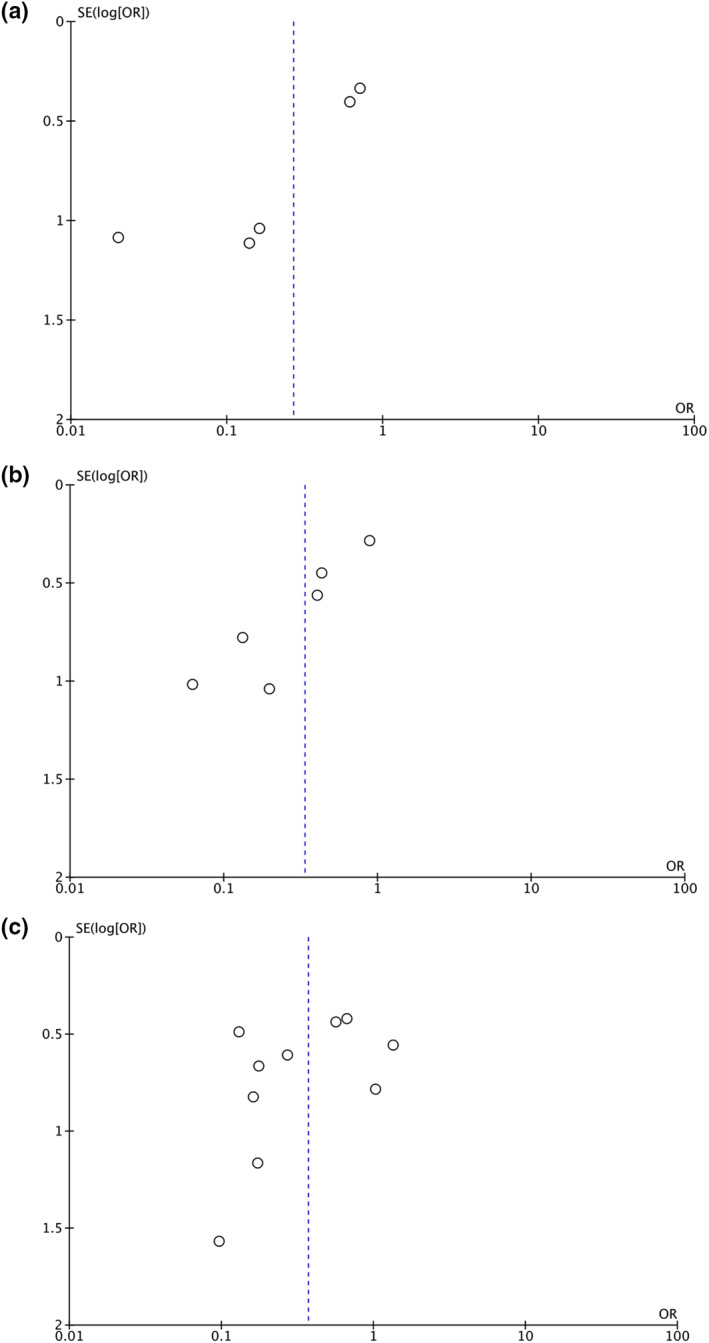
Funnel plot analysis for the association of vitamin D supplementation with ICU admission (a), the need for mechanical ventilation (b) and mortality (c) outcomes
4. DISCUSSION
According to our pooled analysis, vitamin D supplementation had an association with a reduction of ICU admission rate, reduction in the need for ventilators and reduction of mortality from Covid‐19. This meta‐regression reveals that age affects the association between vitamin D supplementation and Covid‐19 mortality.
There are some explanations how vitamin D could affect the prognosis of Covid‐19 patients. The interaction of SARS‐CoV‐2 infection and vitamin D are presented briefly in Figure 5. Both innate and adaptive arms of the immune system, triggered by SARS‐CoV‐2 infection, cause a destructive inflammatory reaction, resulting in local and systemic complications. The host immune or the inflammatory reaction is one of many severity‐determining variables as proven by significant association between inflammatory proteins/indicators and disease severity. 34 , 35 Exhaustion markers (e.g., CD8+ T cells, NK cells, NKG2A) are increased during acute symptomatic period of Covid‐19 and then return to normal in the convalescent period. Cytotoxic lymphocytes and natural killer cells aid control of viral infection, and both may serve as a predictor in determining the severity of the disease. Throughout the course of Covid‐19 infection, Natural killer and cytotoxic lymphocytes will eventually reach functional exhaustion, as indicated by reduced total number. 36 Severe Covid‐19 patients have high levels of various inflammatory proteins such as C‐reactive protein, D‐dimer and cytokines, including IL‐6, IL‐1β, TNF‐α, also known as cytokine storm. 37 IL‐6 can be used as a good indicator of poor outcome in Covid‐19 patients who suffer ARDS. 38 , 39 Cytokine storm leads to a severe pulmonary infiltration by neutrophils and macrophages that causes severe alveolar injury with hyaline membrane formation and alveolar wall thickening. 38 The cytokine storm increases inflammatory mediators and oxidative stress, while concomitantly reducing endothelial nitric oxide synthase. All these processes result in systemic inflammation and endothelial dysfunction, and ultimately cause hemodynamic instability, tissue injury and multiple organ failure. 40
FIGURE 5.
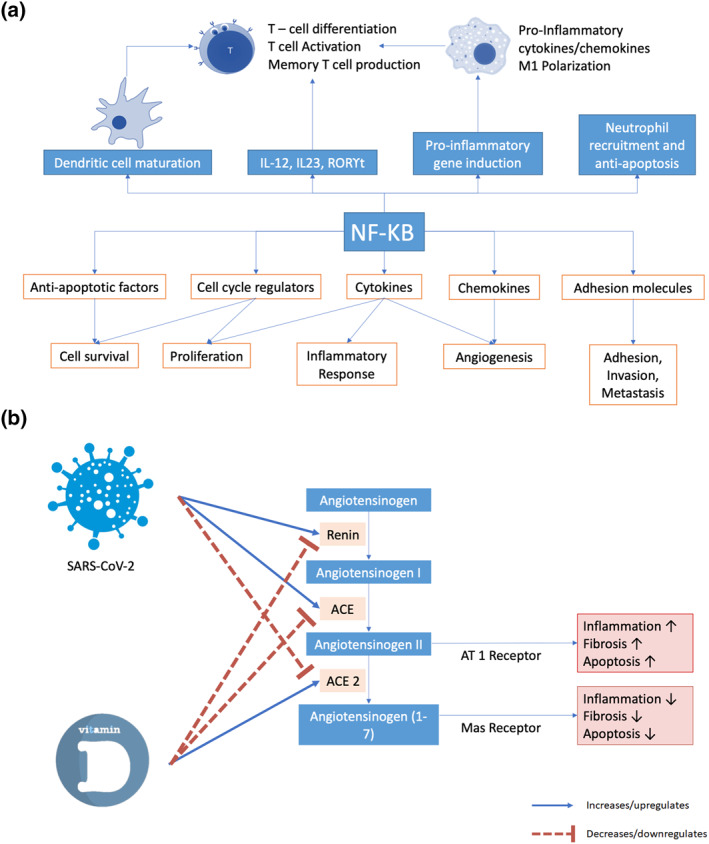
The role of vitamin D in suppressing the nuclear factor kappa B (NF‐κB) signalling pathway (a) and the proposed potential therapeutic effect of vitamin D for COVID‐19 and induced acute respiratory distress syndrome (b)
There are three main mechanisms through which vitamin D may reduce the risk of infection: enhance physical barriers, cellular innate immunity and adaptive immunity. Vitamin D strengthens cellular immunity by induction of antimicrobial peptides, including human cathelicidin LL‐37, and through 1,25‐dihydroxyvitamin D and defensins. 41 Cathelicidins are known to have direct antimicrobial effect towards a variety of pathogens comprising both Gram negative and positive bacteria, non‐enveloped and enveloped viruses, and fungi. 42 Cathelicidins also promote the chemotaxis of cellular immunity to the site of infection and its expression was upregulated by vitamin D especially in respiratory epithelial cells, which play a major role in host defenses. 42 Furthermore, on the cellular level, vitamin D also stimulates gap junction genes, tight junction genes and adherens genes (e.g., E‐cadherin) to strengthen cellular junction integrity and improve cell to cell communication, therefore maintaining the intercellular junctions to prevent further invasion of microorganisms, including viruses, which may lead to further inflammation and tissue damage. 43 , 44 The vitamin D receptor (VDR) is possessed by the majority of immune cells including macrophages, B and T lymphocytes, neutrophils and dendritic cells. VDR activation leads to downstream cell signalling that produces immunomodulatory, anti‐proliferative, and pro differentiative effects. 42 Vitamin D helps to reduce pro‐inflammatory T helper 1 (Th1) cytokines, TNF‐α and interferon (IFN)‐γ and helps the production of Th2 lymphocyte cytokines which indirectly suppress Th1 cells. 41 , 45 Additional evidence that supports immunoregulatory function of vitamin D is that 1,25(OH)2D3 or calcitriol, the active form of vitamin D, is able to induce monocyte differentiation into macrophage‐like form. 42 VDR expressed by monocytes sensitizes them to the differentiating effects of calcitriol (autocrine mechanism for cell maturation). Vitamin D modulates macrophage response, and thus prevents overproduction of inflammatory cytokines and chemokines. 42 Calcitriol downregulates granulocyte‐macrophage colony stimulating factor but stimulates the immunosuppressive prostaglandin E2 production by the macrophages. 41 Importantly, vitamin D deficiency impairs macrophage maturation. 41 Several studies have shown that the risk of Covid‐19 increases in people with vitamin D deficiencies; furthermore, lower concentration also contributes to the development of ARDS. 46 , 47 Aside from that, vitamin D has also been known to induce the production of type I IFNs, which able to suppress and keeping viral replication under control causing the prevention of further inflammatory response. 48 , 49 Several studies have reported that severe form of Covid‐19 is frequently related with increased hypercoagulable state, which could be worsen by excessive inflammation and may accelerates upcoming thrombogenic events. Vitamin D has the potential role to promote antithrombin and thrombomodulin gene expression to suppress that hypercoagulable state, causing significant protection during the course of severe form of Covid‐19. 49 , 50 , 51 Vitamin D supplementation and serum levels above 50 ng/ml have been observed and may help in reducing severity and the course of viral diseases including Covid‐19. 41
Another role of vitamin D in the pathogenesis of Covid‐19 is through its ability to inhibit the RAS and nuclear factor kappa B (NF‐κB) pathway. Because ACE2 has a protective role against lung injuries, interference with that receptor by SARS‐CoV‐2 might be linked with ARDS. Lung injury caused by ischaemia and reperfusion are promoted by the increased expression of ACE/Ang II l and reduced levels of ACE2/Ang‐(1‐7). ACE2 plays its protective function to the lung through Ang‐(1‐7)/MasR pathway and prevents activation of NF‐κB pathway/extracellular signal‐regulated kinase. 52 The 1α,25(OH)2D3, the biological active form of vitamin D, contributes its protective role and reduces lung injury by regulating RAS biosynthesis (renin, ACE/Ang II/AT1R axis and ACE2/Ang‐(1‐7) axis stimulation). 53 The VDR and calcitriol also prevent lung, liver and kidney fibrosis through the downregulation of RAS and the inhibition of NF‐κB and wnt/β‐catenin. 54 , 55
A cohort study that investigates the influence of genetic variation in vitamin D pathway found that three SNP in VDR (rs4334089, rs11568820 and rs7970314) are associated with increased risk of upper respiratory tract infection. 56 A systematic review and meta‐analysis study which evaluates the association between VDR polymorphism and severe Respiratory Syncytial Virus (RSV)‐bronchiolitis supports the association between FokI polymorphism and severe RSV infection. This study also examines the role of six VDR polymorphisms (Cdx, A1012G, FokI, BsmI, ApaI and TaqI) on infection susceptibility to enveloped virus found that a polymorphism at locus rs2228570 (FokI) is associated with viral infections. 57 The TT genotype and T allele were reported to be risk factors for infections with enveloped viruses, including RSV. 58 , 59 Being an enveloped virus, it may be the same case for SARS‐CoV‐2. The mechanism that explains the association of FokI polymorphism with increased viral infection is that the FokI polymorphism creates a shorter VDR proteins that causes higher rate of transcription driven by the NF‐κB, increased IL‐12 and higher lymphocyte proliferation. 56 Therefore, supplementation with vitamin D may help in ameliorating those potentially negative effects from viral infection. All of these properties and roles might explain the benefit of vitamin D on the outcomes of Covid‐19.
This study has some limitations. Significant heterogeneities were identified on most of the outcomes of interests included in this study. This was probably caused by the difference in the given vitamin D doses and co‐administered medications with vitamin D as Covid‐19 treatment. Importantly, we have made rigorous efforts to ensure that only sound studies were included, and several pre‐print studies were included to minimize the risk of publication bias.
5. CONCLUSION
Our meta‐analysis indicates that vitamin D supplementation had an association with favourable outcomes of Covid‐19, compromising reduction in the rate of ICU admission, reduction in mechanical ventilation usage and reduction of mortality rate from Covid‐19. This study suggests that vitamin D might be a potential therapeutic agent for the management of Covid‐19 to give better outcomes for the patients. However, more randomized clinical trial studies are still necessary and should be done to confirming the results of our study. Finally, vitamin D might be considered as an essential drug for future Covid‐19 therapy models.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTION
Conceptualization, methodology, formal analysis, data curation, writing‐original draft, visualization, writing‐review and editing: Timotius Ivan Hariyanto. Conceptualization, methodology, formal analysis, data curation, writing‐original draft, writing‐review and editing: Denny Intan. Conceptualization, validation, supervision, writing‐review and editing: Joshua Edward Hananto. Conceptualization, validation, supervision, writing‐review and editing: Harapan Harapan. Conceptualization, validation, supervision, writing‐review and editing: Andree Kurniawan.
ACKNOWLEDGEMENTS
None.
Hariyanto TI, Intan D, Hananto JE, Harapan H, Kurniawan A. Vitamin D supplementation and Covid‐19 outcomes: a systematic review, meta‐analysis and meta‐regression. Rev Med Virol. 2022;32(2):e2269. 10.1002/rmv.2269
DATA AVAILABILITY STATEMENT
Data analysed in this study were a re‐analysis of existing data, which are openly available at locations cited in the reference section.
REFERENCES
- 1. World Health Organization. Coronavirus disease (COVID‐19): situation report. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19‐‐‐18‐may‐2021. Accessed May 22, 2020.
- 2. Hariyanto TI, Rizki NA, Kurniawan A. Anosmia/Hyposmia is a good predictor of coronavirus disease 2019 (COVID‐19) infection: a meta‐analysis. Int Arch Otorhinolaryngol. 2020;25:e170‐e174. 10.1055/s-0040-1719120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwenandar F, Japar KV, Damay V, et al. Coronavirus disease 2019 and cardiovascular system: a narrative review. Int J Cardiol Heart Vasc. 2020;29:100557. 10.1016/j.ijcha.2020.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Putri C, Hariyanto TI, Hananto JE, Christian K, Situmeang RFV, Kurniawan A. Parkinson's disease may worsen outcomes from coronavirus disease 2019 (COVID‐19) pneumonia in hospitalized patients: a systematic review, meta‐analysis, and meta‐regression. Park Relat Disord. 2021;87:155‐161. 10.1016/j.parkreldis.2021.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hariyanto TI, Rosalind J, Christian K, Kurniawan A. Human immunodeficiency virus and mortality from coronavirus disease 2019: a systematic review and meta‐analysis. South Afr J HIV Med. 2021;22(1):1220. 10.4102/sajhivmed.v22i1.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hariyanto TI, Putri C, Situmeang RFV, Kurniawan A. Dementia is associated with severe coronavirus disease 2019 (COVID‐19) infection. Am J Med Sci. 2020;361(3):394‐295. 10.1016/j.amjms.2020.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gold MS, Sehayek D, Gabrielli S, Zhang X, McCusker C, Ben‐Shoshan M. COVID‐19 and comorbidities: a systematic review and meta‐analysis. Postgrad Med. 2020;132(8):749‐755. 10.1080/00325481.2020.1786964 [DOI] [PubMed] [Google Scholar]
- 8. Hariyanto TI, Kurniawan A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID‐19) pneumonia: a systematic review and meta‐analysis. Sleep Med. 2021;82:47‐53. 10.1016/j.sleep.2021.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hariyanto TI, Kurniawan A. Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID‐19) in diabetic patients: a systematic review, meta‐analysis, and meta‐regression. J Diabetes Metab Disord. 2021;20:1‐550. 10.1007/s40200-021-00777-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hariyanto TI, Hardyson W, Kurniawan A. Efficacy and safety of tocilizumab for coronavirus disease 2019 (Covid‐19) patients: a systematic review and meta‐analysis. Drug Res (Stuttg). 2020;71:265‐274. 10.1055/a-1336-2371 [DOI] [PubMed] [Google Scholar]
- 11. Ivan Hariyanto T, Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol. 2021;93(3):1832‐1836. 10.1002/jmv.26698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hariyanto TI, Halim DA, Jodhinata C, Yanto TA, Kurniawan A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. Clin Exp Pharmacol Physiol. 2021;48(6):823‐830. 10.1111/1440-1681.13488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hariyanto TI, Halim DA, Rosalind J, Gunawan C, Kurniawan A. Ivermectin and outcomes from Covid‐19 pneumonia: a systematic review and meta‐analysis of randomized clinical trial studies. Rev Med Virol. 2021:e2265. 10.1002/rmv.2265 [DOI] [Google Scholar]
- 14. Murdaca G, Pioggia G, Negrini S. Vitamin D and Covid‐19: an update on evidence and potential therapeutic implications. Clin Mol Allergy. 2020;18(1):23. 10.1186/s12948-020-00139-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jakovac H. COVID‐19 and vitamin D‐Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318(5):E589. 10.1152/ajpendo.00138.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dancer RC, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70(7):617‐624. 10.1136/thoraxjnl-2014-206680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID‐19 patients. Aging Clin Exp Res. 2020;32(10):2141‐2158. 10.1007/s40520-020-01677-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weir EK, Thenappan T, Bhargava M, Chen Y. Does vitamin D deficiency increase the severity of COVID‐19? Clin Med (Lond). 2020;20(4):e107‐e108. 10.7861/clinmed.2020-0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang J, Wang X, Chen X, Song Q, Liu W, Lu L. Perioperative antibiotics to prevent acute endophthalmitis after ophthalmic surgery: a systematic review and meta‐analysis. PLoS One. 2016;11(11):e0166141. 10.1371/journal.pone.0166141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 21. Annweiler G, Corvaisier M, Gautier J, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID‐19 patients: the GERIA‐COVID quasi‐experimental study. Nutrients. 2020;12(11):3377. 10.3390/nu12113377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Annweiler C, Hanotte B, Grandin de l'Eprevier C, Sabatier JM, Lafaie L, Célarier T. Vitamin D and survival in COVID‐19 patients: a quasi‐experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. 10.1016/j.jsbmb.2020.105771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cangiano B, Fatti LM, Danesi L, et al. Mortality in an Italian nursing home during COVID‐19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests. Aging (Albany NY). 2020;12(24):24522‐24534. 10.18632/aging.202307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castillo ME, Costa LME, Barrios JMV, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID‐19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. 10.1016/j.jsbmb.2020.105751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giannini S, Passeri G, Tripepi G, et al. Effectiveness of in‐hospital cholecalciferol use on clinical outcomes in comorbid COVID‐19 patients: a hypothesis‐generating study. Nutrients. 2021;13(1):219. 10.3390/nu13010219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernández JL, Nan D, Fernandez‐Ayala M, et al. Vitamin D status in hospitalized patients with SARS‐CoV‐2 infection. J Clin Endocrinol Metab. 2021;106(3):e1343‐e1353. 10.1210/clinem/dgaa733 [DOI] [PubMed] [Google Scholar]
- 27. Jevalikar G, Mithal A, Singh A, et al. Lack of association of baseline 25‐hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID‐19. Sci Rep. 2021;11(1):6258. 10.1038/s41598-021-85809-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ling SF, Broad E, Murphy R, et al. High‐dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID‐19: a cross‐sectional multi‐centre observational study. Nutrients. 2020;12(12):3799. 10.3390/nu12123799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murai IH, Fernandes AL, Sales LP, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID‐19: a randomized clinical trial. J Am Med Assoc. 2021;325(11):1053‐1060. 10.1001/jama.2020.26848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan CW, Ho LP, Kalimuddin S, et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID‐19). Nutrition. 2020;79‐80:111017. 10.1016/j.nut.2020.111017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vasheghani M, Jannati N, Baghaei P, Rezaei M, Marjani M. The association of 25 (OH) vitamin D levels and severity and outcome of COVID‐19: a cross‐sectional study. Research Square. 2021. 10.21203/rs.3.rs-141034/v1 [DOI] [Google Scholar]
- 32. Thornton A, Lee P. Publication bias in meta‐analysis: its causes and consequences. J Clin Epidemiol. 2000;53(2):207‐216. 10.1016/s0895-4356(99)00161-4 [DOI] [PubMed] [Google Scholar]
- 33. Terrin N, Schmid CH, Lau J, Olkin I. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22(13):2113‐2126. 10.1002/sim.1461 [DOI] [PubMed] [Google Scholar]
- 34. Gong J, Dong H, Xia QS, et al. Correlation analysis between disease severity and inflammation‐related parameters in patients with COVID‐19: a retrospective study. BMC Infect Dis. 2020;20(1):963. 10.1186/s12879-020-05681-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hariyanto TI, Japar KV, Kwenandar F, et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID‐19 infection: a systematic review and meta‐analysis. Am J Emerg Med. 2021;41:110‐119. 10.1016/j.ajem.2020.12.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):533‐535. 10.1038/s41423-020-0402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buonaguro FM, Ascierto PA, Morse GD, et al. Covid‐19: time for a paradigm change. Rev Med Virol. 2020;30(5):e2134. 10.1002/rmv.2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID‐19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613‐628. 10.1007/s10735-020-09915-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Malaguarnera L. Vitamin D3 as potential treatment adjuncts for COVID‐19. Nutrients. 2020;12(11):3512. 10.3390/nu12113512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Harten RM, van Woudenbergh E, van Dijk A, Haagsman HP. Cathelicidins: immunomodulatory antimicrobials. Vaccines (Basel). 2018;6(3):63. 10.3390/vaccines6030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55(1):96‐108. 10.1002/mnfr.201000174 [DOI] [PubMed] [Google Scholar]
- 44. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID‐19 infections and deaths. Nutrients. 2020;12(4):988. 10.3390/nu12040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alappat L, Valerio M, Awad AB. Effect of vitamin D and β‐sitosterol on immune function of macrophages. Int Immunopharm. 2010;10(11):1390‐1396. 10.1016/j.intimp.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 46. Jayawardena R, Jeyakumar DT, Francis TV, Misra A. Impact of the vitamin D deficiency on COVID‐19 infection and mortality in Asian countries. Diabetes Metab Syndr. 2021;15(3):757‐764. 10.1016/j.dsx.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pinzon RT, Angela, Pradana AW. Vitamin D deficiency among patients with COVID‐19: case series and recent literature review. Trop Med Health. 2020;48(1):102. 10.1186/s41182-020-00277-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gauzzi MC, Fantuzzi L. Reply to Jakovac: COVID‐19, vitamin D, and type I interferon. Am J Physiol Endocrinol Metab. 2020;319(2):E245‐E246. 10.1152/ajpendo.00315.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kralj M, Jakovac H. Vitamin D and COVID‐19 in an immunocompromised patient with multiple comorbidities‐A Case Report. Clin Case Rep. 2021;9(4):2269‐2275. 10.1002/ccr3.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID‐19: systematic review and meta‐analysis. Crit Rev Food Sci Nutr. 2020:1‐9. 10.1080/10408398.2020.1841090 [DOI] [PubMed] [Google Scholar]
- 51. Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID‐19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10(1):20191. 10.1038/s41598-020-77093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hariyanto TI, Japar KV, Damay V, Kwenandar F, Sieto NL, Kurniawan A. The use of ACE inhibitor/ARB in SARS‐CoV‐2 patients: a comprehensive narrative review. Asian J Med Sci. 2020;11(6):113‐120. 10.3126/ajms.v11i6.29911 [DOI] [Google Scholar]
- 53. Malek Mahdavi A. A brief review of interplay between vitamin D and angiotensin‐converting enzyme 2: implications for a potential treatment for COVID‐19. Rev Med Virol. 2020;30(5):e2119. 10.1002/rmv.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mirković K, de Borst MH. Beyond the RAAS: dissecting the antifibrotic effects of vitamin D analogues. Lab Invest. 2012;92(12):1666‐1669. 10.1038/labinvest.2012.150 [DOI] [PubMed] [Google Scholar]
- 55. Liu T, Zhang L, Joo D, Sun SC. NF‐κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jolliffe DA, Greiller CL, Mein CA, et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br J Nutr. 2018;120(8):891‐900. 10.1017/S000711451800209X [DOI] [PubMed] [Google Scholar]
- 57. McNally JD, Sampson M, Matheson LA, Hutton B, Little J. Vitamin D receptor (VDR) polymorphisms and severe RSV bronchiolitis: a systematic review and meta‐analysis. Pediatr Pulmonol. 2014;49(8):790‐799. 10.1002/ppul.22877 [DOI] [PubMed] [Google Scholar]
- 58. Hashemi SMA, Thijssen M, Hosseini SY, Tabarraei A, Pourkarim MR, Sarvari J. Human gene polymorphisms and their possible impact on the clinical outcome of SARS‐CoV‐2 infection. Arch Virol. 2021:1‐20. 10.1007/s00705-021-05070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Laplana M, Royo JL, Fibla J. Vitamin D Receptor polymorphisms and risk of enveloped virus infection: a meta‐analysis. Gene. 2018;678:384‐394. 10.1016/j.gene.2018.08.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analysed in this study were a re‐analysis of existing data, which are openly available at locations cited in the reference section.


