Abstract
Metarrhizium anisopliae is a common pathogen of insects and has even been used to control insect populations. It is rarely isolated from human or animal sources, but recently, there have been three reported cases of disease, two in humans and one in a cat. We present our experience with five isolates from human sources, including two that were the apparent causes of two cases of sinusitis in immunocompetent hosts. The first patient was a 36-year-old male with frontal and ethmoid sinusitis, and the second was a 79-year-old female with chronic sinusitis. Both patients underwent surgery, and pathology of the surgical specimens revealed branching hyphae. Cultures grew only Metarrhizium species. Neither patient received antifungal therapy, and both did well postoperatively. The other three isolates were cultured from bronchoalveolar lavage specimens but were not felt to be clinically significant. Antifungal susceptibility testing using the National Committee for Clinical Laboratory Standards macrobroth method revealed that all isolates were resistant to amphotericin B, 5-flucytosine, and fluconazole. Itraconazole and newer azole compounds were more active. Metarrhizium species may cause disease in humans, even those without evidence of immunosuppression, and are apparently highly resistant to amphotericin B in vitro.
Metarrhizium anisopliae is an entomopathogenic fungus with a wide range of host species (5). It has a worldwide distribution in soil as well (5). It was described first (under the name Entomophthora anisopliae) as a pathogen of the wheat cockchafer in 1879 by Metschnikoff and later as M. anisopliae by Sorokin in 1883 (12). It has since been used to control a variety of insect populations (3). Until recently, it has not been reported as a cause of disease in animals or humans. In 1997, it was reported as a cause of keratitis in an 18-year-old healthy male from Colombia, who was successfully treated with topical natamycin (2). A case in Australia involved a 9-year-old boy with leukemia who developed skin lesions that repeatedly grew M. anisopliae, though multiple blood cultures were all negative (1). Treatment with amphotericin B plus 5-flucytosine (5-FC) was ineffective and the patient expired. Interestingly, in vitro susceptibility testing (tablet diffusion method) suggested the organism was sensitive to amphotericin B but resistant to 5-FC, fluconazole, ketoconazole, and itraconazole. Another case in Australia involved a cat with invasive rhinitis due to M. anisopliae (8). No susceptibility tests were performed, but the cat was treated with itraconazole and had clinical resolution of the infection. After itraconazole was discontinued, however, the infection relapsed. We report our experience with two M. anisopliae isolates that caused sinusitis in immunocompetent hosts and the susceptibility testing of these and three additional clinical isolates.
CASE REPORTS
UTHSCSA 97-1782.
A 36-year-old male from Michigan with a history of trauma to the left sinus several years ago presented with complaints of nasal congestion and difficulty breathing through the left nostril. Computerized tomography scan of the head showed opacification of the frontal and ethmoid air cells. The patient underwent surgery, and a yellow, hard gritty material was removed from the sinus. Pathology showed fungal hyphae on silver stain, with cultures growing M. anisopliae. No antifungal treatment was given, and the patient recovered without complications.
UTHSCSA 95-143.
A 79-year-old female from Minnesota with a history of allergic rhinitis presented with chronic sinusitis of several months’ duration. She had been treated with multiple courses of antibiotics but continued to have nasal congestion and postnasal drip. At the time of surgery, a green, cheesy material along with a polypoid mass was removed from the ethmoid sinus. Pathology showed branching hyphae, and culture was positive for M. anisopliae. No antifungal treatment was given and the patient recovered without complications.
UTHSCSA isolates 97-864, 94-1246, and 93-1475 were all recovered from bronchoalveolar lavage specimens and were not considered clinically significant by the managing physicians.
MATERIALS AND METHODS
Isolates.
We reviewed our experience at the University of Texas Health Science Center at San Antonio (UTHSCSA) with M. anisopliae and found the following five human clinical isolates (source): UTHSCSA 97-1782 (frontal sinus biopsy), UTHSCSA 95-143 (ethmoid sinus biopsy), UTHSCSA 97-864 (bronchoalveolar lavage), UTHSCSA 94-1246 (bronchoalveolar lavage), and UTHSCSA 93-1475 (bronchoalveolar lavage). Clinical histories were obtained from physicians managing the patients and are presented above in summary form for two of the patients.
Susceptibility testing.
Susceptibility testing was performed according to the National Committee for Clinical Laboratory Standards (NCCLS) macrobroth method M27-A (9), modified for filamentous fungi (10) (Table 1). Briefly, isolates were grown on potato flake agar slants which were prepared in-house at 25°C to induce conidial formation. Sterile, distilled water was added to the cultures, and the colonies were gently scraped to produce a suspension. Using a hemacytometer, a final inoculum of 104 conidia/ml was prepared (10). Antibiotic Medium 3 (Difco Laboratories, Detroit, Mich.) was used for testing amphotericin B (Fungizone; Squibb, Princeton, N.J.) (0.03 to 16 μg/ml), and RPMI 1640 buffered with morpholinepropanesulfonic acid (MOPS) (American Biorganics, Niagara Falls, N.Y.) was used for testing 5-FC (Hoffman-La Roche, Nutley, N.J.) (0.125 to 64 μg/ml), fluconazole (Diflucan; Pfizer, Sandwich, England) (0.125 to 64 μg/ml), itraconazole (Janssen, Beerse, Belgium) (0.03 to 16 μg/ml), voriconazole (Pfizer, Sandwich, England) (0.03 to 16 μg/ml), and SCH 56592 (Schering, Kenilworth, N.J.) (0.03 to 16 μg/ml). Amphotericin B and fluconazole were prepared from pharmaceutical solutions in sterile water. 5-FC was prepared from powder to a stock solution of 6,400 μg/ml in sterile water. Itraconazole, voriconazole, and SCH 56592 were prepared from powder to stock solutions of 1,600 μg/ml in 100% polyethylene glycol. Tubes were incubated at room temperature (25°C) and read at 48 and 72 h.
TABLE 1.
Susceptibility testing of M. anisopliae by the NCCLS macrobroth method
| UTHSCSA isolate | MFCa (μg/ml) of amphotericin Bb | MIC (μg/ml) ofb:
|
|||||
|---|---|---|---|---|---|---|---|
| Amphotericin B | 5-FC | Fluconazole | Itraconazole | Voriconazole | SCH 56592 | ||
| 97-1782 | >16/>16 | 8/>16 | >64/>64 | >64/>64 | 0.5/2 | 0.25/1 | 1/1 |
| 95-143 | >16/>16 | 16/>16 | >64/>64 | 64/>64 | 0.25/2 | 0.25/0.5 | 0.5/1 |
| 97-864 | >16/>16 | 8/>16 | >64/>64 | 64/>64 | 1/1 | 1/1 | 1/2 |
| 94-1246 | >16/>16 | 2/16 | >64/>64 | >64/>64 | 0.06/0.125 | 0.25/0.25 | 0.125/0.5 |
| 93-1475 | >16/>16 | 16/16 | >64/>64 | 32/64 | 0.125/0.125 | 0.5/1 | 0.125/0.125 |
MFC, minimum fungicidal concentration.
Values are the readings taken after 48 and 72 h, respectively.
RESULTS
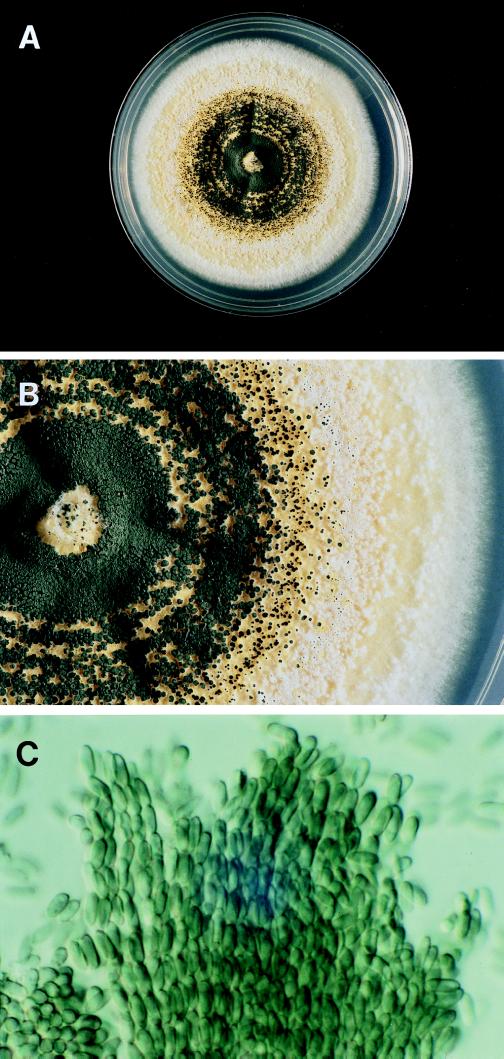
M. anisopliae belongs to the class Hyphomycetes (11). Isolates were identified based on colony and microscopic morphology. On Sabouraud dextrose agar, colonies were initially white to tan and then generally turned a dark olive green as conidia were produced in visible columns (Fig. 1A and B). A yellow pigment was produced. There are two subspecies, variety (var.) anisopliae and var. major, distinguished primarily on the basis of conidial size (12). M. anisopliae var. anisopliae has smaller conidia (5.0 to 8.0 μm) than var. major (10.0 to 14.0 μm) (12). In our isolates, identified as M. anisopliae var. anisopliae, phialoconidia were elongate and green, had one or both ends rounded, measured 6.0 to 8.0 by 2.5 to 3.5 μm, and were arranged in long chains and columns (Fig. 1C). Although slow, growth was best at 25°C, with colonies reaching 7.0 cm after 3 weeks at 25°C on Sabouraud dextrose agar. Growth was slower on potato flake agar, with colonies reaching 4.2 cm after 3 weeks, with minimal color production. There was little to no growth at 35°C.
FIG. 1.
(A) Single colony of M. anisopliae var. anisopliae grown on Sabouraud dextrose agar at 25°C for 3 weeks. Colony size is 7.0 cm. (B) Close-up view of colony from panel A showing visible columns of conidia. (C) Photomicrograph of chains of conidia of M. anisopliae var. anisopliae (magnification, ×920). Reprinted from reference 11 with permission of the publisher.
DISCUSSION
M. anisopliae is a well-known insect pathogen that has recently been the subject of case reports of disease in humans and a cat. We present two cases of sinusitis in humans due to this organism and susceptibility testing using the NCCLS macrobroth method.
Fungal sinusitis has only recently become well characterized. Several types are recognized, though these may be divided into invasive and noninvasive (4, 7). Aspergillus is the most common organism implicated, though dematiaceous fungi such as Curvularia, Alternaria, and Bipolaris are commonly seen in cases of allergic fungal sinusitis (4, 7). To our knowledge, Metarrhizium species have not previously been reported as etiologic agents.
It is apparent that, though rare, M. anisopliae is able to cause disease in both immunocompromised and immunocompetent individuals. How infection becomes established in these cases is not clear, however. As it has a worldwide distribution in soil, exposure may be common. In addition, M. anisopliae has been used to control insect populations, though whether this may lead to increased cases of human disease is not clear. There have been no documented cases associated with local use in insect control. To our knowledge, the cases presented in this report were not associated with use of M. anisopliae for insect control.
In most cases of noninvasive fungal sinusitis, antifungal therapy is not needed, and surgery with or without steroids (allergic fungal sinusitis) is effective (4). If needed, amphotericin B or itraconazole are generally used, though newer azole agents may prove effective in the future. In the cases of sinusitis presented here, surgery alone was effective, and antifungal treatment was not needed.
Susceptibility testing using the NCCLS macrobroth method modified for filamentous fungi suggests that M. anisopliae may be resistant to amphotericin B, 5-FC, and fluconazole. Even minimal fungicidal concentrations for amphotericin B were >16 μg/ml. This is supported by the observation that treatment with amphotericin B and 5-FC in a case of disseminated disease did not produce any clinical improvement. In that report, the authors used a tablet diffusion method and obtained MIC results that suggested that the organism was susceptible to amphotericin B but resistant to azole agents. As antifungal susceptibility testing of filamentous fungi has not yet been standardized and many variables affect the results, it is not possible to directly compare these methods. The NCCLS is currently evaluating broth methods in order to develop a standard similar to that approved for yeasts (6).
Using the modified NCCLS method, MICs of itraconazole are lower, and this may be a more effective agent. In the case report of a cat with invasive disease, itraconazole did produce a clinical response, though relapse occurred after the drug treatment was discontinued. In addition, the newer azoles voriconazole and SCH 56592 also demonstrated in vitro activity comparable to that of itraconazole, and these and other new antifungal agents may be useful in the future. Further testing and experience is necessary before more definitive recommendations can be made regarding treatment of this unusual pathogen.
REFERENCES
- 1.Burgner D, Eagles G, Burgess M, Procopis P, Rodgers M, Muir D, Pritchard R, Hocking A, Priest M. Disseminated invasive infection due to Metarrhizium anisopliae in an immunocompromised child. J Clin Microbiol. 1998;36:1146–1150. doi: 10.1128/jcm.36.4.1146-1150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cepero de Garcia M C, Arboleda M L, Barraquer F, Grose E. Fungal keratitis caused by Metarhizium anisopliae var. anisopliae. J Med Vet Mycol. 1997;35:361–363. [PubMed] [Google Scholar]
- 3.Clarkson J M, Charnley A K. New insights into the mechanisms of fungal pathogenesis in insects. Trends Microbiol. 1996;4:197–203. doi: 10.1016/0966-842x(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 4.de Shazo R D, Chapin K, Swain R E. Fungal sinusitis. N Engl J Med. 1997;337:254–259. doi: 10.1056/NEJM199707243370407. [DOI] [PubMed] [Google Scholar]
- 5.Domsch K H, Gams W, Anderson T H. Compendium of soil fungi. London, United Kingdom: Academic Press; 1980. pp. 413–415. [Google Scholar]
- 6.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morpeth J F, Rupp N T, Dolen W K, Bent J P, Kuhn F A. Fungal sinusitis: an update. Ann Allergy Asthma Immunol. 1996;76:128–140. doi: 10.1016/S1081-1206(10)63411-4. [DOI] [PubMed] [Google Scholar]
- 8.Muir D, Martin P, Kendall K, Malik R. Invasive hyphomycotic rhinitis in a cat due to Metarhizium anisopliae. Med Mycol. 1998;36:51–54. [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Rinaldi M G, Howell A W. Antifungal antimicrobics: laboratory evaluation. In: Wentworth B, editor. Diagnostic procedures for mycotic and parasitic infections. 7th ed. Washington, D.C: American Public Health Association; 1988. pp. 325–356. [Google Scholar]
- 11.Sutton D A, Fothergill A W, Rinaldi M G. Guide to clinically significant fungi. Baltimore, Md: Williams & Wilkins; 1998. pp. 238–239. [Google Scholar]
- 12.Tulloch M. The genus Metarhizium. Trans Br Mycol Soc. 1976;66:407–411. [Google Scholar]