CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript.
To the Editor:
A few months ago, we presented the results from a case‐control study evaluating solid organ transplantation (SOT) recipients’ outcomes after COVID‐19 requiring hospitalization, which showed a trend toward higher mortality among SOT patients compared to controls. 1 Other authors reported similar results regarding mortality, 2 whereas Chavarto et al found no increased risk of death among kidney recipients compared to controls. 3 We hypothesize that COVID‐19 might affect long‐term survival of SOT recipients, drug‐drug interactions (DDI) might contribute to graft rejection, and COVID‐19 unknown long‐term effects might specially affect immunosuppressed patients 4 ; here, we aim to evaluate the long‐term outcomes of SOT patients affected by COVID‐19.
The characteristics of the 46 SOT recipients admitted to our hospital as a result of COVID‐19 from March 11th to April 25th, 2020, have been described previously. 1 Seventeen (37%) patients died within 28 days after COVID‐19 diagnosis and 29 survivors have been followed up for 7‐8 months. Monitoring included creatinine and tacrolimus trough plasma concentration (FK‐Cmin), chest X‐ray, spirometry, and cytomegalovirus and BK virus. During the follow‐up, two (6.9%) kidney recipients died, one of them had respiratory insufficiency 6 months after hospital admission and the other suffered hyperparathyroidism requiring parathyroidectomy that led to cardiorespiratory arrest during the post‐operative. One (3.4%) patient suffered respiratory insufficiency related to COVID‐19 that required hospitalization and one (3.4%) patient developed pulmonary embolism attributable to COVID‐19 hypercoagulability; both patients were kidney recipients and have recovered.
At the end of the follow‐up, 20 patients showed a complete radiologic resolution, whereas 9 patients have residual ground‐glass opacifications or interstitial thickening. Spirometry controls showed that lung recipients kept a stable lung function and other SOT recipients have a normal lung function.
Three lung recipients have persistent mild myalgia and dyspnea, with no requirement of supplemental oxygen, and two kidney recipients reported that their physical performance was lower than prior to COVID‐19. We evaluated FK‐Cmin of the 27 patients receiving it (Figure 1), and found that the variability during the follow‐up was high (coefficient of variation >30%) in 16 (59.3%) of them. 5 Six (22.2%) patients have FK‐Cmin <3 ng/mL at some point over the follow‐up and there was one case (3.4%) of kidney transplantation failure, which preceded COVID‐19. Two patients presented FK‐Cmin >12 ng/mL.
FIGURE 1.
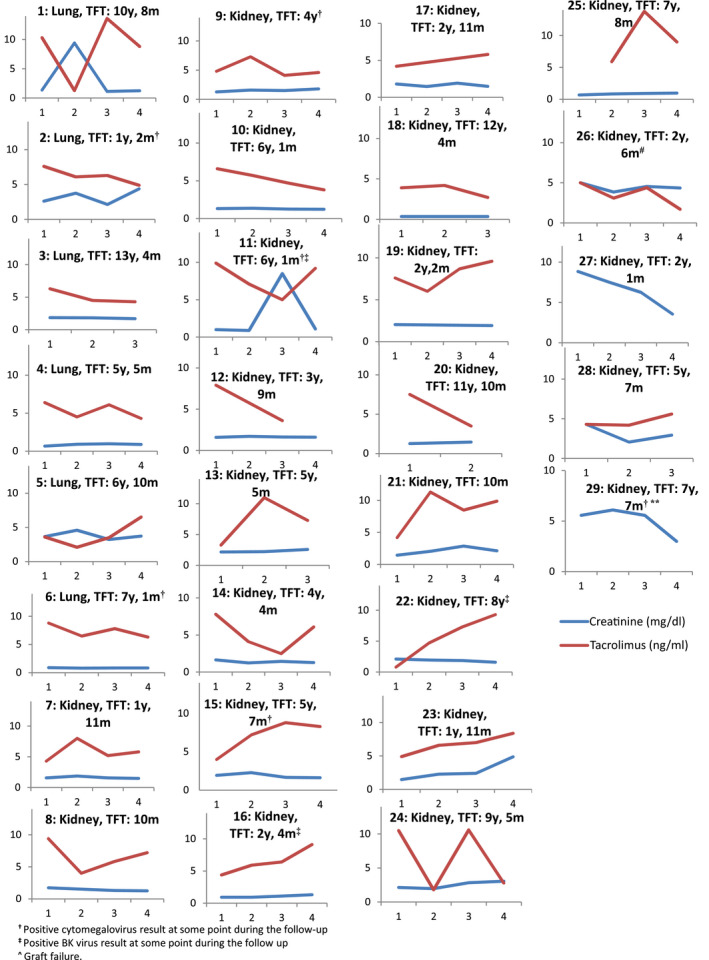
Creatinine and tacrolimus trough plasma concentration of SOT recipients during the follow up (X axis represent the correlative number of blood sample during the follow up, m: month, SOT: solid organ transplantation, TFT: time from transplantation, y: years). Patients 27 and 29 did not receive tacrolimus
There were 6 (20.6%) newly detected asymptomatic replications of cytomegalovirus (median time from transplantation 58 months, interquartile range 10‐91), and 3 (10.3%) asymptomatic viruria of BK virus (median time from transplantation 28 [10‐96] months); one patient was positive for both viruses. Four of 8 patients affected by opportunistic infections during the follow up have received tocilizumab during hospitalization because of COVID‐19; one patient was not receiving tacrolimus, while the others have FK‐Cmin >7 ng/mL at some point. Twenty‐six (96.3%) of 27 patients treated with tacrolimus have had DDI involving tacrolimus during hospitalization.
SOT recipients might have higher susceptibility to COVID‐19‐persistent symptoms; moreover, COVID‐19 and drugs used to treat it might enhance graft rejection and opportunistic infections, even if we did not detect any case of rejection. Further information is required to completely understand COVID‐19 long‐term impact in general population and specifically on SOT recipients.
Larrosa‐Garcia M, Garcia‐Garcia S, Los‐Arcos I, et al. Long‐term effects of COVID‐19 in solid organ transplantation recipients. Transpl Infect Dis. 2021;23:e13677. 10.1111/tid.13677
FUNDING INFORMATION
The authors received no financial support for the research, authorship, and/or publication of this article.
REFERENCES
- 1. Miarons M, Larrosa‐García M, García‐García S, et al. COVID‐19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management. Transplantation. 2021;105(1):138‐150. [DOI] [PubMed] [Google Scholar]
- 2. Trapani S, Masiero L, Puoti F, et al. Incidence and outcome of SARS‐CoV‐2 infection on solid organ transplantation recipients: a nationwide population‐based study. [published online ahead of print December 5, 2020].Am J Transplant. 10.1111/ajt.16428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chavarot N, Gueguen J, Bonnet G, et al. Critical COVID‐19 France Investigators. COVID‐19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant. 2021;21(3):1285‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leung TYM, Chan AYL, Chan EW, et al. Short‐ and potential long‐term adverse health outcomes of COVID‐19: a rapid review. Emerg Microbes Infect. 2020;9(1):2190‐2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leino AD, King EC, Jiang W, et al. Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: Establishing baseline values. Am J Transplant: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2019;19(5):1410‐1420. [DOI] [PubMed] [Google Scholar]


