Abstract
Plant‐derived bioactive molecules display potential antiviral activity against various viral targets including mode of viral entry and its replication in host cells. Considering the challenges and search for antiviral agents, this review provides substantiated data on chemical constituents of edible fruits with promising antiviral activity. The bioactive constituents like naringenin, mangiferin, α‐mangostin, geraniin, punicalagin, and lectins of edible fruits exhibit antiviral effect by inhibiting viral replication against IFV, DENV, polio, CHIKV, Zika, HIV, HSV, HBV, HCV, and SARS‐CoV. The significance of edible fruit phytochemicals to block the virulence of various deadly viruses through their inhibitory action against the entry and replication of viral genetic makeup and proteins are discussed. In view of the antiviral property of active constituents of edible fruits which can strengthen the immune system and reduce oxidative stress, they are suggested to be diet supplements to combat various viral diseases including COVID‐19.
Practical applications
Considering the increasing threat of COVID‐19, it is suggested to examine the therapeutic efficacy of existing antiviral molecules of edible fruits which may provide prophylactic and adjuvant therapy with their potential antioxidant, anti‐inflammatory, and immune‐modulatory effects. Several active molecules like geraniin, naringenin, (2R,4R)‐1,2,4‐trihydroxyheptadec‐16‐one, betacyanins, mangiferin, punicalagin, isomangiferin, procyanidin B2, quercetin, marmelide, jacalin lectin, banana lectin, and α‐mangostin isolated from various edible fruits have showed promising antiviral properties against different pathogenic viruses. Especially flavonoid compounds extracted from edible fruits possess potential antiviral activity against a wide array of viruses like HIV‐1, HSV‐1 and 2, HCV, INF, dengue, yellow fever, NSV, and Zika virus infection. Hence taking such fruits or edible fruits and their constituents/compounds as dietary supplements could deliver adequate plasma levels in the body to optimize the cell and tissue levels and could lead to possible benefits for the preventive measures for this pandemic COVID‐19 situation.
Keywords: COVID‐19, immune‐modulators, phytochemicals, therapeutics, viral replication
Constituents of edible fruits (EFCs) improve the immunity of body and act as immune‐stimulants.The bioactives like naringenin, mangiferin, isomangiferin, α‐mangostin, geraniin, punicalagin, betacyanins, marmelide and some lectins isolated from the edible fruits are revealed as potent antiviral agents.The regular intake of EFCs leads to strengthening the immune cells and reduces the oxidative stress in the host body which in turn inhibits the viral attachment and replication on the host cell.
Abbreviations
- apoB
apolipoprotein B
- CHIKV
chikungunya virus
- CV‐B3
coxsackievirus B3
- DENV
dengue virus
- DNA
deoxyribonucleic acid
- EFCs
edible fruits and their constituents/compounds
- EV71
human enterovirus 71
- HBV
hepatitis B virus
- hCMV
human cytomegalovirus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HSV‐1
herpes simplex virus‐1
- HSV‐2
herpes simplex virus‐2
- IFN‐α
interferon alpha
- IFN‐γ
interferon gamma
- IFV‐A
influenza A virus
- IFV‐B
influenza B virus
- MDCK
Madin‐Darby canine kidney
- MERS‐CoV
Middle East respiratory syndrome virus
- NSV
sindbis neurovirulent strain
- PBMC
peripheral blood mononuclear cell
- PEDV
porcine epidemic diarrhea virus
- RNA
ribonucleic acid
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RSV
respiratory syncytial virus
- SARS
severe acute respiratory syndrome
- Th1
T helper type 1
- Th2
T helper type 2
- TNF‐α
tumor necrosis factor alpha
- VLDL
very low density lipoprotein
- VZV
Varicella‐zoster virus
- WHO
World Health Organization
1. INTRODUCTION
Viruses are one of the major causes of morbidity and mortality around the world that affect about five million people annually (Andersen et al., 2020). There is an ever increase in the number of cases of human immunedeficiency virus, influenza, hepatitis C virus, and herpes simplex viruses which are leading causes of morbidity and death in human (Khwaza et al., 2018). The respiratory tract infections caused by different viral pathogens especially influenza viruses are considered a critical public health issue and causing millions of deaths worldwide annually, particularly with lower respiratory tract infections (Farrag et al., 2019). To overcome this, novel molecules of natural products which hold effective therapeutic potential against such viral diseases are the prime focus to prevent the widespread infections and identification of antiviral mechanisms from these drugs is attracted the researchers throughout the World (Patel et al., 2021).
Many viral diseases have so far been emerged from different parts of the world. Most of these viral diseases are infectious and pose continuous challenge that can emerge or re‐emerge at unpredictable times in diverse climatic conditions. An emerging viral disease is broadly referred as one of the newly evolved infectious disease or recently recognized or have not been witnessed earlier within a specific population or geographical region (Gedif Meseret, 2020). Smallpox emerged from Asia and spread to Europe in 5th century, yellow fever emerged from America during the 16th century, dengue fever from South‐east Asia, Africa, and North America during the 18th century, Spanish flu during 1918–1919 in almost all countries which killed about 40 million people and HIV originated from Africa in the second half of the 20th century which kills nearly 300,000 people every year (Chastel, 2007).
Since 1980s, the world has frequently been facing newly developed/formed pandemic viral infections which continue as a major threat to the human population. In the late 1990s, a highly pathogenic and deadly avian influenza A virus (H5N1) with several subtypes like H7N9, H9N2, and H7N3 spread from poultry to human which becomes a pandemic disease (Luo (George) & Gao, 2020). Nipah virus (paramyxovirus) has been identified as a major cause of severe encephalitis in South‐east Asian countries (Chua, 2000).
Severe acute respiratory syndrome (SARS), a respiratory disease caused by a novel coronavirus (SARS‐CoV) was first identified from China in early 2000 that affected people in 37 countries with over 8,000 infections and 774 deaths (Fouchier et al., 2003). Other viral diseases emerged in the beginning of 21st century are the pandemic influenza in 2009 (caused by swine H1N1 influenza A virus), the Middle East respiratory syndrome virus (MERS‐CoV) in 2012, thrombocytopenia syndrome causing SFTS bunyavirus in 2010, Ebola in West Africa during 2014–2016 and Zika virus in 2015 (Baseler et al., 2017; Bogoch et al., 2016; Vijaykrishna et al., 2010; Yu et al., 2011; Zumla et al., 2015). Of these, SARS‐CoV and MERS‐CoV are caused by the coronaviruses, the major pathogens which target the primary respiratory system in humans and pose severe threat during the last two decades.
Most recent viral emergence is SARS‐CoV‐2, coronavirus disease 2019 (COVID‐2019, a large group of ssRNA viruses) originated in the middle of December 2019 from the city of Wuhan in China and now spreading to almost 220 countries with 178,503,429 confirmed cases including 3,872,457 deaths as of 05:44 pm CEST, 22 June 2021 as reported to World Health Organization (http://who.sprinklr.com). The SARS‐CoV‐2 is transmitted from human to human by respiratory droplets, close contact with diseased persons, and possibly by oral and aerosol contact of diseased patients and airborne transmission is extremely virulent and represents a foremost route to spread of disease (dos Santos, 2020). The major routes of transmission of virus are reported by new infections within the family members, health care workers, and small to larger communities. Because of rapid spread and nature of transmission, the WHO declared the COVID‐19 as a pandemic disease with public health emergency of international concern (Chikhale et al., 2020; Walls et al., 2020).
The common symptoms of COVID‐19 in humans include fever, cough, dyspnea (shortness of breath), and in severe cases, the infection causes SARS, kidney failure, and pneumonia which leads to death (Xian et al., 2020). Like SARS‐CoV, the SARS‐Cov‐2 affects the elderly people with underlying comorbidities and cause complications with bilateral interstitial pneumonia, acute respiratory distress syndrome, acute cardiac injury, and secondary super‐infections (Liu et al., 2020). The potential cellular and molecular level pathogenesis of SARS‐CoV‐2 and its mechanisms responsible for different malfunctions in the body are still unidentified (Filardo et al., 2020). The recent pieces of evidence prove that the spike glycoproteins of SARS‐CoV‐2 have structural similarity to the SARS‐CoV (Lin et al., 2020; Walls et al., 2020).
The foremost step and important phenomenon in the coronavirus infection is a viral entry into the host cell (interaction of host cell through viral spike protein) followed by replication and spread into healthy cells. The natural therapeutic agents which block the entry of viral DNA/RNA can be considered as potential antiviral therapeutics (Sayed et al., 2020; Zahedipour et al., 2020). There are many resemblances between the genetic makeup of MERS‐CoV, SARS‐CoV, and SARS‐CoV‐2 (all three belong to a β‐coronavirus family with ssRNA, closer genome sequence homology, and almost same pathogenesis mechanism) and existing plant‐based antiviral therapies used for SARS‐CoV can also be examined against the COVID‐19 (Bhuiyan et al., 2020).
With the speedy spread of COVID‐19, it is considered a major alarming threat for almost all the countries and there is an increased demand to carry out extensive research in developing effective vaccines or antiviral agents against the various strains of coronaviruses (Bhuiyan et al., 2020). The currently available drugs which are effective against some of the coronavirus strains can also be used against COVID‐19, but this is not the perfect solution to overcome this pandemic situation (Wilder‐Smith et al., 2020).
1.1. Natural products as antiviral agents
Natural products have made great contributions to human health. Since ancient times, natural products from plant resources are used in treating various diseases including viral infections. Subsequently, many active constituents have been identified, isolated and their mechanisms of action in host organism have been elucidated by various researchers (Liu & Du, 2012). Plants have the potential to produce diverse secondary metabolites and maintain human health by inhibiting virus attachment, penetration, replication, and interfering intracellular signal activation pathways. So, it is essential to develop potential antiviral agents from natural products that have been traditionally used as antiviral agents which can be expected to prolong the efficacy of drug therapy.
Although the antiviral activity of several natural products against various infectious viruses has been investigated, there is a lacuna in the development of natural drugs as antiviral therapies against the coronaviruses which cause many diseases like bronchitis, gastroenteritis, hepatitis, pneumonia and leads to death in birds, bats, cats, and humans (Denaro et al., 2020; Islam et al., 2020). There are several classes of phytochemicals present in various parts of plants which are reported to have many active principles with antiviral activity (Ghildiyal et al., 2020). The phytochemicals like phenolic compounds, alkaloids, flavonoids, saponins, quinines, tannins, terpenes, proanthocyanidins, proanthocyanins, lignins, glycosides, steroids, organic acids, coumarins thiosulfonates with a broad spectrum of pharmacokinetics, are reported to have antiviral activity (Chukwu Odimegwu & Gospel Ukachukwu, 2020; Liu & Du, 2012).
The bioactive plant compounds like rutin, quercetin, myricetin and baicalin, mangiferin, naringenin are effective against avian IFV, HSV‐1, HSV‐2, IFV, rhinovirus, DENV, poliovirus, adenovirus, Epstein‐Barr virus, Mayaro virus, Japanese encephalitis virus, respiratory syncytial virus, HCV, enterovirus, Newcastle disease virus, HIV, HBV, and Zika virus (Ben‐Shabat et al., 2020). The mechanism of action of these antiviral compounds is also well addressed. In the last few decades, the success of plant‐based effective therapies led to much attention in the identification of antiviral lead molecules of plant origin (Lee et al., 2013). With the continuous search on antiviral agents, plant‐based natural compounds such as caffeic acid, griffithsin, isobavachalcone, myricetin, psoralidin, quercetin, saikosaponin B2, scutellarein, silvestrol, and tryptanthrin showed promising results against the deadly viruses like coronaviruses in humans (Mani et al., 2020; Sinha et al., 2020).
Research on antiviral agents from plant extracts has gained momentum since 1950s when some of the traditional medicinal plants have proven effective against some pathogenic viruses. Antiviral synthetic medicines administered for exterminating these infectious viruses also impose side effects on human health thus demanding intervention of antiviral agents of plant origin. Post‐exposure of human system to different viral infections requires effective therapeutic approaches to overcome severe infections and it is vital to develop novel antiviral agents from natural products (Chukwu Odimegwu & Gospel Ukachukwu, 2020). Plant‐derived bioactives display antiviral activity against the SARS viral targets including mode of viral entry and viral replication into host cells (Sayed et al., 2020).
Given the facts of the ability of edible fruit constituents in improving body immunity, we have tried to consolidate previously published data on such research with antiviral potential. The objective of this review was to gather information on antiviral properties of bioactive constituents isolated from edible fruits, and efforts to obtain their efficient delivery. Since secondary metabolites of edible fruits are reported to possess antiviral properties, these can also be utilized in combating COVID‐19 in the current pandemic situation.
1.2. Review methodology
Several online bibliographical databases including PubMed, Scopus, and Web of Science were used to search literature on the antiviral effect of edible fruit constituents. An extensive literature search was conducted in the above databases with the terms “edible fruits and phytoconstituents”, edible fruit constituents, “antiviral activity” along with “viral infections”, “viral replication”, “immuno‐modulators”, and “immune boosters”. Only the reports that were in English were considered and included while compiling the data. The related articles linked to the retrieval list of articles were auto‐prompted during the literature search and reviewed for relevance. The references cited in the retrieved articles were also searched to get further results. Chemical structures of compounds were drawn using ACD/ChemSketch software (ACD/Labs Release 2012, File version 14.01, Build 65,894).
1.3. Phytoconstituents (from edible fruits) as source of antiviral agents
Bioactive constituents like flavonoids and polyphenols extracted from various fruits shown to alleviate an inflammatory response in adipocytes, macrophages, and other immune cells and improve several metabolic disorders by modulating their mechanism (Li et al., 2020). Among the reported phytochemicals from edible fruits, phenolic compounds were extensively studied to reduce the risk of life‐threatening diseases like cancer, heart problem, and diabetes along with antimicrobial, antiviral, anti‐inflammatory, and antiallergenic properties (Shahidi & Ambigaipalan, 2015). Several plants of nutraceutical significance and their phytochemicals are reported to be effective against diverse viral respiratory infections and altered the immune stimulation and inflammation modulating effects (Patel et al., 2021). Due to the revival of interest in herbal medicines and novel compounds of nutraceutical plants, the AYUSH (https://www.ayush.gov.in/) systems of medicine promote lifestyle modification and dietary management in day‐to‐day life in the prevention of COVID‐19 by improving immunity in the body.
Antioxidants present in edible fruits can scavenge reactive oxygen species and inhibit the NF‐kB‐mediated inflammation which leads to inhibit oxidative stress (Wood & Gibson, 2009). The excess amount of free radicles produced in the body by various factors could cause oxidative stress which leads to chronic diseases, so the increased consumption of edible fruits with a rich amount of antioxidants in the daily diet will prevent or slow down the oxidative stress (Sun et al., 2002). Several flavonoid compounds extracted from edible fruits possess antiviral activity against a wide array of viruses like HIV‐1, HSV‐1 and 2, HCV, INF, dengue, yellow fever, NSV, and Zika virus infection (Cataneo et al., 2019).
Various bioactive components present in the edible fruits could have complementary and overlapping mechanisms of action by performing as antioxidants, stimulant factor in the immune system, molecular level regulators of gene expression in cell proliferation and apoptosis, hormone metabolism as well as antimicrobial and antiviral agents (Liu, 2013). The phytochemicals present in the edible fruits act as immune‐modulators by enhancing the cell‐mediated immunity and activate non‐specific immune responses and activation of necessary immune cells in the body result in the production of vital components like interferons, cytokines, and chemokines which are served as stimulators in the immune responses (Yang & Wang, 2020). The phytochemicals tested for antiviral activity are abundant in various nuts, fruits, berries, and its active components possibly have the capacity to diminish infection and replication of many viruses (Brijesh et al., 2009).
The review also provided an overview of likely effects of the intake of edible fruits constituents to strengthen the immune cells by reducing the oxidative stress in host body system which in turn inhibit the viral attachment and replication on the host cell. Several active molecules from the fruits like geraniin from rambutan, naringenin from citrus and grapes, (2R,4R)‐1,2,4‐trihydroxyheptadec‐16‐one from avocado, betacyanin from red dragon, mangiferin and isomangiferin from mango, procyanidin B2 and quercetin from apple, punicalagin from pomegranate, marmelide from bael, jacalin lectin from jackfruit, and a banana lectin from banana have been isolated and showed promising antiviral properties against different pathogenic viruses (Table 1). Several of these phytochemicals of edible fruits have complementary and overlapping mechanisms of action including potential antiviral effect by either inhibiting the formation of viral DNA/RNA or inhibiting the activity of viral reproduction inside the host cell.
TABLE 1.
List of phytochemicals isolated from edible fruits with antiviral properties
| Phytochemical | Viruses studied | Observation | References |
|---|---|---|---|
| Naringenin | HBV | Infectivity of viruses in the host cell by targeting viral envelope by reverse transcriptases | Alam et al. (2017) |
| HCV | Reduction in lipid profile and liver enzyme aspartate transaminase with a decreased infectivity and spread of infection | Goncalves et al. (2017) | |
| DENV‐1,2,3 & 4 | Reduction in Huh‐7.5 DENV‐1 infected cells and CD14+ cells in human PBMCs | Frabasile et al. (2017) | |
| DENV‐2 | 50% of reduction in viral RNA infection | Zandi et al. (2011) | |
| ZIKV | Reduction in the number of infected hmd‐DCs by impairing the viral replication | Cataneo et al. (2019) | |
| HCV | Minimum binding energy with NS2 protease and target protein for antiviral activity were analysed by in silico method | Sajitha Lulu et al. (2016) | |
| CHIKV | Production of viral proteins was prevented in a host cell by which leads to killing 50% of infected cells | Ahmadi et al. (2016) | |
| NSV | 80% of viral replication was inhibited by blocking the infectivity | Paredes et al. (2003) | |
| HCV | 70% of inhibition in the secretion of HCV core and HCV RNA which leads to inhibition of apoB100‐dependent HCV secretion | Nahmias et al. (2008) | |
| HCV | Assembly of infectious HCV particles blocked by activating the peroxisome proliferator‐activated receptor‐α and reduction in VLDL production | Goldwasser et al. (2011) | |
| Mangiferin | HSV‐1 | 56.8% of plaque reduction was observed with decreased viral replication | Zheng and Lu (1990) |
| HIV | The significant cytopathic effect in infected cells | Guha et al. (1996) | |
| Poliovirus | Increased virulence and replication of the virus in host cells was observed in different stages of poliovirus | Rechenchoski et al. (2018) | |
| HSV‐2 | Viral replication was significantly reduced with decreased plaque formation in HeLa cells | Zhu et al. (1993) | |
| HIV‐1IIIB & HIV‐1RF | The inhibitory activity is effective against peptidic protease inhibitor‐resistant strains in lower concentrations | Wang et al. (2011) | |
| Isomangiferin | HSV‐1 | 69.5% of plaque reduction with decreased viral replication | Zheng et al. (1990) |
| α‐Mangostin | HIV‐1 | Significant inhibitory activity with decreased viral replication | Chen et al. (1996) |
| DENV‐2 | Viral replication was reduced by more than 50% and increased gradually by post‐infection | Sugiyanto et al. (2019) | |
| DENV‐1,2,3 & 4 | The infection rate was reduced by 47%–55% by inhibiting the cytokine and chemokine transcription | Tarasuk et al. (2017) | |
| Geraniin | DENV‐2 | 100% inhibition of viral replication was noticed by preventing the viral attachment at early stages of infection | Abdul Ahmad et al. (2017) |
| EV71 | Addition of geraniin at 2 hr post EV71 infection on human rhabdomyosarcoma cells inhibited infectious virus yield and viral RNA replication | Yang, Zhang, et al. (2012) | |
| Geraniin significantly enhanced the survival rate of EV71 infected mice and decreased viral replication in muscle tissues | Yang, Zhang, et al. (2012) | ||
| DENV‐1,2 | The compound inhibited the replication of HSV‐2 with an IC50 and IC90 of 18.4 ± 2.0 and 37.6 ± 2.3 µM, respectively, and inhibited the replication of HSV‐1 with an IC50 of 35.0 ± 4.2 µM. | Yang et al. (2007) | |
| HIV‐1 | Significant inhibitory effect against the replication of HIV‐1 showed with IC50 and EC50 of 1.8 ± 0.5 µg/ml and 0.24 ± 0.10 µg/ml respectively in MAGI cells | Notka et al. (2003) | |
| The significant anti‐HIV activity was recorded in CD4+ lymphoid cells MT4 at even low concentrations | Notka et al. (2003) | ||
| Punicalagin | EV71 | Viral replication was inhibited with reduced cytopathic effect on rhabdomyosarcoma cells | Yang, Zhang, et al. (2012) |
| HSV‐1 | Viral infectivity and replication were reduced 8‐fold with no toxicity | Houston et al. (2017) | |
| HSV‐2 | 100% inhibition of viral infection was recorded in Hep‐2 cells of human epithelial tissue | Arunkumar and Rajarajan (2018) | |
| HBV | Punicalagin down regulated the level of cccDNA formation in HBV without inhibiting viral RNA transcription and DNA replication in the host cells. | Liu et al. (2016) | |
| IFV‐A | The compound significantly inhibiting the agglutination of chicken RBCs in the IFV‐A and prevents the proliferation of IFV‐A in both single and multiple cycle growth conditions in infected MDCK cells | Haidari et al. (2009) | |
| (2R, 4R)‐1,2,4‐trihydroxyheptadec‐16‐one | DENV‐1,2,3 & 4 | The survival rate of DENV infected mice was increased by stimulating the NF‐κB‐ mediated IFN responses with no cytotoxicity | Wu et al. (2019) |
| BanLec (Banana lectin) | HIV‐1 | Attachment of virus into the host cell was prevented in very low concentrations | Mazalovska and Kouokam (2018) |
| HIV | Mitogenicity and significant antiviral properties were observed | Swanson et al. (2015) | |
| Influenza A, B | Engineered BanLec exhibited antiviral activity at 10 μg/ml | Covés‐Datson et al. (2020) | |
| HIV | Penetration of viral particles and viral envelopes into the host cells was observed with less toxicity | Akkouh et al. (2015) | |
| HIV | Inhibition of viral entry into the host cell and spread of infection was reduced by binding to the glycosylated viral envelope | Swanson et al. (2010) | |
| Jacalin (Jackfruit lectin) | HIV‐1 | Inhibition of viral replication by binding to membrane molecules together with CD4 | Favero et al. (1993) |
| HSV‐2, VZV, hCMV | 50% of inhibition and viral replication was noticed with mitogenic action for NK lymphocyte | Wetprasit et al. (2000) | |
| Lectin like compounds (Japanese plum) | H1N1, H3N2 | Prevents the attachment of viral haemagglutination in host MDCK cells before viral adsorption | Yingsakmongkon et al. (2008) |
| Betacyanin (Fractions) | DEN‐V | 95% of viral replication and infection was inhibited with no cytotoxicity | Chang et al. (2020) |
| Marmelide | Coxsackievirus B3 | Loss of infectivity was observed with inactivating the viruses at 125 µg/ml for 1 hr at 37℃ | Badam et al. (2002) |
The EFCs possessed a vast range of antiviral activity through different mode of action (e.g., inhibition of viral replication, reducing oxidative damage and improving the immune system) and it can also be effective antiviral agents against COVID‐19. The existing evidences suggest that a number of phytoconstituents derived from fruits, spices, herbals, and roots possess a significant antioxidant, anti‐inflammatory and virucidal functions and can reduce the risk or severity of a wide range of viral infections by boosting the immune response mostly in people with deficient dietary sources (Mrityunjaya et al., 2020).
Many functional foods and substances naturally possess diverse bioactive compounds that have been scientifically proven to have immune‐boosting properties and antioxidant present in such foods or edible fruits can be directly obtained as dietary supplements (López‐Varela et al., 2002). For example, hesperidin from orange (one of the renowned source for its vitamin and flavonoids content) attracted the attention of researchers, since the compound has a low binding energy, both with the SARS‐CoV‐2 spike protein and with main protease which transforms the early proteins of virus into a complex responsible for viral replication in host cells as evidenced by computational methods (Bellavite & Donzelli, 2020). Flavonoid compounds have the capacity to bind with functional domains of the SARS‐CoV‐2 protein which referred as the viral surface glycoprotein that required for early attachment and internalization of viruses into the host cells (Patel et al., 2021).
1.4. Naringenin, a potent antiviral compound
The flavonoid group of compounds are effective against several viruses mainly HCV by blocking its entry into host cell and can alleviate HCV infection by reducing apolipoprotein B100 (apoB100) secretion which is required for infection of HCV (Hernández‐Aquino & Muriel, 2018). Naringenin (4′,5,7‐trihydroxy flavanone; Figure 1a) is one of the most important naturally occurring flavonoids widely distributed in various fruits and vegetables and one of a promising drug candidate in the development of anti‐COVID‐19 therapy (Tutunchi et al., 2020).
FIGURE 1.
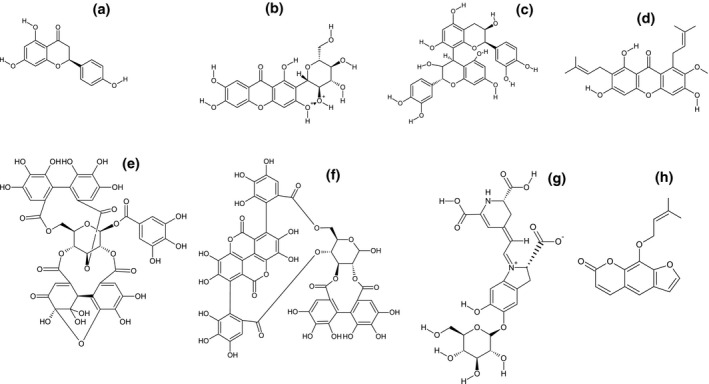
Major phytochemicals isolated from edible fruits with potential antiviral properties. (a) Naringenin; (b) Mangiferin; (c) Isomangiferin; (d) α‐Mangostin; (e) Geraniin; (f) Punicalagin; (g) Betacyanins; (h) Marmelide
The addition of naringenin at 250, 125, and 62.5 μM to Huh‐7.5 cells infected with DENV 1,2,3, and 4 serotypes proved the ability to impair and reduce the DENV replication and its maturation with effectiveness similar to IFN‐α 2A (a well‐known antiviral cytokine) and ribavirin treatments (Frabasile et al., 2017). After 24 hr of naringenin treatment at 62.5 μM concentration (identified as non‐toxic concentration), it effectively reduced the percentage of Huh‐7.5 DENV‐1 infected cells and reduced the number of infected CD14+ cells in human PBMCs and number of infectious virus particles in the culture. It was also revealed that, after 6 hr of naringenin treatment, the DENV‐1 infection reduced the DENV‐titer which confirms the potential anti‐DENV activity of this flavanone compound.
Naringenin impairs the ZIKV replication and efficiently reduced the number of ZIKV infected human monocyte‐derived dendritic cells at 125 μM (Cataneo et al., 2019). From the molecular modelling data, naringenin’s ability to interact with viral protease (allosteric inhibitors) is analyzed and the molecular target of naringenin is NS2B‐NS3 protease. It is proved that naringenin displays strong antiviral activity by affecting viral replication or assembly of viral particles especially post‐infection (Cataneo et al., 2019). Antiviral activity of naringenin is analyzed by protein prediction study using molecular modelling protocol, in which it showed minimum binding energy of 7.97 kcal/mol with NS2 protease and all the observed ligands present within the binding pocket of target protein (Sajitha Lulu et al., 2016).
Naringenin extracted from the citrus fruits possesses significant antiviral activity by inhibiting the CHIKV replication in a host cell and down‐regulating the production of viral proteins that are involved in viral replication (Ahmadi et al., 2016). Administration of naringenin at 25 μg/ml in baby hamster cells 21 clone 15 (BHK‐21) infected with sindbis neurovirulent strain (NSV) inhibited the viral replication up to 80% by blocking the infectivity of the NSV (Paredes et al., 2003).
The HCV is the foremost cause for chronic liver diseases associated with circulating lipoproteins, cholesterol, and lipid pathways and it progresses to a chronic state in about 70% of patients, ultimately causing cirrhosis and hepatocellular carcinoma (Nahmias et al., 2008). Their study also suggested to take naringenin as supplementation with the diet to HCV patients since, the compound has the ability of reduction in HCV viral load by inhibiting viral secretion and possibly by allowing uninfected cells to regenerate which potentially increasing the overall rate of viral clearance in the patients. The HCV replication depends on the expression of apoB100 and assembly very‐low‐density lipoprotein (VLDL) in host cells especially in human hepatocytes (Huang et al., 2007).
The Huh‐7.5.1 cells infected with HCV administered with 200 μM naringenin for 24 hr inhibits the secretion of HCV core and HCV positive‐strand RNA followed by inhibition of apoB100‐dependent HCV secretion in a dose‐dependent manner (Nahmias et al., 2008). Naringenin obtained from grapefruit [Vitis vinifera L., Vitaceae] repressed VLDL secretion, microsomal triglyceride transfer protein activity, and transcription of 3‐hydroxy‐3‐methyl‐glutarl‐coenzyme A reductase and acyl‐coenzyme A cholesterol acyl‐transferase leading to a reduction in 80% of HCV by silencing the apoB mRNA in infected cells and caused a 70% reduction in the release of apoB100 as well as HCV replication (Nahmias et al., 2008).
Naringenin obtained from the grapefruit significantly inhibited the secretion of apoB and HCV RNA followed by a decreased level of HCV core protein secretion (Goldwasser et al., 2011). Naringenin inhibits the HCV secretion by blocking the assembly of infectious HCV particles and the antiviral activity is mediated by activating peroxisome proliferator‐activated receptor‐α led to a reduction in VLDL production which is necessary for the secretion of HCV particles in host cells.
Naringenin exhibited significant virucidal activity against the DENV‐2 type with the IC50 of 52.64 μg/ml by inhibiting the DENV‐2 RNA level at 50 μg/ml concentration (Keivan et al., 2014). The presence of 0.14 μg/mg concentration of naringenin in ethanolic leaf extracts of the plant Guiera senegalensis J.F. Gmel. (Combretaceae) showed significant anti‐HBV activity by RP‐HPTLC method which supports the possible role of the compound in inhibition of HBV gene expressions and DNA replication (Alam et al., 2017).
1.5. Mangiferin against HSV‐1, 2, HIV, and poliovirus
Mangiferin (1,3,6,7‐tetrahydroxy‐C2‐β‐D‐glucoside) is a xanthone glucoside found in significant level in higher plants and a major compound in various parts of Mango [Mangifera indica L., Anacardiaceae] such as fruit peel, kernel, stalk, leaf, bark, and seed. It possesses immune‐modulating effects on different oxidative mechanisms in curing various disorders (Wang et al., 2011). Mangiferin (Figure 1b) and isomangiferin (Figure 1c) isolated from the fruit pulp of mango have significant antiviral property against HSV‐1 with 56.8% and 69.5% of average plaque reduction rates respectively and have comparable results over the standard drugs like acyclovir, idoxuridine, and cyclocytidine (Zheng & Lu, 1990). Mangiferin isolated from the leaves of mango showed an EC50 and EC99 at the concentration of 33 and 80 mg/ml against HSV‐2 plaque formation in HeLa cells and reduces viral replication efficiently in the late event of HSV‐2 replication (Zhu et al., 1993).
Mangiferin purified from the fruits of Mango at a concentration of 10 µg/ml significantly reduced the cytopathic effect of HIV in susceptible human leukemia cells and showed a EC50 of the drug at 3.59 µg/ml and compound did not found any substantial change in the uninfected human leukemia cells when added with the different doses of mangiferin (Guha et al., 1996). Mangiferin purified from the root and rhizome extracts of anemarrhena (Anemarrhena asphodeloides Bunge, Asparagaceae) exhibited dose‐dependent anti‐HIV inhibitory activity on HIV‐1 induced syncytium formation in HIV‐1IIIB and HIV‐1RF with the EC50 of 7.13 μg/ml (16.90 μM) and 15.45 μg/ml (36.61 μM) respectively (Wang et al., 2011). In the time‐of‐addition assay, when mangiferin was added at various times after HIV‐1IIIB infected C8166 cells, the compound blocked HIV‐1 p24 antigen production after 12 hr. It is also observed that mangiferin is effective against HIV‐1 peptidic protease inhibitor‐resistant strains and less effective against the protease inhibitor‐resistant strains of HIV‐1.
Mangiferin isolated from the fruit peels of mango showed good inhibitory property against poliovirus‐1 with 97.8%, 84.2%, and 57.1% of viral inhibition at 200, 100, and 50 μg/ml concentrations, respectively (Rechenchoski et al., 2018). The overall IC50 was recorded as 53.5 μg/ml and after 12 hr of mangiferin treatment 62.7% of viral inhibition was recorded at the concentration of 100 μg/ml. However, during the late stages of viral replication, even at a low concentration (25 μg/ml) mangiferin exhibited strong virucidal activity with 100% of viral inhibition of viral adsorption.
1.6. α‐Mangostin against DENV, HIV, and CHIKV
The α‐mangostin (Figure 1d) is the major xanthone extracted from the pericarps and bark of the mangosteen (Garcinia mangostana L., Clusiaceae) and holds a wide range of biological activities such as antimicrobial, antiparasitic, antioxidant, anti‐inflammatory, anti‐tumor, antiobesity, cardioprotective, and antidiabetic (Ibrahim et al., 2016). This xanthone compound showed noteworthy pharmacological effects in in vitro studies as well as in experimental animal models through various mechanisms of action.
The ethanolic extract of mangosteen fruit is considered as a potent inhibitor of HIV‐1 protease activity and the antiviral property of this fruit is due to the presence of purified molecules like α‐mangostin and γ‐mangostin (Chen et al., 1996). The administration of α‐mangostin (obtained from the pericarps of mangosteen) to dengue patients during the acute phase of illness may decrease infection severity by activating the host’s immune response through mechanism of interfering DENV NS5 protein activity which is essential for DENV replication and reducing transcriptional responses of cytokines (Sugiyanto et al., 2019).
The effect of α‐mangostin against the DENV infection and post‐infection in PBMCs, TNF‐α and IFN‐γ cytokines revealed that increasing concentration of α‐mangostin inhibits the viral replication by more than 50% with the IC50 of 5.47 and 5.77 μM for 24 and 48 hr of post‐treatments, respectively (Sugiyanto et al., 2019). The α‐mangostin inhibits DENV production in cultured hepatocellular carcinoma HepG2 and Huh‐7 cells, and cytokine/chemokine expression in HepG2 cells (Tarasuk et al., 2017). DENV virus‐infected cells treated with α‐mangostin considerably reduced the infection rates 47%–55%, and complete inhibition in production of DENV‐1 and DENV‐3 was recorded by decreasing the cytokine (IL‐6 and TNF‐α) and chemokine (RANTES, MIP‐1β, and IP‐10) transcription. Therefore, α‐mangostin can be considered as a potent antiviral agent than the synthetic ribavirin due to its superior activity. In the countries where all the four DENV serotypes are common which leads to severe secondary infections, α‐mangostin is ideal and an effective antiviral drug (Sugiyanto et al., 2019).
In vitro prophylactic effect of α‐mangostin (extracted from the pericarps of mangosteen) was significantly inhibited the replication of CHIKV in Vero E6 cells with the 100% reduction of virus titer at 8 μM concentration under cotreatment condition (Patil et al., 2021). Also, reduction of CHIKV replication in serum and muscles of infected mice models showed nearly 99% of reduction in CHIKV RNA copies with low and high dose of α‐mangostin. The molecular docking study revealed that α‐mangostin interacts with the E2‐E1 heterodimeric glycoprotein and the outcomes suggest that α‐mangostin can possibly inhibit the replication of CHIKV infection through multiple target proteins (Patil et al., 2021).
1.7. Geraniin against DENV‐2, EV71, HSV‐1,2, and HIV
Geraniin (Figure 1e) is an ellagitannin with a complex chemical structure belongs to a hydrolysable tannin group and commonly found in edible fruits like common berries (raspberries, strawberries, and blackberries), pomegranate, almonds, rambutan, and walnuts (Palanisamy et al., 2011). Gareniin has a wide range of pharmacological activities and possesses antioxidant, antibacterial, antifungal, antitumour, antihypertensive, antinociceptive, radioprotective, and antiviral properties (Yang, Zhang, et al., 2012).
Geraniin isolated from the rambutan [Nephelium lappaceum L., Sapindaceae] fruit rind reduces the infectivity of DENV‐2, represses the viral attachment, and prevents DENV‐2 replication upto 100% of inhibition during early stages of viral infection (Abdul Ahmad et al., 2017). Geraniin effectively reduced the DENV‐2 infectivity at 3.28 μM of concentration in a dose‐dependent manner. It was also observed that the mechanism of geraniin is either through the binding or disruption to the DENV‐E protein by inhibiting attachment of the virus to cellular receptors and binding of geraniin to E‐DIII prevents protein‐protein interaction between host cells and DENV‐2 thus preventing viral attachment and entry of DENV into the host cells (Abdul Ahmad et al., 2017). Geraniin produced a low cytotoxicity on human rhabdomyosarcoma cells in plaque reduction assay and addition of 20 μg/ml concentration of geraniin (with an IC50 of 10 μg/ml) at 2 hr post EV71 infection positively inhibited the infectious virus yield and viral RNA replication (Yang, Zhang, et al., 2012). In an in vivo study, the treatment of 0.4 and 1.0 mg/kg doses of geraniin significantly enhanced the survival rate of EV71 infected mice with 35% and 40%, respectively with a reduction in mortality and decreased viral replication in muscle tissues (Yang, Zhang, et al., 2012).
Geraniin extracted from the whole plant parts of Phyllanthus urinaria L. [Phyllanthaceae] showed promising anti‐HSV‐1 and HSV‐2 activity (Yang et al., 2007). The compound efficiently inhibited the replication of HSV‐2 with the IC50 and IC90 of 18.4 ± 2.0 µM and 37.6 ± 2.3 µM concentrations, respectively, and showed strong inhibitory effect against the replication of HSV‐1 with the IC50 of 35.0 ± 4.2 µM concentration of geraniin. The purified fraction of Phyllanthus emblica leaves enriched with geraniin showed significant inhibitory effect against the replication of HIV‐1 with the IC50 and EC50 values of 1.8 ± 0.5 µg/ml (at a concentration of 1.9 ± 0.5 µM) and 0.24 ± 0.10 µg/ml (at a concentration of 0.25 ± 0.1 µM), respectively, in MAGI cells (Notka et al., 2003). Likewise, geraniin showed decent anti‐HIV‐1 activity in the CD4+ lymphoid cells MT4 with the EC50 and CC50 values of 0.46 ± 0.17 µg/ml (0.48 ± 0.18 µM concentration) and 13.53 ± 2.30 µg/ml (14.20 ± 2.41 µM concentration) respectively.
1.8. Punicalagin against IFV‐A, EV71, HSV‐1, 2, and HBV
Punicalagin (Figure 1f) is a large polyphenolic compound which belongs to the hydrolysable tannins and identified as a major active constituent of pomegranate [Punica granatum L. Lythraceae] fruits especially in fruit rinds (Yang, Xiu, et al., 2012). The compound is considered as potential free radical scavengers (antioxidant) which have superoxide anion, singlet oxygen, and hydroxyl radical scavenging abilities with lipid peroxidation inhibitory activity along with several health benefits (Kulkarni et al., 2007). Punicalagin also possess a wide range of pharmacological effects including antimicrobial, antioxidant, chemo‐preventive, hepatoprotective, immune‐stimulant, antiproliferative, apoptotic, and antiviral activities (Yang, Xiu, et al., 2012).
Punicalagin is the active anti‐influenza component of the fruit rind extract of pomegranate which increases the inhibitory effect synergistically with oseltamivir by inhibiting the agglutination of chicken RBCs in the IFV‐A (Haidari et al., 2009). The compound also has the potential to inhibit the proliferation of IFV‐A in both single and multiple cycle growth conditions in the infected MDCK cells. The in vitro and in vivo analysis on the antiviral effect of punicalagin (isolated from fruit rind extract of pomegranate) on EV71 showed that the compound decreases the cytopathic effect on rhabdomyosarcoma cells after treatment with 15 µg/ml of punicalagin at 2 hr post EV71 infection significantly inhibits the viral replication (Yang, Xiu, et al., 2012). The punicalagin also impressively prolonged the survival time and reduced the mortality of mice models when administered with dose of 0.4, 1, or 5 mg/kg body weight which recorded 20, 40, and 38% of long‐term survivor of infected mice.
Punicalagin (extracted from fruit rind of pomegranate) at very low concentration shows a strong virucidal log reduction of 8.93 ± 0.35 (at 0.05 mg/ml concentration) on a mass to mass basis and reduces infectivity of the HSV‐1 by inhibiting the viral replication in T75 of Vero cells infected with HSV‐1 (Houston et al., 2017). It was also observed that, when the punicalagin was added with ZnSO4 at 0.14 mg/ml concentration it potentially increased the virucidal action with a resulting log reduction of 4.6 ± 0.16 mg/ml from 2.4 ± 0.9 mg/ml. Punicalagin exhibits a strong anti‐HSV‐2 activity with 100% inhibition at 31.25 μg/ml and 50% of inhibition at 15.625 μg/ml in HEp‐2 cells of human epithelial tissue‐specific (Arunkumar & Rajarajan, 2018). Liu et al. (2016) made an attempt to examine the anti‐HBV activity of punicalagin through cell‐based cccDNA (covalanetly closed circular DNA which formed from circular partially double‐strand DNA) accumulation and stability assay in HepDES19 and HepG2.117 cell lines. Their study revealed that, punicalagin efficiently down regulated the level of cccDNA formation in HBV without inhibiting RNA transcription and DNA replication in host cells that indicates the role of punicalagin in cccDNA metabolism.
1.9. (2R,4R)‐1,2,4‐Trihydroxyheptadec‐16‐one against DENV
The compound (2R,4R)‐1,2,4‐trihydroxyheptadec‐16‐one (THHY) extracted and purified from the unripe avocado [Persea americana Mill., Lauraceae] fruits possesses anti‐DENV activity with the EC50 of 14.61 ± 2.4, 10.98 ± 1.9, 12.87 ± 1.7, and 14.61 ± 2.1 µM on DENV‐1, 2, 3, and 4 serotypes, respectively, with no observable cytotoxicity to host cell by stimulating the NF‐κB‐mediated IFN responses against the studied four serotypes (Wu et al., 2019). THHY also prevents DENV replication in a time‐dependent manner and treatment of virus‐infected mice with THHY increased the survival rate ensuring that this fruit as a potential dietary resource to develop a supplement to treat DENV and related viral diseases.
1.10. Lectins against Flu, HIV‐1 & 2, HSV‐2, VZV, and CMC
Lectins are principally proteins that bind to specific carbohydrate structures and different lectins isolated from plants, animals, fungi, and microbes are reported to be effective against HCV, influenza A/B, HSV‐1 & 2, Japanese encephalitis virus, and coronaviruses with more focus on the anti‐HIV property (Mazalovska & Kouokam, 2018). In general, the lectins of plant origin encloses tandem repeats within the primary sequence and exist in the form of monomeric and higher multimeric states comprised of β‐sheets (Mitchell et al., 2017). In antiviral therapies, lectins can neutralize different viruses including influenza and HIV making them potential targets in developing novel antiviral drugs. As natural proteins, lectins target the sugar moieties of a SARS‐CoV spike protein (glycoprotein) and primarily mannose binding lectins indicated their interference with virus attachment to SARS‐CoV spike protein making them early entry inhibitors (Keyaerts et al., 2007).
The lectin (BanLec) isolated from the banana fruits [Musa acuminata Colla, Musaceae] is one of the jacalin‐related (lectin isolated from Jackfruit) lectins which have the affinity towards high‐mannose structures. BanLec can inhibit various HIV‐1 isolates in low nano‐molar range with a concentration‐dependent manner thus preventing the attachment of virus into host cell (Mazalovska & Kouokam, 2018). BanLec is one of the potent mitogens form murine T‐cells and when a mutation happens within sugar‐binding site of BanLec, it reduces mitogenic activity without affecting HIV neutralization and mitogenicity, besides antiviral activities of BanLec requires association with N‐glycans (Swanson et al., 2015).
The H84T BanLec, an engineered banana lectin is effective against an array of influenza virus strains that possesses significant antiviral activity better than most of the previously tested lectins (Covés‐Datson et al., 2020). The engineered lectin‐like H84T BanLec is a broad‐spectrum lectin capable of inhibiting both influenza A and B type viruses, which could be used as a potent therapeutic agent against a diverse array of viral strains. The antiviral lectins can prevent penetration of viral pathogens into the host cells which are suitable for topical applications with lower toxicity than other commonly practiced antiviral therapies and the glycoprotein covered retroviruses like HIV and other viruses having similar type envelopes (Akkouh et al., 2015). BanLec binds to glycosylated viral envelope and inhibits the cellular entry of the virus into a host cell and suppresses HIV‐1 infection. This activity is identical to the existing snowdrop lectin‐like Griffithsin, known anti‐HIV drugs like maraviroc and T‐20 (Swanson et al., 2010). Likewise, intranasal administration of H84T BanLec after 4 hr post infection of mice model with H1N1 efficiently blocked viral infection with about 75% of survival rate with even at lower dose of 0.03 mg (Swanson et al., 2010).
The jacalin, obtained from the fruits of jackfruit [Artocarpus heterophyllus Lam. Moraceae] and a jacalin‐α chain‐derived peptide inhibits HIV‐1 infection in human T lymphoid cells exerts its anti‐HIV activity through binding to membrane molecules together with CD4 and as like other lectins the jacalin may not interact with external glycoprotein of the virus (Favero et al., 1993). Lectin derived from jackfruit seeds inhibits HSV‐2, VZV, and hCMV and shows mitogenic action for NK lymphocytes (CD16+/CD56+) (Wetprasit et al., 2000).
Lectin like molecules isolated from the concentrated fruit juice of Japanese Plum [Prunus mume Siebold & Zucc., Rosaceae] exhibits antiviral activity against the human influenza A viruses like A/PR/8/34 (H1N1), A/Aichi/2/68 (H3N2) and A/Memphis/1/71 (H3N2) in host Mardin‐Darby canine kidney (MDCK) cells before viral adsorption (Yingsakmongkon et al., 2008). The concentrated fruit juice extract of Japanese Plum prevents and reduces the infectivity of human influenza A virus and it may be due to the presence of lectin‐like compounds (with high molecular weight) which inhibits the attachment of viral hemagglutination on host cell surfaces.
1.11. Betacyanins against DENV‐1
Betacyanin is a red‐violet pigment belongs to betalains (pigments group) which are water‐soluble, nitrogen containing pigments and can exist as the red‐violet betacyanins or yellow betaxanthins (Gengatharan et al., 2015). These groups of compounds are commonly available in several fruit crops like red dragon fruit, cacti fruit and also in red beetroot (as a red betacyanin and a yellow betaxanthin), various green amaranth species. Betacyanins extracted from various sources of fruits and other plant parts show a wide range of pharmacological effects including antioxidant, antibacterial, antifungal, anticancer, and antilipidemic activities (Gengatharan et al., 2015). The betacyanin (Figure 1g) fractions obtained from red dragon fruit (Hylocereus costaricensis [F.A.C. Weber] Britton & Rose, Cactaceae) shows a significant virucidal effect against the DENV‐2 with 95% of inhibition at 379.5 µg/ml (with an IC50 of 126.70 μg/ml) without any cytotoxicity supporting its efficiency as a potential antiviral agent (Chang et al., 2020). Their study on the virucidal effect of betacyanin fractions showed the ability of the compound to inactivate the extracellular DENV‐2 particles and decrease the viral infection in dose‐dependent manner.
1.12. Marmelide against CV‐B3
The exposure of CV‐B3 to marmelide (Figure 1h) isolated from the bael [Aegle marmelos Corr., Rutaceae] fruits causes a total loss of infectivity by inhibiting the formation of viral cells followed by the inactivation of viruses. The addition of marmelide at a concentration of 125 μg/ml for 1 hr on CV‐B3 in Vero cells resulted in increased inhibition of infectivity that signifies the effect of the compound (Badam et al., 2002). Therefore, marmelide could be a novel virucidal agent against the CV‐B3 by further studying the mode of action by molecular level studies, since the mode of action marmelide could be associated with the earlier and later stages of virus replication in the host cell.
2. CONCLUDING REMARKS
Among the reported antiviral compounds of edible fruits, naringenin, mangiferin, and lectins are much effective against more number of viruses as reported by various researchers. Figure 2 displays the antiviral activity of phytochemicals isolated from the edible fruits with a different mode of action. The components α‐mangostin, betacyanins, geraniin, naringenin, and (2R,4R)‐1,2,4‐trihydroxyheptadec‐16‐one shown potential candidates against dengue virus serotypes. The figure also clearly shows the antiviral effect of these constituents by inhibiting the viral replication, plaque formation, viral replication, attachment of the virus into a host cell, blocks the assembly of viral particles, down‐regulating the production of viral proteins which in turn lead to a reduction in viral infectivity in host cells.
FIGURE 2.
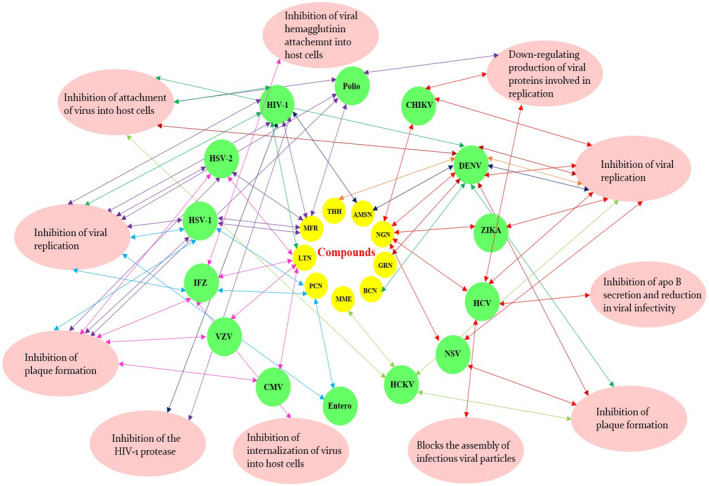
Compounds isolated from edible fruits and its multi‐target antiviral activity with a described mechanism. The different colored double arrow represents specific phytochemicals with respective antiviral properties with various actions in a host cell. LTN (Lectins)–Pink; PCN (Punicalagin)–Light blue; MME (Marmelide)–Light Green; BCN (Betacyanins)–Green; GRN (Geraniin)–Dark Red; NGN (Naringenin)–Red; AMSN (α‐mangostin)–Dark Blue; THH ([2R, 4R]‐1,2,4‐trihydroxyheptadec‐16‐one)–Orange; MFR (Mangiferin)–Purple
The anticipated role of EFCs in the management of COVID‐19 as evidenced by their efficacy against other viral diseases is shown in Figure 3. It was evident that, as soon as the SARS‐CoV‐2 virus crosses the respiratory tract, it spreads into the lung cells with its specific spike protein which can couple with ACE‐2 receptors of the host cell. The EFCs may directly damage the virus structure and inhibit the entry of viruses by disturbing the connections between viral spike proteins and host ACE‐2 receptors. Also, EFCs may inhibit the internalization of the virus into the host cell. The EFCs may inhibit viral replication by suppressing the protease (Mpro), RNA‐dependent RNA‐polymerase (RdRp), and protein synthesis.
FIGURE 3.
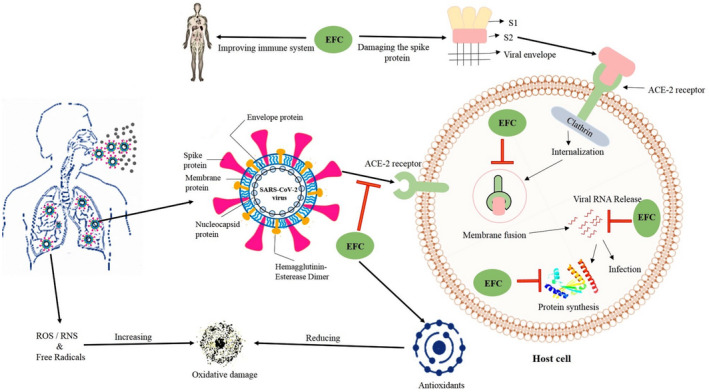
Possible binding mechanism of constituents of edible fruit extracts against the COVID‐19 virus. The figure depicts the regular intake of edible fruits and their constituents/compounds leads to strengthening the immune cells and reduces the oxidative stress in the host body system which in turn inhibits the viral attachment and replication on the host cell
The figure also evidences the effect of EFCs in reducing oxidative damage caused during COVID‐19 infection via surplus ROS/RNS and free radicals. It was reported that, increased utilization of free radicals might have impacted negatively in the COVID‐19 patients who are prone to depleted levels of antioxidants like vitamins, enzymes and some mineral elements (Muhammad et al., 2021). Likewise, lectins of different plant origin (including edible fruits) interfere with the SARS‐CoV glycoprotein during the initial entry and viral release into the host cell which piloted the further research on anti‐ SARS‐CoV activity and anti‐COVID‐19 therapies using these plant lectins (Keyaerts et al., 2007). Similarly, we hypothesized that EFCs may improve the immune system thereby suppress the COVID 19 infection, since antioxidants present in EFCs such as vitamin A, C, D, and E are reported to improve the immune response against COVID‐19 (Khanna et al., 2021).
The overall recommendation of EFCs in COVID‐19 treatment is due to their ability to improve the immune system as well as reducing the oxidative stress in the host cell machinery which helps to prevent the viral entry. As reported earlier, seasonal epidemics with coronavirus outbreaks like SARS‐CoV and MERS CoV in the recent past and other major life threatening viral diseases like HCV and INF were successfully treated by various herbal drugs and phytoconstituents of nutraceuticals (Patel et al., 2021). It was also advised that the prevention of viral respiratory related infections requires intake of vitamin C in the form of commercial drugs or regular consumption of fruits like orange, lemon, etc. which contains hesperidin as major compound (Bellavite & Donzelli, 2020). Hence taking such fruits or EFCs as dietary supplements delivers adequate plasma levels in the body to optimize the cell and tissue levels which led to possible benefits for the preventive measures for this pandemic COVID‐19 situation.
The use of dietary supplements from various food sources and nutraceuticals as coadjutant therapeutics for the prevention and treatment of COVID‐19 infection could be a useful strategy, since food‐derived antioxidants has a key role in prevention of oxidative stress and inflammation which are plays a significant cause in the progression of COVID‐19 (Lammi & Arnoldi, 2021). The results of the recent studies also suggested that antioxidant rich vitamins like vitamin C and D are effectively activated the immune response which leads to reducing the risk of respiratory tract infections by noticeably balancing the inflammatory reaction which suggests the use of those vitamins as nutritional supplements for COVID‐19 patients (Lammi & Arnoldi, 2021).
Despite a few vaccines available against Covid‐19 and several vaccines in the pipeline, psychological stigma against this viral infection remains the same which warrants extensive research on herbal medications in the cure of Covid‐19. The current situation led the scientists to develop new drugs and immune‐modulators from natural resources which reveal promising efficacy to promote immune system in the treatment and management of Covid‐19. These continuing researches on preclinical and clinical trials on nutraceuticals and food supplements of various plant resources could provide novel drugs against the emerging viral infections with better therapeutic benefits, less expensive, and minor adverse reactions. Considering the increasing threat of COVID‐19, it is opined to examine the therapeutic efficacy of existing antiviral molecules of edible fruits discussed in this review as food supplements. Such focused research may provide prophylactic and adjuvant therapy against the COVID‐19 because of their potential antioxidant, anti‐inflammatory and immune‐modulatory effects against the other emerging viral infections.
CONSENT FOR PUBLICATION
The manuscript does not have any data copied from other sources and the mechanism flowchart provided in the manuscript is drawn by us.
CONFLICT OF INTEREST
The authors declared that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Data curation; Formal analysis; Writing‐original draft: V.P. Santhi. Conceptualization; Writing‐original draft: P. Masilamani. Formal analysis; Resources; Writing‐original draft: Venkatraman Sriramavaratharajan. Data curation; Formal analysis; Writing‐review & editing: Ramar Murugan. Data curation; Validation; Writing‐review & editing: Shailendra S. Gurav. Data curation; Formal analysis; Methodology: V.P. Sarasu. Data curation; Resources: S. Parthiban. Conceptualization; Funding acquisition; Project administration; Supervision; Validation; Writing‐review & editing: Muniappan Ayyanar.
ETHICS APPROVAL
The manuscript is a review article and do not need any ethical approval.
Santhi, V. P., Masilamani, P., Sriramavaratharajan, V., Murugan, R., Gurav, S. S., Sarasu, V. P., Parthiban, S., & Ayyanar, M. (2021). Therapeutic potential of phytoconstituents of edible fruits in combating emerging viral infections. Journal of Food Biochemistry, 45, e13851. 10.1111/jfbc.13851
Funding information
The writing of this manuscript was supported by the grant received from the Science and Engineering Research Board (SERB), Department of Science & Technology (DST), Government of India, New Delhi, India (Grant No. EMR/2016/007164)
REFERENCES
- Abdul Ahmad, S. A., Palanisamy, U. D., Tejo, B. A., Chew, M. F., Tham, H. W., & Syed Hassan, S. (2017). Geraniin extracted from the rind of Nephelium lappaceum binds to dengue virus type‐2 envelope protein and inhibits early stage of virus replication. Virology Journal, 14, 1–13. 10.1186/s12985-017-0895-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi, A., Hassandarvish, P., Lani, R., Yadollahi, P., Jokar, A., Bakar, S. A., & Zandi, K. (2016). Inhibition of chikungunya virus replication by hesperetin and naringenin. RSC Advances, 6, 69421–69430. 10.1039/C6RA16640G [DOI] [Google Scholar]
- Akkouh, O., Ng, T., Singh, S., Yin, C., Dan, X., Chan, Y., Pan, W., & Cheung, R. (2015). Lectins with anti‐HIV activity: A review. Molecules, 20, 648–668. 10.3390/molecules20010648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam, P., Parvez, M. K., Arbab, A. H., & Al‐Dosari, M. S. (2017). Quantitative analysis of rutin, quercetin, naringenin, and gallic acid by validated RP‐ and NP‐HPTLC methods for quality control of anti‐HBV active extract of Guiera senegalensis . Pharmaceutical Biology, 55, 1317–1323. 10.1080/13880209.2017.1300175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, P. I., Ianevski, A., Lysvand, H., Vitkauskiene, A., Oksenych, V., Bjørås, M., Telling, K., Lutsar, I., Dumpis, U., Irie, Y., Tenson, T., Kantele, A., & Kainov, D. E. (2020). Discovery and development of safe‐in‐man broad‐spectrum antiviral agents. International Journal of Infectious Diseases, 93, 268–276. 10.1016/j.ijid.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunkumar, J., & Rajarajan, S. (2018). Study on antiviral activities, drug‐likeness and molecular docking of bioactive compounds of Punica granatum L. to Herpes simplex virus‐2 (HSV‐2). Microbial Pathogenesis, 118, 301–309. 10.1016/j.micpath.2018.03.052 [DOI] [PubMed] [Google Scholar]
- Badam, L., Bedekar, S. S., Sonawane, K. B., & Joshi, S. P. (2002). In vitro antiviral activity of bael (Aegle marmelos Corr) upon human coxsackieviruses B1–B6. Journal of Communicable Diseases, 34, 88–99. [PubMed] [Google Scholar]
- Baseler, L., Chertow, D. S., Johnson, K. M., Feldmann, H., & Morens, D. M. (2017). The pathogenesis of Ebola virus disease. Annual Review of Pathology: Mechanisms of Disease, 12, 387–418. 10.1146/annurev-pathol-052016-100506 [DOI] [PubMed] [Google Scholar]
- Bellavite, P., & Donzelli, A. (2020). Hesperidin and SARS‐CoV‐2: New light on the healthy function of citrus fruits. Antioxidants, 9, 1–18. 10.3390/antiox9080742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Shabat, S., Yarmolinsky, L., Porat, D., & Dahan, A. (2020). Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Delivery and Translational Research, 10, 354–367. 10.1007/s13346-019-00691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan, F. R., Howlader, S., Raihan, T., & Hasan, M. (2020). Plants metabolites: Possibility of natural therapeutics against the COVID‐19 pandemic. Frontiers in Medicine, 7(7), 444. 10.3389/fmed.2020.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoch, I. I., Brady, O. J., Kraemer, M. U. G., German, M., Creatore, M. I., Kulkarni, M. A., Brownstein, J. S., Mekaru, S. R., Hay, S. I., Groot, E., Watts, A., & Khan, K. (2016). Anticipating the international spread of Zika virus from Brazil. The Lancet (London, England), 387, 335–336. 10.1016/S0140-6736(16)00080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brijesh, S., Daswani, P., Tetali, P., Antia, N., & Birdi, T. (2009). Studies on the antidiarrhoeal activity of Aegle marmelos unripe fruit: Validating its traditional usage. BMC Complementary and Alternative Medicine, 9, 1–12. 10.1186/1472-6882-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataneo, A. H. D., Kuczera, D., Koishi, A. C., Zanluca, C., Silveira, G. F., Arruda, T. B. D., Suzukawa, A. A., Bortot, L. O., Dias‐Baruffi, M., Verri, W. A., Robert, A. W., Stimamiglio, M. A., Duarte dos Santos, C. N., Wowk, P. F., & Bordignon, J. (2019). The citrus flavonoid naringenin impairs the in vitro infection of human cells by Zika virus. Scientific Reports, 9, 1–15. 10.1038/s41598-019-52626-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y. J., Pong, L. Y., Hassan, S. S., & Choo, W. S. (2020). Antiviral activity of betacyanins from red pitahaya (Hylocereus polyrhizus) and red spinach (Amaranthus dubius) against dengue virus type 2 (GenBank accession no. MH488959). Access Microbiology, 2(1), acmi000073. 10.1099/acmi.0.000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastel, C. (2007). Les virus bougent: périls planétaires. Bulletin de l'Académie Nationale de Médecine, 191, 1563–1577. 10.1016/S0001-4079(19)32908-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S.‐X., Wan, M., & Loh, B.‐N. (1996). Active constituents against HIV‐1 protease from Garcinia mangostana . Planta Medica, 62, 381–382. 10.1055/s-2006-957916 [DOI] [PubMed] [Google Scholar]
- Chikhale, R. V., Gurav, S. S., Patil, R. B., Sinha, S. K., Prasad, S. K., Shakya, A., Shrivastava, S. K., Gurav, N. S., & Prasad, R. S. (2020). Sars‐cov‐2 host entry and replication inhibitors from Indian ginseng: An in‐silico approach. Journal of Biomolecular Structure & Dynamics, 1–12. 10.1080/07391102.2020.1778539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua, K. B. (2000). Nipah virus: A recently emergent deadly paramyxovirus. Science (80‐), 288, 1432–1435. 10.1126/science.288.5470.1432 [DOI] [PubMed] [Google Scholar]
- Chukwu Odimegwu, D., & Gospel Ukachukwu, U. (2020). Antiviral natural products against hepatitis‐A virus. In Streba C. (Ed.), Hepatitis A and other associated hepatobiliary diseases (pp. 1–17). IntechOpen. 10.5772/intechopen.91869 [DOI] [Google Scholar]
- Covés‐Datson, E. M., King, S. R., Legendre, M., Gupta, A., Chan, S. M., Gitlin, E., Kulkarni, V. V., Pantaleón García, J., Smee, D. F., Lipka, E., Evans, S. E., Tarbet, E. B., Ono, A., & Markovitz, D. M. (2020). A molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. Proceedings of the National Academy of Sciences USA, 117, 2122–2132. 10.1073/pnas.1915152117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaro, M., Smeriglio, A., Barreca, D., De Francesco, C., Occhiuto, C., Milano, G., & Trombetta, D. (2020). Antiviral activity of plants and their isolated bioactive compounds: An update. Phytotherapy Research, 34, 742–768. 10.1002/ptr.6575 [DOI] [PubMed] [Google Scholar]
- dos Santos, W. G. (2020). Natural history of COVID‐19 and current knowledge on treatment therapeutic options. Biomedicine & Pharmacotherapy, 129, 110493. 10.1016/j.biopha.2020.110493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrag, M. A., Hamed, M. E., Amer, H. M., & Almajhdi, F. N. (2019). Epidemiology of respiratory viruses in Saudi Arabia: Toward a complete picture. Archives of Virology, 164, 1981–1996. 10.1007/s00705-019-04300-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero, J., Corbeau, P., Nicolas, M., Benkirane, M., Travé, G., Dixon, J. F. P., Aucouturier, P., Rasheed, S., Parker, J. W., Liautard, J. P., Devaux, C., & Dornand, J. (1993). Inhibition of human immunodeficiency virus infection by the lectin jacalin and by a derived peptide showing a sequence similarity with gp120. European Journal of Immunology, 23, 179–185. 10.1002/eji.1830230128 [DOI] [PubMed] [Google Scholar]
- Filardo, S., Di Pietro, M., Mastromarino, P., & Sessa, R. (2020). Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacology & Therapeutics, 214, 107613. 10.1016/j.pharmthera.2020.107613 [DOI] [PubMed] [Google Scholar]
- Fouchier, R. A. M., Kuiken, T., Schutten, M., van Amerongen, G., van Doornum, G. J. J., van den Hoogen, B. G., Peiris, M., Lim, W., Stöhr, K., & Osterhaus, A. D. M. E. (2003). Koch’s postulates fulfilled for SARS virus. Nature, 423, 240. 10.1038/423240a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frabasile, S., Koishi, A. C., Kuczera, D., Silveira, G. F., Verri, W. A., Duarte dos Santos, C. N., & Bordignon, J. (2017). The citrus flavanone naringenin impairs dengue virus replication in human cells. Scientific Reports, 7, 1–10. 10.1038/srep41864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedif Meseret, A. (2020). Emerging and re‐emerging viral diseases: The case of coronavirus disease‐19 (COVID‐19). International Journal of Virology and AIDS, 7, 1–13. 10.23937/2469-567x/1510067 [DOI] [Google Scholar]
- Gengatharan, A., Dykes, G. A., & Choo, W. S. (2015). Betalains: Natural plant pigments with potential application in functional foods. LWT‐Food Science and Technology, 64, 645–649. 10.1016/j.lwt.2015.06.052 [DOI] [Google Scholar]
- Ghildiyal, R., Prakash, V., Chaudhary, V. K., Gupta, V., & Gabrani, R. (2020). Phytochemicals as antiviral agents: Recent updates. In Swamy M. (Ed.), Plant‐derived bioactives (pp. 279–295). Singapore: Springer. 10.1007/978-981-15-1761-7_12 [DOI] [Google Scholar]
- Goldwasser, J., Cohen, P. Y., Lin, W., Kitsberg, D., Balaguer, P., Polyak, S. J., Chung, R. T., Yarmush, M. L., & Nahmias, Y. (2011). Naringenin inhibits the assembly and long‐term production of infectious hepatitis C virus particles through a PPAR‐mediated mechanism. Journal of Hepatology, 55, 963–971. 10.1016/j.jhep.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, D., Lima, C., Ferreira, P., Costa, P., Costa, A., Figueiredo, W., & Cesar, T. (2017). Orange juice as dietary source of antioxidants for patients with hepatitis C under antiviral therapy. Food and Nutrition Research, 61(1), 1296675. 10.1080/16546628.2017.1296675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha, S., Ghosal, S., & Chattopadhyay, U. (1996). Antitumor, immunomodulatory and anti‐HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy, 42, 443–451. 10.1159/000239478 [DOI] [PubMed] [Google Scholar]
- Haidari, M., Ali, M., Ward Casscells, S., & Madjid, M. (2009). Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine, 16, 1127–1136. 10.1016/j.phymed.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Hernández‐Aquino, E., & Muriel, P. (2018). Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World Journal of Gastroenterology, 24, 1679–1707. 10.3748/wjg.v24.i16.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston, D. M. J., Bugert, J. J., Denyer, S. P., & Heard, C. M. (2017). Correction: Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against herpes simplex virus (HSV) when co‐administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir‐resistant HSV. PLoS One, 12, 1–15. 10.1371/journal.pone.0188609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H., Sun, F., Owen, D. M., Li, W., Chen, Y., Gale, M., & Ye, J. (2007). Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low‐density lipoproteins. Proceedings of the National Academy of Sciences, 104, 5848–5853. 10.1073/pnas.0700760104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, M. Y., Hashim, N. M., Mariod, A. A., Mohan, S., Abdulla, M. A., Abdelwahab, S. I., & Arbab, I. A. (2016). α‐Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arabian Journal of Chemistry, 9, 317–329. 10.1016/j.arabjc.2014.02.011 [DOI] [Google Scholar]
- Islam, M. T., Sarkar, C., El‐Kersh, D. M., Jamaddar, S., Uddin, S. J., Shilpi, J. A., & Mubarak, M. S. (2020). Natural products and their derivatives against coronavirus: A review of the non‐clinical and pre‐clinical data. Phytotherapy Research, 34(10), 2471–2492. 10.1002/ptr.6700 [DOI] [PubMed] [Google Scholar]
- Keivan, Z., Boon‐Teong, T., Sing‐Sin, S., Pooi‐Fong, W., Mohd, R. M., & Sazaly, A. (2014). In vitro antiviral activity of fisetin, rutin and naringenin against dengue virus type‐2. Journal of Medicinal Plants Research, 8, 307–312. 10.5897/jmpr11.1046 [DOI] [Google Scholar]
- Keyaerts, E., Vijgen, L., Pannecouque, C., Van Damme, E., Peumans, W., Egberink, H., Balzarini, J., & Van Ranst, M. (2007). Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Research, 75, 179–187. 10.1016/j.antiviral.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, K., Kohli, S. K., Kaur, R., Bhardwaj, A., Bhardwaj, V., Ohri, P., Sharma, A., Ahmad, A., Bhardwaj, R., & Ahmad, P. (2021). Herbal immune‐boosters: Substantial warriors of pandemic Covid‐19 battle. Phytomedicine, 85, 153361. 10.1016/j.phymed.2020.153361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaza, V., Oyedeji, O., & Aderibigbe, B. (2018). Antiviral activities of oleanolic acid and its analogues. Molecules, 23, 2300. 10.3390/molecules23092300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, A. P., Mahal, H. S., Kapoor, S., & Aradhya, S. M. (2007). In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. Journal of Agriculture and Food Chemistry, 55, 1491–1500. 10.1021/jf0626720 [DOI] [PubMed] [Google Scholar]
- Lammi, C., & Arnoldi, A. (2021). Food‐derived antioxidants and COVID‐19. Journal of Food Biochemistry, 45, 1–6. 10.1111/jfbc.13557 [DOI] [PubMed] [Google Scholar]
- Lee, M. H., Lee, B.‐H., Lee, S., & Choi, C. (2013). Reduction of hepatitis A virus on FRhK‐4 cells treated with Korean red ginseng extract and ginsenosides. Journal of Food Science, 78, M1412–M1415. 10.1111/1750-3841.12205 [DOI] [PubMed] [Google Scholar]
- Li, D., Zhang, T., Lu, J., Peng, C., & Lin, L. (2020). Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Critical Reviews in Food Science and Nutrition, 61(12), 1947–1965. 10.1080/10408398.2020.1768044 [DOI] [PubMed] [Google Scholar]
- Lin, L., Lu, L., Cao, W., & Li, T. (2020). Hypothesis for potential pathogenesis of SARS‐CoV‐2 infection–a review of immune changes in patients with viral pneumonia. Emerging Microbes & Infections, 9, 727–732. 10.1080/22221751.2020.1746199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, A.‐L., & Du, G.‐H. (2012). Antiviral properties of phytochemicals. In Patra A. K. (Ed.), Dietary phytochemicals and microbes (pp. 93–126). Springer. 10.1007/978-94-007-3926-0_3 [DOI] [Google Scholar]
- Liu, C., Cai, D., Zhang, L., Tang, W., Yan, R., Guo, H., & Chen, X. (2016). Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antiviral Research, 134, 97–107. 10.1016/j.antiviral.2016.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Zheng, X., Tong, Q., Li, W., Wang, B., Sutter, K., Trilling, M., Lu, M., Dittmer, U., & Yang, D. (2020). Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. Journal of Medical Virology, 92, 491–494. 10.1002/jmv.25709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R. H. (2013). Health‐promoting components of fruits and vegetables in the diet. Advances in Nutrition, 4, 384S–392S. 10.3945/an.112.003517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Varela, S., González‐Gross, M., & Marcos, A. (2002). Functional foods and the immune system: A review. European Journal of Clinical Nutrition, 56, S29–S33. 10.1038/sj.ejcn.1601481 [DOI] [PubMed] [Google Scholar]
- Luo, G. (George), & Gao, S. (2020). Global health concerns stirred by emerging viral infections. Journal of Medical Virology, 92, 399–400. 10.1002/jmv.25683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani, J. S., Johnson, J. B., Steel, J. C., Broszczak, D. A., Neilsen, P. M., Walsh, K. B., & Naiker, M. (2020). Natural product‐derived phytochemicals as potential agents against coronaviruses: A review. Virus Research, 284, 197989. 10.1016/j.virusres.2020.197989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazalovska, M., & Kouokam, J. C. (2018). Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. BioMed Research International, 2018, 1–12. 10.1155/2018/3750646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, C. A., Ramessar, K., & O’Keefe, B. R. (2017). Antiviral lectins: Selective inhibitors of viral entry. Antiviral Research, 142, 37–54. 10.1016/j.antiviral.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrityunjaya, M., Pavithra, V., Neelam, R., Janhavi, P., Halami, P. M., & Ravindra, P. V. (2020). Immune‐boosting, antioxidant and anti‐inflammatory food supplements targeting pathogenesis of COVID‐19. Frontiers in Immunology, 11, 1–12. 10.3389/fimmu.2020.570122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad, Y., Kani, Y. A., Iliya, S., Muhammad, J. B., Binji, A., El‐Fulaty Ahmad, A., Kabir, M. B., Umar Bindawa, K., & Ahmed, A. (2021). Deficiency of antioxidants and increased oxidative stress in COVID‐19 patients: A cross‐sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Medicine, 9, 205031212199124. 10.1177/2050312121991246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias, Y., Goldwasser, J., Casali, M., van Poll, D., Wakita, T., Chung, R. T., & Yarmush, M. L. (2008). Apolipoprotein B‐dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology, 47, 1437–1445. 10.1002/hep.22197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notka, F., Meier, G. R., & Wagner, R. (2003). Inhibition of wild‐type human immunodeficiency virus and reverse transcriptase inhibitor‐resistant variants by Phyllanthus amarus . Antiviral Research, 58, 175–186. 10.1016/S0166-3542(02)00213-9 [DOI] [PubMed] [Google Scholar]
- Palanisamy, U. D., Ling, L. T., Manaharan, T., & Appleton, D. (2011). Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti‐hyperglycemic activity. Food Chemistry, 127, 21–27. 10.1016/j.foodchem.2010.12.070 [DOI] [Google Scholar]
- Paredes, A., Alzuru, M., Mendez, J., & Rodríguez‐Ortega, M. (2003). Anti‐sindbis activity of flavanones hesperetin and naringenin. Biological &/and Pharmaceutical Bulletin, 26, 108–109. 10.1248/bpb.26.108 [DOI] [PubMed] [Google Scholar]
- Patel, B., Sharma, S., Nair, N., Majeed, J., Goyal, R. K., & Dhobi, M. (2021). Therapeutic opportunities of edible antiviral plants for COVID‐19. Molecular and Cellular Biochemistry, 476, 2345–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, P., Agrawal, M., Almelkar, S., Jeengar, M. K., More, A., Alagarasu, K., Kumar, N. V., Mainkar, P. S., Parashar, D., & Cherian, S. (2021). In vitro and in vivo studies reveal α‐mangostin, a xanthonoid from Garcinia mangostana, as a promising natural antiviral compound against chikungunya virus. Virology Journal, 18, 47. 10.1186/s12985-021-01517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajitha Lulu, S., Thabitha, A., Vino, S., Mohana Priya, A., & Rout, M. (2016). Naringenin and quercetin – potential anti‐HCV agents for NS2 protease targets. Natural Product Research, 30, 464–468. 10.1080/14786419.2015.1020490 [DOI] [PubMed] [Google Scholar]
- Sayed, A. M., Khattab, A. R., AboulMagd, A. M., Hassan, H. M., Rateb, M. E., Zaid, H., & Abdelmohsen, U. R. (2020). Nature as a treasure trove of potential anti‐SARS‐CoV drug leads: A structural/mechanistic rationale. RSC Advances, 10, 19790–19802. 10.1039/D0RA04199H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi, F., & Ambigaipalan, P. (2015). Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. Journal of Functional Foods, 18, 820–897. 10.1016/j.jff.2015.06.018 [DOI] [Google Scholar]
- Sinha, S. K., Prasad, S. K., Islam, M. A., Gurav, S. S., Patil, R. B., AlFaris, N. A., Aldayel, T. S., Alkehayez, N. M., Wabaidur, S. M., & Shakya, A. (2020). Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS‐CoV‐2 spike glycoprotein and non‐structural protein‐15: A pharmacoinformatics study. Journal of Biomolecular Structure & Dynamics, 1–15. 10.1080/07391102.2020.1779132. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyanto, Z., Yohan, B., Hadisaputro, S., Dharmana, E., Suharti, C., Djamiatun, K., Rahmi, F. L., & Sasmono, R. T. (2019). Inhibitory effect of alpha‐mangostin to dengue virus replication and cytokines expression in human peripheral blood mononuclear cells. Natural Products and Bioprospecting, 9, 345–349. 10.1007/s13659-019-00218-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J., Chu, Y.‐F., Wu, X., & Liu, R. H. (2002). Antioxidant and antiproliferative activities of common fruits. Journal of Agriculture and Food Chemistry, 50, 7449–7454. 10.1021/jf0207530 [DOI] [PubMed] [Google Scholar]
- Swanson, M. D., Boudreaux, D. M., Salmon, L., Chugh, J., Winter, H. C., Meagher, J. L., André, S., Murphy, P. V., Oscarson, S., Roy, R., King, S., Kaplan, M. H., Goldstein, I. J., Tarbet, E. B., Hurst, B. L., Smee, D. F., de la Fuente, C., Hoffmann, H.‐H., Xue, Y. I., … Markovitz, D. M. (2015). Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell, 163, 746–758. 10.1016/j.cell.2015.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, M. D., Winter, H. C., Goldstein, I. J., & Markovitz, D. M. (2010). A lectin isolated from bananas is a potent inhibitor of HIV replication. Journal of Biological Chemistry, 285, 8646–8655. 10.1074/jbc.M109.034926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasuk, M., Songprakhon, P., Chimma, P., Sratongno, P., Na‐Bangchang, K., & Yenchitsomanus, P.‐T. (2017). Alpha‐mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Research, 240, 180–189. 10.1016/j.virusres.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Tutunchi, H., Naeini, F., Ostadrahimi, A., & Hosseinzadeh‐Attar, M. J. (2020). Naringenin, a flavanone with antiviral and anti‐inflammatory effects: A promising treatment strategy against COVID‐19. Phytotherapy Research, 34(12), 3137–3147. 10.1002/ptr.6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaykrishna, D., Poon, L. L. M., Zhu, H. C., Ma, S. K., Li, O. T. W., Cheung, C. L., Smith, G. J. D., Peiris, J. S. M., & Guan, Y. (2010). Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science (80‐), 328, 1529–1529. 10.1126/science.1189132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls, A. C., Park, Y.‐J., Tortorici, M. A., Wall, A., McGuire, A. T., & Veesler, D. (2020). Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell, 181, 281–292.e6. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R.‐R., Gao, Y.‐D., Ma, C.‐H., Zhang, X.‐J., Huang, C.‐G., Huang, J.‐F., & Zheng, Y.‐T. (2011). Mangiferin, an anti‐HIV‐1 agent targeting protease and effective against resistant strains. Molecules, 16, 4264–4277. 10.3390/molecules16054264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetprasit, N., Threesangsri, W., Klamklai, N., & Chulavatnatol, M. (2000). Jackfruit lectin: Properties of mitogenicity and the inhibition of herpesvirus infection. Japanese Journal of Infectious Diseases, 53, 156–161. [PubMed] [Google Scholar]
- Wilder‐Smith, A., Chiew, C. J., & Lee, V. J. (2020). Can we contain the COVID‐19 outbreak with the same measures as for SARS? The Lancet Infectious Diseases, 20, e102–e107. 10.1016/S1473-3099(20)30129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, L. G., & Gibson, P. G. (2009). Dietary factors lead to innate immune activation in asthma. Pharmacology & Therapeutics, 123, 37–53. 10.1016/j.pharmthera.2009.03.015 [DOI] [PubMed] [Google Scholar]
- Wu, Y.‐H., Tseng, C.‐K., Wu, H.‐C., Wei, C.‐K., Lin, C.‐K., Chen, I.‐S., Chang, H.‐S., & Lee, J.‐C. (2019). Avocado (Persea americana) fruit extract (2R,4R)‐1,2,4‐trihydroxyheptadec‐16‐yne inhibits dengue virus replication via upregulation of NF‐κB–dependent induction of antiviral interferon responses. Scientific Reports, 9, 1–10. 10.1038/s41598-018-36714-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian, Y., Zhang, J., Bian, Z., Zhou, H., Zhang, Z., Lin, Z., & Xu, H. (2020). Bioactive natural compounds against human coronaviruses: A review and perspective. Acta Pharmaceutica Sinica B, 10, 1163–1174. 10.1016/j.apsb.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C.‐M., Cheng, H.‐Y., Lin, T.‐C., Chiang, L.‐C., Lin, C.‐C. (2007). The in vitro activity of geraniin and 1,3,4,6‐tetra‐O‐galloyl‐β‐d‐glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. Journal of Ethnopharmacology, 110(3), 555–558. 10.1016/j.jep.2006.09.039 [DOI] [PubMed] [Google Scholar]
- Yang, P., & Wang, X. (2020). COVID‐19: A new challenge for human beings. Cellular & Molecular Immunology, 17, 555–557. 10.1038/s41423-020-0407-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Xiu, J., Zhang, L., Qin, C., & Liu, J. (2012). Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Phytomedicine, 20, 67–70. 10.1016/j.phymed.2012.08.012 [DOI] [PubMed] [Google Scholar]
- Yang, Y., Zhang, L., Fan, X., Qin, C., & Liu, J. (2012). Antiviral effect of geraniin on human enterovirus 71 in vitro and in vivo. Bioorganic & Medicinal Chemistry Letters, 22, 2209–2211. 10.1016/j.bmcl.2012.01.102 [DOI] [PubMed] [Google Scholar]
- Yingsakmongkon, S., Miyamoto, D., Sriwilaijaroen, N., Fujita, K., Matsumoto, K., Jampangern, W., Hiramatsu, H., Guo, C.‐T., Sawada, T., Takahashi, T., Hidari, K., Suzuki, T., Ito, M., Ito, Y., & Suzuki, Y. (2008). In vitro inhibition of human influenza A virus infection by fruit‐juice concentrate of Japanese plum (Prunus mume Sieb. et Zucc). Biological and Pharmaceutical Bulletin, 31, 511–515. 10.1248/bpb.31.511 [DOI] [PubMed] [Google Scholar]
- Yu, X.‐J., Liang, M.‐F., Zhang, S.‐Y., Liu, Y., Li, J.‐D., Sun, Y.‐L., Zhang, L., Zhang, Q.‐F., Popov, V. L., Li, C., Qu, J., Li, Q., Zhang, Y.‐P., Hai, R., Wu, W., Wang, Q., Zhan, F.‐X., Wang, X.‐J., Kan, B., … Li, D.‐X. (2011). Fever with thrombocytopenia associated with a novel bunyavirus in China. New England Journal of Medicine, 364, 1523–1532. 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedipour, F., Hosseini, S. A., Sathyapalan, T., Majeed, M., Jamialahmadi, T., Al‐Rasadi, K., Banach, M., & Sahebkar, A. (2020). Potential effects of curcumin in the treatment of COVID‐19 infection. Phytotherapy Research, 34(11), 2911–2920. 10.1002/ptr.6738. ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendrini Rechenchoski, D., Faccin‐Galhardi, L. C., Pacheco Cunha, A., Pontes Silva Ricardo, N. M., Nozawa, C., & Carvalho Linhares, R. E. (2018). Antiviral potential of mangiferin against poliovirus. International Journal of Pharmacological Research, 8, 34. 10.7439/ijpr.v8i4.4706 [DOI] [Google Scholar]
- Zheng, M. S., & Lu, Z. Y. (1990). Antiviral effect of mangiferin and isomangiferin on herpes simplex virus. Chinese Medical Journal (England), 103, 160–165. [PubMed] [Google Scholar]
- Zhu, X. M., Song, J. X., Huang, Z. Z., Wu, Y. M., & Yu, M. J. (1993). Antiviral activity of mangiferin against herpes simplex virus type 2 in vitro. Zhongguo Yao Li Xue Bao, 14, 452–454. [PubMed] [Google Scholar]
- Zumla, A., Hui, D. S., & Perlman, S. (2015). Middle East respiratory syndrome. The Lancet (London, England), 386, 995–1007. 10.1016/S0140-6736(15)60454-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi, K., Teoh, B.‐T., Sam, S.‐S., Wong, P.‐F., Mustafa, M., & Abu Bakar, S. (2011). In vitro antiviral activity of fisetin, rutin and naringenin against dengue virus type‐2. Journal of Medicinal Plants Research, 5, 5534–5539. 10.5897/JMPR11.1046 [DOI] [Google Scholar]


