Abstract
COVID‐19 infection, affecting every one of us from the last year. Emerging reports have indicated thromboembolism in serious cases of COVID‐19. The aspirin is useful to reduce mortality of serious patients with acute respiratory distress syndrome without COVID‐19. Thus, we have conducted a metanalysis to find out the role of aspirin in the mortality of COVID‐19 patients using RevMan 5. A total of 10 studies containing 56 696 COVID‐19 patients were found appropriate for quantitative analysis. The quality of articles was assessed using Newcastle–Ottawa scale. The fixed‐effect model was used to calculate the odds ratio with 95% confidence interval (CI). The odd ratio was found to be 0.70 [0.63, 0.77] which indicates a lesser likelihood of having death in COVID‐19 patients in aspirin group as compared with non‐aspirin group. However, no effect 0.00 [−0.04, 0.04] was observed after the exclusion of outliers. Thus, further clinical evidence is required to make valid conclusion.
What's known?
Thromboembolism is one of the major reason for deaths of serious COVID‐19 patients.
Aspirin is one of the well‐known antiplatelet drugs.
It mainly inhibits the COX that ultimately results in the inhibition of thromboxane A2 that is responsible for thrombo‐inflammation and thrombosis in patients.
Its already known that aspirin is useful to reduce mortality of serious patients with acute respiratory distress syndrome without COVID‐19.
What's new?
The present study was conducted to find out the role of aspirin in the reduction of mortality of COVID‐19 patients
The results of the current study indicates a lesser likelihood of having death in COVID‐19 patients in aspirin group as compared with non‐aspirin group.
1. INTRODUCTION
Recently, a novel coronavirus emerged in China and rapidly spread across the globe. The World Health Organization (WHO) has already declared it as pandemic, and currently, cases are still increasing significantly. Studies have also shown mutation of severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2), and its spread is much more contagious as compared with the earlier strain. The treatment of COVID‐19 infection is symptomatic. Currently, various classes of drugs such as antimalarial, antibiotics, anti‐inflammatory, antiviral, antibodies, steroids, anticoagulants, and so forth are being repurposed for its prophylaxis and treatment depending upon the condition of the individual patient. 1
Emerging studies have indicated the thromboembolic events in high‐risk individuals suffering from COVID‐19 infection particularly in the last stages. 2 , 3 , 4 Laboratory findings have shown the increased level of fibrinogen, C‐reactive protein (CRP), and fibrin d‐dimer in these kinds of patients which finally result in poor outcomes. 5 , 6 The meta‐analysis of clinical studies has also shown a high incidence of venous thromboembolism in COVID‐19 patients. 7 It has been observed that coagulation is the main contributor to the death of patients due to respiratory failure. Studies have also indicated the benefit of using anticoagulants in high‐risk COVID‐19 patients. McBane et al 8 have indicated the role of anticoagulation in COVID‐19 patients. The mortality in severe COVID‐19 patients was decreased after anticoagulant treatment. 9
Aspirin is one of the well‐known antiplatelet drugs, which shows antiplatelet action through inhibition of cyclooxygenase (COX) enzyme that is responsible for activation of thromboxane. Literature has shown the association of aspirin in the reduction of deaths of non‐COVID serious patients suffering from acute respiratory distress syndrome. 10 , 11 Clinical observational studies have also shown reduction of the mortality of COVID‐19 patients in the aspirin group as compared with the non‐aspirin group. To the best of our knowledge, only one meta‐analysis of three observational studies was done so far on this topic, and results are inconclusive. 12 Thus, we have conducted a meta‐analysis of available observational studies to find out the association between uses of aspirin with the mortality of COVID‐19 patients.
2. MATERIAL AND METHODS
The articles published in English were searched in PubMed from inception until March 2021. The search terms include the following: “(COVID19 OR COVID‐19 virus infection OR COVID‐19 infection OR 2019 novel corona virus infection OR 2019‐nCoV infection OR 2019‐nCoV disease AND aspirin or acetylsalicylic acid or antiplatelet)”. The PRISMA guideline was followed to conduct this study. 13
2.1. Inclusion and exclusion criteria
The studies were included as per the inclusion and exclusion criteria. The inclusion criteria include observational studies, COVID‐19 patients, age above 18 years, and use of aspirin alone or in combinations, and the primary outcome was death. The reviews, systematic reviews, meta‐analysis, case reports, animal studies, and the outcome where no death was reported were excluded.
2.2. Quality assessment
RS and AK separately have done a quality assessment of all included studies using the Newcastle–Ottawa scale (NOS). 14 The NOS is subdivided into three major headings: selection, comparability, and outcome. Each subheading has items that are as follows: four for selection, three items for the outcome, and two items for comparability subheadings. Based on NOS, studies can be categorized as: Good, Fair, and Poor quality according to the item points.
2.3. Data extraction
The data were extracted from 10 studies. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 The extracted data were included in an excel sheet that contains the columns like authors' first names, year of study, type of study, sex, the total number of subjects (non‐aspirin), the number of subjects survived (non‐aspirin), the total number of subjects (aspirin), and the number of subjects survived (aspirin) as mentioned in Table 1.
TABLE 1.
Characteristics of included studies
| References | Country | Study design | Sample size | Sex | Aspirin group | Non‐aspirin group | |||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Number of Patients | Death | Number of Patients | Death | ||||
| Chow et al 15 | USA | Retrospective | 412 | 244 | NR | 98 | 26 | 314 | 73 |
| Osborne et al 16 | USA | Retrospective | 54 696 | 48 763 | NR | 13 166 | 445 | 41 530 | 2042 |
| Liu et al 17 | China | Retrospective | 232 | 117 | NR | 28 | 3 | 204 | 20 |
| Yuan et al 19 | China | Retrospective | 183 | 99 | 84 | 52 | 11 | 131 | 29 |
| Merzon et al 21 | Israel | Retrospective | 112 | 62 | NR | 21 | 1 | 91 | 6 |
| Alamdari et al 23 | Iran | Retrospective | 459 | 320 | 139 | 53 | 9 | 406 | 54 |
| Kevorkian et al 20 | France | Cohort | 68 | 53 | NR | 28 | 0 | 40 | 2 |
| Lodigiani et al 22 | Italy | Retrospective | 28 | 17 | 11 | 6 | 2 | 22 | 5 |
| Sahai et al 24 | USA | Retrospective | 496 | NR | NR | 248 | 33 | 248 | 38 |
| Viecca et al 18 | Italy | Retrospective | 10 | 8 | 2 | 5 | 1 | 5 | 3 |
Abbreviation: NR, not reported.
2.4. Data analysis
RevMan 5 was used for the analysis of the data. Mantel–Haenszel odds ratio (OR) with its 95% confidence intervals was calculated. The fixed‐effects model was used due to less variation among studies with respect to study design, study population, and so forth. The P value < .1 was considered as significant heterogeneity among studies. The heterogenicity among studies was also estimated using the χ 2 test and the I 2 statistic. The I 2 values of 25% considered low, 50% as moderate, and 75% as high heterogenicity. The funnel plot was created for the qualitative assessment of publication bias.
3. RESULTS
3.1. Search results
The initial search strategy identified 7803 studies, from inception until March 2021. One duplicate was removed. The 7717 articles were excluded based on titles. Further, 33 articles were excluded based on abstracts. Finally, the full text of 52 articles was downloaded, and 42 articles were excluded based on irrelevant information as shown in Figure 1. Finally, 10 articles were included in a quantitative synthesis that involved 56 696 COVID‐19 patients from 10 observational studies. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 The one article among these 10 articles was preprint. 24
FIGURE 1.
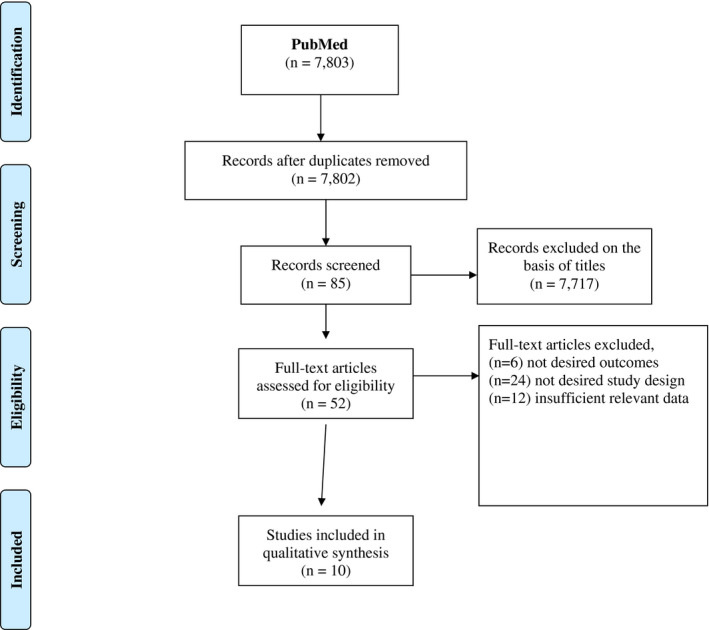
Selection of studies as per the PRISMA Checklist
3.1.1. Study characteristics
Out of 10 studies, three were conducted in the United States, two each in China and Italy, and one each in Israel, Iran, and France. All studies are retrospective except Kevorkian et al 20 that is a prospective observational study. The study characteristics were summarized in Table 1.
3.1.2. Quality assessment
The quality assessment results have shown seven studies were of good quality, and the remaining three were fair, as shown in Table 2.
TABLE 2.
Quality assessment using the Newcastle–Ottawa scale
| References | Selection | Comparability | Outcome | Total Score | Quality of the study |
|---|---|---|---|---|---|
| Chow et al 15 | *** | * | *** | 7 | Good |
| Osborne et al 16 | *** | * | *** | 7 | Good |
| Liu et al 17 | *** | * | *** | 7 | Good |
| Yuan et al 19 | ** | ** | *** | 7 | Fair |
| Merzon et al 21 | *** | * | *** | 7 | Good |
| Alamdari et al 23 | *** | * | *** | 7 | Good |
| Kevorkian et al 20 | *** | * | *** | 7 | Good |
| Lodigiani et al 22 | ** | ** | *** | 7 | Fair |
| Sahai et al 24 | *** | * | *** | 7 | Good |
| Viecca et al 18 | ** | ** | ** | 6 | Fair |
* indicates one criteria was followed, ** two criteria were followed and ***three criteria were followed.
3.2. Aspirin use and mortality of COVID‐19 patients
Ten studies were included, with a total of 56 696 COVID‐19 cases. A total of 13 705 patients were on aspirin, whereas 42 991 patients were in the non‐aspirin group. The fixed‐effect model was used to check the association of aspirin with mortality in COVID‐19 patients. The pooled OR was found to be 0.71 [0.64, 0.78] as shown in Figure 2, which indicates a lesser likelihood of having death in COVID‐19 patients in the aspirin group as compared with the non‐aspirin group.
FIGURE 2.
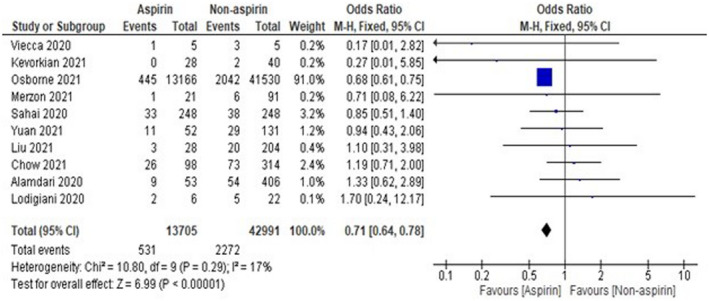
Pooled analysis results using a fixed‐effect model (forest plot)
The sample size of Osborne et al 16 was observed to be high as compared with other included studies, which might affect the results. Thus, analysis was done again after removal of Osborne et al, 16 and the results were found to be 1.00 [0.75‐1.33], which indicates a non‐significant reduction in mortality of COVID‐19 patients in the aspirin group as compared with the non‐aspirin group as shown in Figure 3. On the other hand, the sample size of two studies, that is, Viecca et al 18 and Lodigiani et al, 22 was found to be very low as compared with other included studies. The pooled OR was found to be 0.00 [−0.00, 0.04] after the exclusion of Viecca et al, 18 which indicates no effect of aspirin in reduction of mortality as compared with the non‐aspirin group (Figure 4). The exclusion of Lodigiani et al 22 has also shown a non‐significant 0.99 [0.74, 1.32] reduction of mortality as shown in Figure 5. We have also done the analysis after the removal of Osborne et al, 16 Viecca et al, 18 and Lodigiani et al 22 and found no effect of aspirin in the reduction of mortality of COVID‐19 patients as compared with the non‐aspirin group (Figure 6). The I 2 statistics have shown no heterogeneity among studies after the removal of outliers.
FIGURE 3.
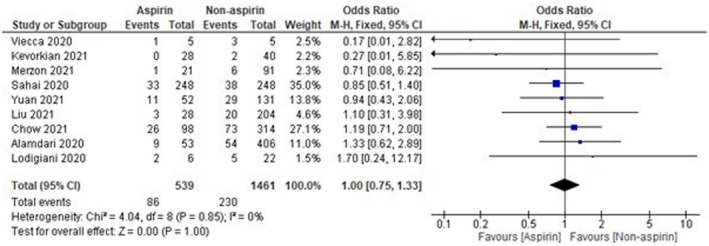
Pooled analysis results using a fixed‐effect model (forest plot) after exclusion of Osborne et al 16
FIGURE 4.
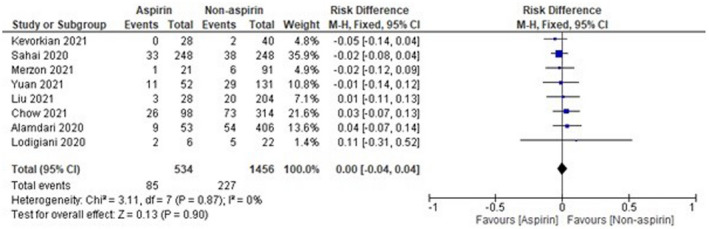
Pooled analysis results using a fixed‐effect model (forest plot) after exclusion of Viecca et al 18
FIGURE 5.
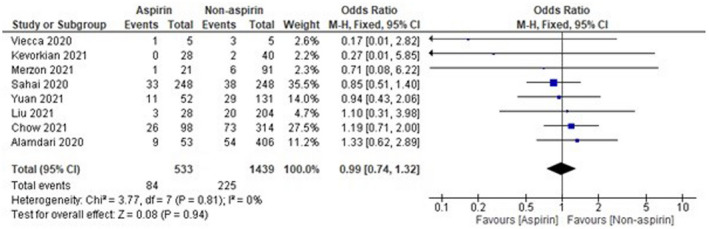
Pooled analysis results using a fixed‐effect model (forest plot) after exclusion of Lodigiani et al 22
FIGURE 6.
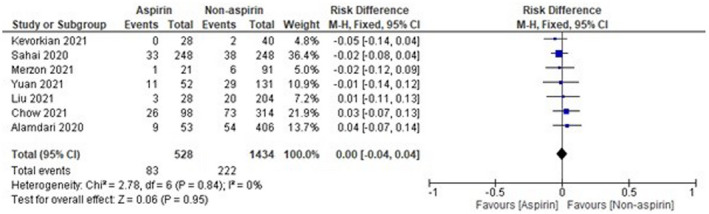
Pooled analysis results using a fixed‐effect model (forest plot) after exclusion of Osborne et al, 16 Viecca et al, 18 and Lodigiani et al 22
3.3. Publication bias assessment
The funnel plot was used for the qualitative assessment of publication bias. The shape of the plot was found to be symmetrical (Figure 7), which indicates minimum publication bias.
FIGURE 7.
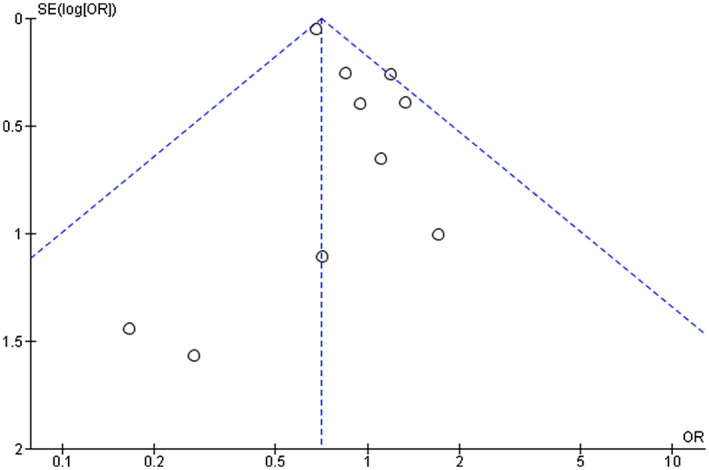
Funnel plot for the assessment of publication bias
4. DISCUSSION
COVID‐19 is already declared by WHO as a pandemic situation, and our main objective today is to save maximum lives from this infection. Studies have shown thromboembolism in serious COVID‐19 patients and a major reason for the deaths of patients. 25 , 26 Thus, regulatory authorities across the globe recommending the use of anticoagulants in serious cases of COVID‐19. 27 , 28 , 29 Aspirin is one of the well‐known antiplatelet drugs, which inhibits platelet aggregation results from the activation of arachidonic acid (AA) pathways. It mainly inhibits the COX that ultimately results in the inhibition of thromboxane A2 that is responsible for thrombo‐inflammation and thrombosis in patients. The low dose (70‐80 mg/day) is sufficient to block COX. The main reason for mortality of COVID‐19 patients is thromboembolism. Thus, the present study was conducted to find out the role of aspirin in the reduction of mortality of COVID‐19 patients. The observational studies have indicated the benefit of aspirin in the reduction of mortality of COVID‐19 patients. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Salah and Mehta 12 have done a metanalysis to find out the role of aspirin in patients with COVID‐19. However, only three relevant studies are available at that time, and results are inconclusive. The results of the present study have done the metanalysis of 10 observational studies and found a lesser likelihood of having death in COVID‐19 patients in the aspirin group as compared with the non‐aspirin group. The heterogeneity among studies was also non‐significant. However, results have shown a non‐significant reduction in mortality after the removal of Osborne et al 16 (large sample size), Viecca et al, 18 and Lodigiani et al 22 (small sample size). Thus, further data are required to make a valid conclusion regarding the use of aspirin in the reduction of mortality of COVID‐19 patients.
4.1. Limitation
We have only searched PubMed, Google Scholar, Clinial trials databases for relevant articles. Further, the subgroup analysis was not done due to a limited number of studies.
5. CONCLUSION
Aspirin is useful in the reduction of mortality of COVID‐19 patients. However, further evidence is required to make a valid conclusion.
DISCLOSURES
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Ritika Srivastava extracted the data and prepared the first draft of the manuscript. Anoop Kumar cross‐check the data, analysed, and prepared the final draft of the manuscript.
ACKNOWLEDGMENTS
The authors are thankful to Vice‐Chancellor, Professor (Dr) R.K. Goyal, Delhi Pharmaceutical Sciences & Research University (DPSRU), New Delhi 110017, India, for his continuous support, motivation, and providing necessary facilities to carry out this work.
Srivastava R, Kumar A. Use of aspirin in reduction of mortality of COVID‐19 patients: A metanalysis. Int J Clin Pract. 2021;75:e14515. 10.1111/ijcp.14515
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in tables of the manuscript.
REFERENCES
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 2. Bozzani A, Arici V, Tavazzi G, et al. Acute arterial and deep venous thromboembolism in COVID‐19 patients: risk factors and personalized therapy. Surgery. 2020;168:987‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porfidia A, Pola R. Venous thromboembolism in COVID‐19 patients. J Thromb Haemost. 2020;18:1516‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dobesh PP, Trujillo TC. Coagulopathy, venous thromboembolism, and anticoagulation in patients with COVID‐19. Pharmacotherapy. 2020;40:1130‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95:834‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med. 2020;58:1116‐1120. [DOI] [PubMed] [Google Scholar]
- 7. Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID‐19 and venous thromboembolism: a meta‐analysis of literature studies. Semin Thromb Hemost. 2020;46:763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McBane II, Robert D, Torres Victor D, et al. Anticoagulation in COVID‐19: a systematic review, meta‐analysis and rapid guidance from the Mayo clinic. In: Mayo Clinic Proceedings. Elsevier, 2020;95:2467‐2486. 10.1016/j.mayocp.2020.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Du F, Jiang P, He S, Song D, Xu F. Antiplatelet therapy for critically ill patients: a pairwise and Bayesian network meta‐analysis. Shock. 2018;49:616‐624. 10.1097/SHK.0000000000001057 [DOI] [PubMed] [Google Scholar]
- 11. Wang L, Li H, Gu X, Wang Z, Liu S, Chen L. Effect of antiplatelet therapy on acute respiratory distress syndrome and mortality in critically ill patients: a meta‐analysis. PLoS One. 2016;11:e0154754. 10.1371/journal.pone.0154754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salah HM, Mehta JL. Meta‐analysis of the effect of aspirin on mortality in COVID‐19. Am J Cardiol. 2021;142:158‐159. 10.1016/j.amjcard.2020.12.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 2, 2021.
- 15. Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in‐hospital mortality in hospitalized patients with coronavirus disease 2019. Anest Analg. 2021;132:930‐941. 10.1213/ANE.0000000000005292 [DOI] [PubMed] [Google Scholar]
- 16. Osborne TF, Veigulis ZP, Arreola DM, Mahajan SM, Röösli E, Curtin CM. Association of mortality and aspirin prescription for COVID‐19 patients at the Veterans Health Administration. PLoS One. 2021;16:e0246825. 10.1371/journal.pone.0246825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Q, Huang N, Li A, et al. Effect of low‐dose aspirin on mortality and viral duration of the hospitalized adults with COVID‐19. Medicine. 2021;100:e24544. 10.1097/MD.0000000000024544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viecca M, Radovanovic D, Forleo GB, Santus P. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid‐19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res. 2020;158:104950. 10.1016/j.phrs.2020.104950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan S, Chen P, Li H, Chen C, Wang F, Wang DW. Mortality and pre‐hospitalization use of low‐dose aspirin in COVID‐19 patients with coronary artery disease. J Cell Mol Med. 2021;25:1263‐1273. 10.1111/jcmm.16198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kevorkian JP, Lopes A, Sène D, et al. Oral corticoid, aspirin, anticoagulant, colchicine, and furosemide to improve the outcome of hospitalized COVID‐19 patients—the COCAA‐COLA cohort study. J Infect. 2021;82:276‐316. 10.1016/j.jinf.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merzon E, Green I, Vinker S, et al. The use of aspirin for primary prevention of cardiovascular disease is associated with a lower likelihood of COVID‐19 infection. The FEBS Journal. 2021;288:5179‐5189. 10.1111/febs.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9‐14. 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alamdari NM, Afaghi S, Rahimi FS, et al. Mortality risk factors among hospitalized COVID‐19 patients in a major referral center in Iran. Tohoku J Exp Med. 2020;252:73‐84. 10.1620/tjem.252.73 [DOI] [PubMed] [Google Scholar]
- 24. Sahai A, Bhandari R, Koupenova M, et al. SARS‐CoV‐2 receptors are expressed on human platelets and the effect of aspirin on clinical outcomes in COVID‐19 patients. Res Sq [Preprint]. 2020. Dec 23:rs.3.rs‐119031. 10.21203/rs.3.rs-119031/v1. [DOI]
- 25. Ornelas‐Ricardo D, Jaloma‐Cruz AR. Coronavirus disease 2019: hematological anomalies and antithrombotic therapy. Tohoku J Exp Med. 2020;251:327‐336. 10.1620/tjem.251.327 [DOI] [PubMed] [Google Scholar]
- 26. Mahajan P, Dass B, Radhakrishnan N, McCullough PA. COVID‐19‐associated systemic thromboembolism: a case report and review of the literature. Cardiorenal Med. 2020;10:462‐469. 10.1159/000511800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Indian Council of Medical Research (ICMR) . Clinical Guidance for Management of COVID‐19 Patients. https://www.icmr.gov.in/pdf/covid/techdoc/COVID19_Management_Algorithm_22042021_v1.pdf. Accessed April 19, 2021. [Google Scholar]
- 28. World Health Organization . WHO Recommends Follow‐up Care, Low‐Dose Anticoagulants for COVID‐19 Patients. https://www.who.int/news‐room/feature‐stories/detail/who‐recommends‐follow‐up‐care‐low‐dose‐anticoagulants‐for‐covid‐19‐patients. Accessed April 20, 2021. [Google Scholar]
- 29. National Institute of Health (NIH) . COVID‐19 Treatment Guidelines. Updated on February 11, 2021. https://www.covid19treatmentguidelines.nih.gov/antithrombotic‐therapy/. Accessed April 20, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in tables of the manuscript.


