Summary
Strict isolation of vulnerable individuals has been a strategy implemented by authorities to protect people from COVID‐19. Our objective was to investigate health‐related quality of life (HRQoL), uncertainty and coping behaviours in solid organ transplant (SOT) recipients during the COVID‐19 pandemic. A cross‐sectional survey of adult SOT recipients undergoing follow‐up at our institution was performed. Perceived health status, uncertainty and coping strategies were assessed using the EQ‐5D‐5L, Short‐form Mishel Uncertainty in Illness Scale (SF‐MUIS) and Brief Cope, respectively. Interactions with COVID‐19 risk perception, access to health care, demographic and clinical variables were assessed. The survey was completed by 826 of 3839 (21.5%) invited participants. Overall, low levels of uncertainty in illness were reported, and acceptance was the major coping strategy (92%). Coping by acceptance, feeling protected, self‐perceived susceptibility to COVID‐19 were associated with lower levels of uncertainty. Health status index scores were significantly lower for those with mental health illness, compromised access to health care, a perceived high risk of severe COVID‐19 infection and higher levels of uncertainty. A history of mental health illness, risk perceptions, restricted healthcare access, uncertainty and coping strategies was associated with poorer HRQoL in SOT recipients during strict isolation. These findings may allow identification of strategies to improve HRQoL in SOT recipients during the pandemic.
Keywords: COVID‐19, health‐related quality of life, isolation, mental health, shielding, transplant
Solid organ transplant recipients are required to take additional precautions to protect themselves from COVID‐19. Perceived health status was significantly lower for those with mental health illness, compromised access to care and a self‐perceived risk of severe COVID‐19. Transplant centres should consider this in their service delivery.

Introduction
Due to the highly contagious nature of COVID‐19, governments have implemented various strategies promoting self‐isolation and social distancing of the general population to mitigate viral spread [1, 2, 3]. The impact of strictly isolating vulnerable individuals during the pandemic to prevent infection with COVID‐19 is unknown at present; however, the well‐being of vulnerable individuals may be at risk during long periods of strict isolation [2, 4, 5].
In March 2020, Public Health England introduced a ‘shielding’ policy, designed to protect individuals deemed clinically extremely vulnerable, such as oncology patients, individuals with respiratory disease or patients taking immunosuppressive medications [6]. The ‘shielding’ guidance advised clinically extremely vulnerable individuals to stay at home at all times between 23 March and 31 July 2020 [7]. Additionally, shielded individuals were asked to stay 2 m away from others as much as possible, even from household members. With the exception of emergencies, they could not physically attend appointments with healthcare providers. Due to the unprecedented nature of the pandemic, these expedient decisions were based on epidemiological principles rather than established evidence.
Evidence from the Middle East respiratory syndrome coronavirus (MERS‐CoV) outbreak previously identified immunosuppression as a risk factor for severe infection and death [8]. The early accounts of COVID‐19 risk in immunosuppressed solid organ transplant (SOT) patients were conflicting. While a protective effect of immunosuppression against the sequalae of the cytokine storm associated with severe COVID‐19 was proposed, others suggested an increased risk of severe COVID‐19 [9, 10, 11, 12]. In agreement with the latter, an increased mortality rate following symptomatic COVID‐19 has been demonstrated in renal transplant recipients, in comparison with patients with renal disease awaiting transplant [13]. However, a multicentre study demonstrated that the severity of illness with COVID‐19 in SOT recipients was related to age and other comorbidities [14]. In addition to the great deal of uncertainty concerning risk of infection and severity of illness, these individuals may also experience anxiety regarding their specific healthcare needs not being met due to restricted access to health care during shielding. Increased anxiety has been demonstrated in other patient populations that were also required to undergo shielding [15, 16]. In addition, Smith et al. [17] demonstrated worsening mental health in patients with asthma undergoing shielding during the pandemic, and individuals with a history of anxiety or depression were more vulnerable. Our study was designed in response to rapidly defined public health research priorities for people experiencing severe distress during the COVID‐19 pandemic [18, 19].
We hypothesized that SOT recipients would have a higher level of self‐perceived risk for severe COVID‐19, leading to greater levels of uncertainty in illness and worse health‐related quality of life (HRQoL). In addition, we hypothesized that social isolation from shielding would lead to higher levels of anxiety and depression and consequently poorer HRQoL. Our aim was to investigate HRQoL, uncertainty and coping behaviours in solid organ transplant (SOT) recipients during the COVID‐19 pandemic, and identify deleterious and advantageous coping strategies used in this population. This will enable identification of at‐risk groups for potential harm during shielding for this or future pandemics, allowing targeted interventions to support these individuals.
Patients and methods
Study design and setting
A cross‐sectional survey of all SOT recipients being managed at a tertiary level transplant centre in the Midlands region of the UK, servicing a population of 10 235 000 people. Patient‐reported outcomes (PROs) were the primary outcome of interest. To optimize the quality of reporting, the SPIRIT‐PRO Extension guidelines were adhered to and reported where possible [20]. The study protocol was approved by the Human Research Ethics Committee (20/HRA/2613) and data acquisition approved by our institution (CARMS‐16123).
Participants
All adult (≥18 years) liver, renal, heart and lung transplant recipients identified through departmental electronic databases that were alive and undergoing follow‐up at our transplant centre in June 2020 were eligible for this study. A sample size calculation was not performed as the entire target population was approached.
Data collection
All eligible transplant recipients were invited to participate via a postal invitation letter at the beginning of July 2020. The invitation included the participant information sheet (PIS), details of a uniform resource locator and participant‐specific login details. This ensured only one online survey could be completed per recipient via the Research Electronic Data Capture (REDCap®) program [21]. A paper‐based version of the consent and survey was provided upon request. Only English language versions of the study survey, including PRO tools, were provided, and the use of a proxy to complete or translate the survey was permitted. The survey remained open for a 28‐day period until the 31 July 2020, which coincided with the last day of the recommended shielding period by Public Health England.
Demographics and clinical data
Demographic, transplant and health characteristics were self‐reported by patients. COVID‐19 infection was defined as reporting a positive COVID RT‐PCR test and suspected COVID‐19 infection (in the absence of a negative or positive test) as a self‐reported illness with the presence of two or more of the following COVID‐19 key symptoms; temperature, persistent cough and anosmia. Self‐reported illness consistent with COVID‐19 was included in our case definition due to lack of routine testing of individuals with mild symptoms.
Both non‐white ethnicity and a lower socio‐economic status have been extensively reported in the scientific literature and media as being associated with poorer COVID‐19 outcomes, and therefore, this group may have additional levels of concern or uncertainty [22, 23]. Data were collected on participants’ self‐reported ethnicity, and the Index of Multiple Deprivation (IMD) was used to assess socio‐economic status. The IMD quintile values, a measure of relative deprivation at small local area level, were calculated from each recipient's residential postcode using the English Indices of Deprivation 2019 [24].
PRO measures
A single measurement with relevant PROs was performed with reference to the period of shielding (March–July 2020). The primary outcome measure was HRQoL using the EQ‐5D‐5L questionnaire [25]. Secondary outcomes comprised levels of uncertainty and coping strategies, measured with the Short‐form Mishel Uncertainty in Illness Scale (SF‐MUIS) and Brief COPE questionnaire [26, 27]. These tools were chosen to minimize the participant response burden.
EQ‐5D‐5L is a standardized, non‐disease‐specific measure of self‐perceived health status widely used around the world in clinical research and population health studies, and real‐world clinical settings, being recommended by several health technology assessment bodies internationally [28]. It incorporates five domains (mobility, self‐care, usual activities, pain and discomfort, and anxiety and depression), further including an assessment of overall health using a visual analogue scale (VAS; best imaginable health is 100, and worst imaginable health is 0). The EQ‐5D‐5L version (using five levels of response; e.g. not, slightly, moderately, severely or extremely) is more sensitive and suffers less ceiling effect than the original 3‐level instrument (EQ‐5D‐3L) [29]. It has been validated in multiple populations across geographical and disease areas [30]. The EQ‐5D‐5L instrument was used according to the published instructions [31]. The five domain scores were used to calculate the health state index scores, ranging from <0 (where 0 is the value of a health state equivalent to dead; negative values representing values as worse than dead) to 1 (the value of full health), which were used in the analysis.
The SF‐MUIS comprises five questions and assesses four components of uncertainty: ambiguity, complexity, inconsistency and unpredictability. This derives a score (range 5–25) with higher levels corresponding to increased levels of uncertainty in illness. In a validation study conducted in the Norwegian breast cancer population, the ordinal coefficient alpha for the SF‐MUIS was 0.70, which is considered reasonably consistent. The correlation coefficient was 0.98, supporting excellent reliability of the scale.
The Brief COPE evaluates 14 coping strategies (Table 4) by answering 28 items on a 4‐point Likert scale (1 = ‘I haven’t been doing this at all’ to 4 = ‘I’ve been doing this a lot’). In the original validation study [27], Cronbach's alpha coefficient of each scale ranged from 0.50 to 0.90, showing acceptable to extremely good reliability [31]. The instrument was used according to the instructions available from the author [32].
Table 4.
Coping strategies used by shielded solid organ transplant recipients (brief COPE).
| Coping strategy | Likert scale | Percentage reporting Likert 2, 3 or 4 points* | ||
|---|---|---|---|---|
| Mean | SD | Mean (%) | SD | |
| Substance abuse | 1.16 | 0.46 | 11.4 | 0.013 |
| Behavioural disengagement | 1.29 | 0.56 | 19.1 | 0.060 |
| Denial | 1.33 | 0.60 | 21.6 | 1.144 |
| Self‐blame | 1.38 | 0.66 | 23.7 | 0.160 |
| Religion | 1.45 | 0.85 | 25.4 | 0.013 |
| Venting | 1.58 | 0.69 | 40.7 | 0.190 |
| Instrumental support | 1.79 | 0.77 | 54.6 | 0.091 |
| Humour | 1.92 | 0.92 | 55.5 | 0.043 |
| Emotional support | 2.23 | 0.89 | 70.9 | 0.056 |
| Positive reframing | 2.23 | 0.92 | 71.0 | 0.053 |
| Planning | 2.31 | 0.92 | 70.8 | 0.076 |
| Self‐distraction | 2.57 | 0.95 | 78.5 | 0.031 |
| Active coping | 2.66 | 0.90 | 78.9 | 0.049 |
| Acceptance | 3.25 | 0.80 | 91.7 | 0.028 |
2 = I’ve been doing this a little bit; 3 = I’ve been doing this a medium amount; 4 = I’ve been doing this a lot.
Participants’ self‐reported COVID‐19 infection status, shielding behaviour, risk perceptions and public trust were assessed using items from the World Health Organizations (WHO) standard protocol: COVID‐19 Snapshot MOnitoring (COSMO Standard), using both Likert scale and VAS [33]. Items regarding the protective behaviour of shielding and public trust were modified to represent the UK‐specific recommendations and healthcare system, as recommended. Access to and perceptions of primary, secondary and tertiary healthcare services were assessed. Participants’ concerns were investigated by allowing selection from a standard list of concerns derived from a general population survey on the mental health impact of the COVID‐19 pandemic [18].
Population comparisons
EQ‐5D‐5L domains and index scores were compared with the general population in England using the Health Survey for England 2017 (HSE), which comprised a multi‐stage, stratified, random probability sample of 7997 adult respondents. Disease‐specific comparison was achieved with four post‐transplant cohorts, after crosswalk from EQ‐5D‐5L to EQ‐5D‐3L according to NICE guidelines [34, 35, 36, 37]. These four cohorts consisted of patients that were in the early or late post‐operative period following either renal or liver transplantation and were compared with subgroups of the study population accordingly [34, 35, 38, 39].
The rate of self‐reported COVID‐19‐positive cases was compared with national registry data on SOT recipients, compiled by the UK transplant regulatory body (NHS Blood and Transplant) and divided into geographic regions [40]. Shielding adherence data were compared with National UK Shielding Behavioural Survey conducted in July 2020 by the Office for National Statistics. This survey included 4081 clinically extremely vulnerable sampled through the National Shielding Helpline [41].
Statistical analysis
Analyses were performed using stata/se v16.1 (College Station, TX, USA: StataCorp LLC). Explanatory variables for primary and secondary outcomes, such as demographics, transplantation and immunosuppression details, shielding behaviours, perceptions of COVID‐19 risk, self‐reported COVID‐19 infection status and access to health care, were pre‐specified.
The relationship between EQ‐5D‐5Lindex score (primary outcome) and explanatory variables was assessed using a backward stepwise selection process with an alpha‐to‐remove of ≥0.1 as criteria for inclusion in a multivariable linear regression model. Age, sex, ethnicity, IMD and BMI were forced into the model. A similar backward stepwise linear regression model was built for SF‐MUIS uncertainty scale.
A two‐sample t‐test or Chi‐square test was performed, as appropriate, to assess for statistically significant differences between comparison population data sets and for differences in age, gender, type of organ transplanted, time since transplantation, ethnicity, first language and IMD between survey responders and non‐responders.
Patient and public involvement
Patient and public involvement was first initiated during the design stage of the study, through consultation and pilot testing. Feedback and opinion on the questionnaire design, methods of administration and time required to participate were obtained.
Results
Baseline demographics
826 of the 3839 (21.5%) SOT recipients invited to participate completed the entire survey and were included in the analysis (Fig. 1). The median age of responders was 60 years, and 57% (470/826) were male (Table 1). Time since transplant was more than five years in 61%, and 72% had undergone a liver transplant. 28% reported ≥2 comorbidities (including renal dialysis, cardiac, respiratory, diabetes, hypertension), and 20% had a history of a mental health illness, with depression being the most frequent (88/826). Most responders were on two or more immunosuppressive medications (67%). Responders were more likely to be older, had longer duration post‐transplantation, be of a white ethnicity and to have a higher IMD (Table S1).
Figure 1.
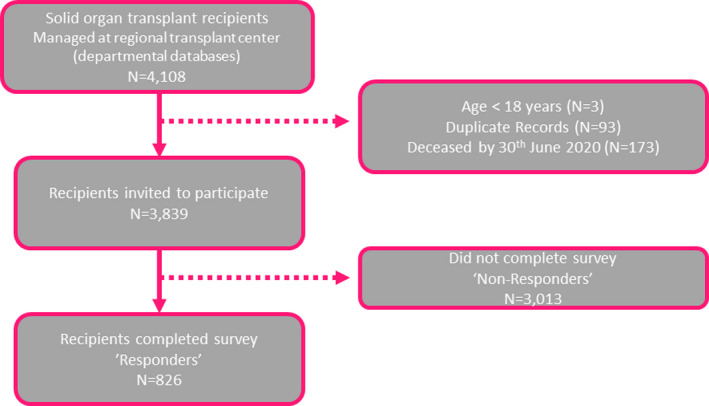
COVID transplant survey consort diagram.
Table 1.
Baseline characteristics of respondents of the COVID transplant survey.
| N (%) | |
|---|---|
| Total number of respondents | 826 |
| Age in years (median, IQR) | 60 (50.67) |
| Sex male | 470 (57) |
| Ethnicity | |
| White | 766 (93) |
| BAME | 54 (7) |
| Prefer not to answer | 6 (1) |
| Index of deprivation | |
| 1 (least deprived) | 111 (13) |
| 2 | 127 (15) |
| 3 | 134 (16) |
| 4 | 155 (19) |
| 5 (most deprived) | 196 (24) |
| Not available | 103 (12) |
| Medical comorbidities | |
| Diabetes | 140 (17) |
| Hypertension | 456 (55) |
| Heart disease | 74 (9) |
| Chronic lung disease | 65 (8) |
| End‐stage renal failure | 6 (1) |
| Number of medical comorbidities per recipient | |
| 0 | 228 (28) |
| 1 | 364 (44) |
| 2 | 173 (21) |
| ≥3 | 61 (7) |
| BMI | |
| Normal weight | 277 (34) |
| Underweight | 14 (2) |
| Overweight | 268 (32) |
| Obese | 203 (25) |
| Invalid entry | 64 (8) |
| Mental health illness (yes) | 166 (20) |
| Anxiety | 16 (2) |
| Depression | 88 (11) |
| PTSD | 43 (5) |
| Other | 19 (2) |
| Organ transplanted | |
| Liver | 593 (72) |
| Kidney | 146 (17) |
| Heart or lung | 87 (11) |
| Time since transplant | |
| <1 year | 58 (7) |
| 1–2 years | 74 (9) |
| 2–5 years | 188 (23) |
| >5 years | 506 (61) |
| Level of immunosuppression | |
| No immunosuppression | 1 (0) |
| Monotherapy | 269 (33) |
| Dual therapy | 360 (44) |
| Triple therapy or more | 196 (23) |
| Steroids (yes) | 312 (38) |
| Missing | 3 (0) |
BAME, Black, Asian and minority ethnic; BMI, body mass index; PTSD, post‐traumatic stress disorder.
Shielding and COVID‐19 infection
The adherence levels to different components of the shielding advice are shown in Table 2. Comparable to national data, communication of official advice to shield for clinically extremely vulnerable individuals had been highly successful in reaching our responders (95% vs. 96%, P 0.17; Table S2) [42]. 96% declared adherence with shielding advice, which was significantly lower than the 99% adherence observed at a national level (P < 0.001). Adherence to the recommendation to stay home at all times was significantly better in our cohort.
Table 2.
Solid organ transplant recipient shielding during the COVID‐19 pandemic: advice received, shielding adherence and elements followed.
| N (%) | |
|---|---|
| Total number of respondents | 826 |
| Received government advice regarding shielding (yes) | 793 (96) |
| No | 26 (3) |
| Unsure | 7 (1) |
| Followed government advice to shield (yes) | 793 (96) |
| Point shielding commenced | |
| Before advice received | 656 (79) |
| After advice received | 149 (18) |
| Decided not to shield | 21 (3) |
| Adherence to all recommended elements of shielding | |
| Yes | 587 (71) |
| No | 13 (2) |
| Partially | 226 (27) |
| Staying home at all times | |
| Yes | 587 (71) |
| No | 13 (2) |
| Partially | 226 (27) |
| Avoided gatherings | |
| Yes | 794 (96) |
| No | 9 (1) |
| Partially | 23 (3) |
| Avoided contact with symptomatic people | |
| Yes | 812 (98) |
| No | 8 (1) |
| Partially | 6 (1) |
| Observed social distancing within household | |
| Yes | 349 (42) |
| No | 321 (39) |
| Partially | 156 (19) |
| Number of members in household | |
| Lives alone | 121 (15) |
| One other person | 425 (51) |
| 3–5 people | 270 (33) |
| 6 or more people | 10 (1) |
Eight (1%) responders tested positive for COVID‐19, and 16 (2%) declared a combination of 2 or more key symptoms for COVID‐19. Twelve patients reported that their symptoms required hospital admission, but no recipients reported admission to the intensive treatment unit (ITU) or need for ventilatory support. A comparison with NHSBT registry data showed no significant difference between our survey and either the national or regional level transplant registry infection rate in SOT recipients (Table S3).
Perception of risk and public trust
The probability of contracting COVID‐19 was perceived as extremely likely or somewhat likely in a minority of responders (27%), and a perceived high level of knowledge regarding how to protect themselves from COVID‐19 was reported [median visual analogue scale (VAS) 94/100] (Table 3). However, a high perceived susceptibility to COVID‐19 infection was reported (median VAS 78/100), and responders believed they would be severely unwell with COVID‐19 (median VAS 91/100). 24% of responders reported their access to health care had been compromised during shielding, putting them at potential risk. Compared to local healthcare facilities and government, SOT recipients had the greatest confidence in their transplant centre to manage COVID‐19 well (median 95/100; Table 3).
Table 3.
COVID‐19 risk perceptions and access to services.
| N (%) | |
|---|---|
| Total number of respondents | 826 |
| What do you consider to be your own probability of getting infected with COVID‐19?* | |
| Extremely likely | 78 (9) |
| Somewhat likely | 145 (18) |
| Neither likely or unlikely | 229 (28) |
| Somewhat unlikely | 251 (30) |
| Extremely unlikely | 123 (15) |
| Perceived risks and beliefs (visual analogue scale 0–100)* | |
| How susceptible do you consider yourself to be to an infection with COVID‐19? † | 78.0 (50–95) |
| How severe do you think contracting COVID‐19 would be for you? ‡ | 91.0 (80–100) |
| Do you know how to protect yourself from COVID‐19? § | 94.0 (83–100) |
| For me avoiding an infection with COVID‐19 in the current situation is? ¶ | 75.0 (50–88) |
| During shielding for COVID‐19 I had safe and reliable access to | |
| Getting my prescriptions | |
| Yes | 721 (87) |
| No | 17 (2) |
| Partially | 88 (11) |
| Visiting my GP | |
| Yes | 266 (32) |
| No | 53 (6) |
| Did not attend | 507 (61) |
| Visiting the healthcare facilities at my local hospital | |
| Yes | 223 (27) |
| No | 46 (6) |
| Did not attend | 557 (67) |
| Visiting the healthcare facilities at my transplant unit | |
| Yes | 125 (15) |
| No | 37 (4) |
| Did not attend | 630 (76) |
| Not applicable as local hospital is transplant unit | 34 (4) |
| How much confidence do you have in the below individuals and organizations that they can handle COVID‐19 well? (visual analogue scale 0–100)* | |
| The specialist doctors and nurses of the transplant unit?** | 95.0 (80–100) |
| Your own family doctor/GP?** | 75.0 (50–90) |
| Your local hospital?** | 75.0 (50–90) |
| Department of Health?** | 52.0 (41–80) |
| The Government?** | 50 (22–72) |
| Has your access to health care been compromised due to shielding, putting you at potential risk? | |
| Yes | 201 (24) |
| No | 625 (76) |
Questions adapted from the World Health Organizations (WHO) tool for behavioural insights on COVID‐19 to assess risk perceptions, behaviours, trust and knowledge.
0 = not susceptible, 100 = very susceptible.
0 = not severely unwell, 100 = severely unwell.
0 = don’t know at all, 100 = know very well.
0 = extremely difficult, 100 = extremely easy.
0 = no confidence, 100 = very confident.
Uncertainty levels and coping strategies
Median uncertainty in illness during shielding for COVID‐19, measured by the SF‐MUIS score, was 11 (range 5–24) and was considered low. The frequency of coping strategies used is shown in Table 4. Acceptance was the most frequently used coping strategy (92%, mean 3.25, SD 0.8, on a 4‐point Likert scale), followed by active coping, self‐distraction and planning. The least reported coping strategies were substance abuse, behavioural disengagement, denial and self‐blame. The Cronbach‐alpha value for the SF‐MUIS and Brief COPE responses was 0.66 and 0.77, respectively.
Perceived health status
Comparing EQ‐5D‐5L domains and index scores for shielded SOT recipients to age‐matched UK population controls, health was equivalent in the youngest and eldest cohorts (18–24 and >75 years), however, significantly poorer for SOT recipients in the 35–74 age range for the majority of health domains (Table 5). Median EQ‐5D‐5Lindex score for age categories was consistently lower in SOT recipients but did not reach statistical significance (Fig. 2). The Cronbach‐alpha value for the EQ‐5D‐5L was 0.88.
Table 5.
Health‐related quality of life in shielded solid organ transplant (SOT) recipients compared with UK population † (using EQ‐5D‐5L ‡ ).
| EQ‐5D domains | 18–24 years | 25–34 years | 35–44 years | 45–54 years | 55–64 years | 65–74 years | >75 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID transplant | UK population | COVID transplant | UK population | COVID transplant | UK population | COVID transplant | UK population | COVID transplant | UK population | COVID transplant | UK population | COVID transplant | UK population | |
| N = 15 (%) | N = 422 (%) | N = 50 (%) | 977 (%) | N = 70 (%) | 1196 (%) | N = 162 (%) | 1218 (%) | N = 232 (%) | 1243 (%) | N = 247 (%) | 1124 (%) | N = 49 (%) | 851 (%) | |
| Mobility | ||||||||||||||
| 1 | 15 (100) | 377 (89) | 41 (82) | 887 (91)* | 47 (67) | 1042 (87)* | 109 (67) | 975 (80)* | 140 (60) | 919 (74)* | 138 (56) | 743 (66)* | 19 (39) | 417 (49) |
| 2 | 0 (0) | 31 (7) | 4 (8) | 61 (6) | 9 (13) | 89 (7) | 23 (14) | 118 (10) | 32 (14) | 162 (13) | 49 (20) | 163 (15) | 11 (22) | 180 (21) |
| 3 | 0 (0) | 10 (2) | 5 (10) | 20 (2) | 11 (16) | 35 (3) | 24 (15) | 64 (5) | 38 (16) | 84 (7) | 42 (17) | 124 (11) | 8 (16) | 129 (15) |
| 4 | 0 (0) | 3 (1) | 0 (0) | 8 (1) | 2 (3) | 26 (2) | 5 (3) | 51 (4) | 20 (9) | 69 (6) | 18 (7) | 86 (8) | 9 (18) | 112 (13) |
| 5 | 0 (0) | 1 (0) | 0 (0) | 1 (0) | 1 (1) | 4 (0) | 1 (1) | 10 (1) | 2 (1) | 9 (1) | 0 (0) | 8 (1) | 2 (4) | 13 (2) |
| Self‐care | ||||||||||||||
| 1 | 14 (93) | 409 (97) | 46 (92) | 944 (97) | 56 (80) | 1146 (96)* | 134 (83) | 1121 (92)* | 177 (76) | 1108 (89)* | 205 (83) | 990 (88)* | 37 (76) | 714 (84) |
| 2 | 1 (7) | 5 (1) | 3 (6) | 22 (2) | 6 (9) | 24 (2) | 14 (9) | 39 (3) | 26 (11) | 62 (5) | 25 (10) | 69 (6) | 3 (6) | 69 (8) |
| 3 | 0 (0) | 6 (1) | 1 (2) | 8 (1) | 4 (6) | 15 (1) | 9 (6) | 40 (3) | 25 (11) | 44 (4) | 12 (5) | 50 (4) | 7 (14) | 42 (5) |
| 4 | 0 (0) | 2 (0) | 0 (0) | 3 (0) | 3 (4) | 9 (1) | 4 (2) | 14 (1) | 4 (2) | 20 (2) | 2 (1) | 14 (1) | 1 (2) | 18 (2) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 2 (0) | 1 (1) | 4 (0) | 0 (0) | 9 (1) | 3 (1) | 1 (0) | 1 (2) | 8 (1) |
| Usual activities | ||||||||||||||
| 1 | 13 (87) | 368 (87) | 35 (70) | 872 (89)* | 41 (59) | 1040 (87)* | 108 (67) | 981 (81)* | 126 (54) | 931 (75)* | 138 (56) | 787 (70)* | 21 (43) | 489 (57) |
| 2 | 1 (7) | 41 (10) | 8 (16) | 67 (7) | 13 (19) | 82 (7) | 26 (16) | 112 (9) | 44 (19) | 160 (13) | 58 (23) | 173 (15) | 11 (22) | 172 (20) |
| 3 | 0 (0) | 8 (2) | 6 (12) | 28 (3) | 10 (14) | 43 (4) | 21 (13) | 64 (5) | 38 (16) | 91 (7) | 34 (14) | 97 (9) | 11 (22) | 115 (14) |
| 4 | 1 (7) | 4 (1) | 1 (2) | 9 (1) | 3 (4) | 25 (2) | 5 (3) | 48 (4) | 13 (6) | 46 (4) | 12 (5) | 50 (4) | 6 (12) | 54 (6) |
| 5 | 0 (0) | 1 (0) | 0 (0) | 1 (0) | 3 (4) | 6 (1) | 2 (1) | 13 (1) | 11 (5) | 15 (1) | 5 (2) | 17 (2) | 0 (0) | 21 (2) |
| Pain and discomfort | ||||||||||||||
| 1 | 10 (67) | 316 (75) | 33 (66) | 720 (74) | 28 (40) | 742 (62)* | 73 (45) | 657 (54)* | 85 (37) | 599 (48)* | 107 (43) | 445 (40) | 17 (35) | 312 (37) |
| 2 | 5 (33) | 64 (15) | 12 (24) | 175 (18) | 21 (30) | 303 (25) | 51 (31) | 342 (28) | 72 (31) | 374 (30) | 75 (30) | 378 (34) | 17 (35) | 246 (29) |
| 3 | 0 (0) | 34 (8) | 4 (8) | 56 (6) | 15 (21) | 103 (9) | 30 (19) | 148 (12) | 52 (22) | 185 (15) | 51 (21) | 203 (18) | 11 (22) | 205 (24) |
| 4 | 0 (0) | 7 (2) | 1 (2) | 21 (2) | 4 (6) | 35 (3) | 8 (5) | 48 (4) | 21 (9) | 62 (5) | 13 (5) | 85 (8) | 3 (6) | 73 (9) |
| 5 | 0 (0) | 1 (0) | 0 (0) | 5 (1) | 2 (3) | 13 (1) | 0 (0) | 23 (2) | 2 (1) | 23 (2) | 1 (0) | 13 (1) | 1 (2) | 15 (2) |
| Anxiety and depression | ||||||||||||||
| 1 | 7 (47) | 265 (63) | 19 (38) | 669 (68)* | 30 (43) | 825 (69)* | 70 (43) | 833 (68)* | 116 (50) | 879 (71)* | 149 (60) | 831 (74)* | 36 (73) | 609 (72) |
| 2 | 2 (13) | 89 (21) | 17 (34) | 182 (19) | 22 (31) | 235 (20) | 64 (40) | 222 (18) | 70 (30) | 217 (17) | 67 (27) | 193 (17) | 7 (14) | 157 (18) |
| 3 | 5 (33) | 48 (11) | 10 (20) | 96 (10) | 12 (17) | 96 (8) | 22 (14) | 104 (9) | 37 (16) | 95 (8) | 24 (10) | 83 (7) | 6 (12) | 65 (8) |
| 4 | 1 (7) | 14 (3) | 4 (8) | 28 (3) | 2 (3) | 31 (3) | 4 (2) | 37 (3) | 6 (3) | 35 (3) | 6 (2) | 11 (1) | 0 (0) | 17 (2) |
| 5 | 0 (0) | 6 (1) | 0 (0) | 2 (0) | 4 (6) | 9 (1) | 2 (1) | 22 (2) | 3 (1) | 17 (1) | 1 (0) | 6 (1) | 0 (0) | 3 (0) |
P‐value < 0.05.
UK population data derived from Health Survey England 2017.
Representing five levels of response for each domain; e.g. not = 1, slightly, moderately, severely or extremely = 5.
Figure 2.

Age‐matched shielded solid organ transplant recipient health‐related quality of life compared with the general population of UK (Health Survey England 2017) (median EQ‐5D‐5Lindex score and interquartile range).
Comparing overall EQ‐5D‐3L index scores, perceived health status was worse for SOT recipients during shielding, than for pre‐pandemic age‐matched general population cohorts. Comparing perceived health status with disease‐specific controls did not reveal consistent results. EQ‐5D‐3L VAS in liver transplant recipients during shielding was equivalent to pre‐pandemic controls in the early post‐transplant period (<2 years), but with a trend to worse outcomes than the pre‐pandemic group in the late period (>2 years after liver transplantation). Conversely, perceived health status (EQ‐5D index score) showed a trend towards better EQ‐5D VAS scores in a smaller subgroup of renal transplant recipients during shielding compared with a pre‐pandemic renal transplant cohort ≥2 years after transplantation (Fig. 3) [34, 35, 36].
Figure 3.
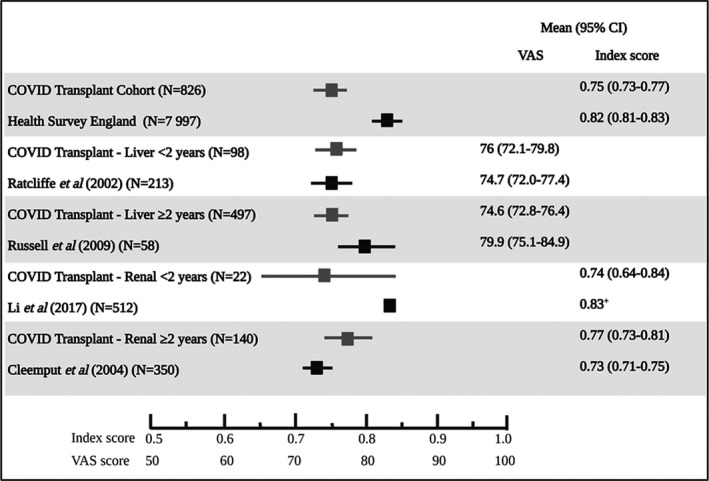
Shielded solid organ transplant recipient mean EQ‐5D‐3Lindex or VAS scores compared with relevant published data. The entire COVID Transplant cohort EQ‐5D‐3Lindex was lower than the general population of England as per Health Survey England results. The group of patients in the study cohort that had undergone a liver transplantation were compared with the results of Ratcliffe et al. in which all 213 participants were surveyed at 24 months post‐liver transplant. The patients in the COVID transplant cohort that were more than 2 years from transplant are compared with Russell et al. (>36 months post‐transplant. Similar comparisons were done with the renal transplant subgroup with Li et al. (6 months post‐transplant) and Cleemput (median 16.7 months post‐transplant, IQR 7.9–38.6).
Multivariable linear regression model of EQ‐5D‐5Lindex scores revealed a significant relationship between several explanatory variables, as shown in Table 6. A previous history of mental health illness and being underweight or obese were significantly associated with lower EQ‐5D‐5Lindex scores. Additionally, reporting not knowing whether they had been infected with COVID‐19, perception of high likelihood of severe COVID‐19 infection, compromised access to health care, higher levels of uncertainty in illness and a coping strategy of behavioural disengagement were also associated with lower EQ‐5D‐5Lindex scores. Conversely, increasing deprivation index, chronic respiratory comorbidity, a coping strategy of self‐distraction and positive reframing and perceived safe access to their hospital despite not needing to attend were associated with higher EQ‐5D‐5Lindex scores.
Table 6.
Linear regression analysis (ordinary least squares) of health‐related quality of life (EQ‐5D‐5Lindex) for shielded solid organ transplant recipients during the COVID‐19 pandemic.
| Variables (r 2 0.41) (n = 826) | Category | EQ‐5D‐5L index | ||
|---|---|---|---|---|
| β (Coef.) | 95% CI | P‐value | ||
| Age distribution (years) (18–24 years = reference group) | 25–34 | 0.02 | −0.08 to 0.12 | 0.715 |
| 35–44 | −0.05 | −0.15 to 0.04 | 0.298 | |
| 45–54 | −0.02 | −0.11 to 0.07 | 0.699 | |
| 55–64 | −0.07 | −0.16 to 0.02 | 0.141 | |
| 65–74 | −0.04 | −0.13 to 0.04 | 0.324 | |
| >75 | −0.09 | −0.19 to 0.01 | 0.066 | |
| Sex (female = reference group) | Male | −0.01 | −0.03 to 0.02 | 0.553 |
| Ethnicity (white = reference group) | BAME | 0.02 | −0.03 to 0.07 | 0.414 |
| Prefer not to answer | 0.05 | −0.09 to 0.18 | 0.515 | |
| Index of multiple deprivation (1 = reference group; least deprived) | 2 | 0.00 | −0.04 to 0.05 | 0.936 |
| 3 | 0.05 | 0.01–0.10 | 0.017 | |
| 4 | 0.05 | 0.01–0.09 | 0.026 | |
| 5 (most deprived) | 0.07 | 0.02–0.11 | 0.002 | |
| Not available | 0.00 | −0.04 to 0.05 | 0.898 | |
| Body mass index (kg/m2) (normal weight = reference group) | Underweight | −0.10 | −0.19 to −0.01 | 0.028 |
| Overweight | −0.00 | −0.03 to 0.03 | 0.838 | |
| Obese | −0.05 | −0.08 to −0.02 | 0.004 | |
| Self‐reported comorbidities | End‐stage renal disease (dialysis) | 0.23 | 0.09–0.37 | 0.001 |
| Mental health illness (yes) | −0.12 | −0.16 to −0.09 | 0.000 | |
| Chronic respiratory disease | 0.03 | 0.01–0.05 | 0.014 | |
| Self‐reported COVID infection (no = reference group) | Don’t know | −0.04 | −0.07 to −0.00 | 0.031 |
| Uncertainty | Mishel score (SF‐MUIS) | −0.01 | −0.01 to −0.00 | <0.001 |
| Coping strategies (brief COPE) | Self‐distraction | 0.02 | 0.00–0.03 | 0.033 |
| Positive reframing | 0.02 | 0.00–0.03 | 0.022 | |
| Disengagement | −0.09 | −0.11 to −0.06 | 0.000 | |
| Perceptions | ||||
| Compromised access to health care (no = reference group) | Yes | −0.05 | −0.08 to −0.02 | 0.001 |
| Safe and reliable access to hospital (no = reference group) | Yes | 0.06 | 0.00–0.12 | 0.040 |
| Did not attend | 0.10 | 0.05–0.15 | <0.001 | |
| Trust in local hospital | <0.01 | 0.00–0.00 | 0.013 | |
β coefficient rounded off to two decimal places.
Uncertainty in illness
Regression analysis revealed an association between increasing uncertainty and compromised access to health care, coping strategies of denial, substance abuse, behavioural disengagement and planning (Table 7). Moderate deprivation, renal transplant recipients, higher EQ‐5D‐5Lindex scores, a low perceived risk of contracting COVID‐19, public trust and an acceptance coping strategy were associated with lower levels of uncertainty.
Table 7.
Linear regression analysis of uncertainty in illness (SF‐MUIS) for shielded solid organ transplant recipients during the COVID‐19 pandemic.
| Variables (r 2 0.35) (n = 826) | Category | Mishel score | ||
|---|---|---|---|---|
| β (Coef.) ‡ | 95% CI | P‐value | ||
| Age distribution (years) (18–24 years = reference group) | 25–34 | −0.38 | −2.04 to 1.28 | 0.653 |
| 35–44 | −0.74 | −2.35 to 0.87 | 0.369 | |
| 45–54 | −1.42 | −2.96 to 0.11 | 0.068 | |
| 55–64 | −0.34 | −1.85 to 1.16 | 0.654 | |
| 65‐74 | 0.22 | −1.29 to 1.73 | 0.778 | |
| >75 | −0.70 | −2.37 to 0.97 | 0.411 | |
| Sex (female = reference group) | Male | −0.27 | −0.68 to 0.14 | 0.197 |
| Ethnicity (white = reference group) | BAME | 0.45 | −0.38 to 1.28 | 0.292 |
| Prefer not to answer | 1.63 | −0.67 to 3.93 | 0.166 | |
| Index of multiple deprivation (1 = reference group; least deprived) | 2 | 0.09 | −0.65 to 0.83 | 0.817 |
| 3 | −0.74 | −1.49 to 0.00 | 0.051 | |
| 4 | −0.67 | −1.40 to 0.06 | 0.073 | |
| 5 (most deprived) | −0.28 | −0.99 to 0.43 | 0.434 | |
| Not available | −0.18 | −0.98 to 0.62 | 0.667 | |
| Body mass index (kg/m2) (normal weight = reference group) | Underweight | 0.31 | −1.25 to 1.87 | 0.698 |
| Overweight | −0.02 | −0.50 to 0.47 | 0.950 | |
| Obese | −0.07 | −0.61 to 0.47 | 0.795 | |
| Missing | −0.72 | −1.50 to 0.07 | 0.075 | |
| Organ transplanted | Kidney | −0.66 | −1.17 to −0.14 | 0.012 |
| Number of comorbidities | ≥3 | 0.88 | 0.12–1.65 | 0.023 |
| Symptoms: muscle aches | 2.54 | 0.22–4.86 | 0.032 | |
| Health‐related quality of life | EQ‐5D‐5L index | −1.96 | −3.05 to −0.87 | <0.001 |
| Coping strategies (brief COPE) | Behavioural disengagement | 0.57 | 0.15–0.99 | 0.008 |
| Substance abuse | 0.49 | 0.05–0.92 | 0.028 | |
| Denial | 0.48 | 0.11–0.85 | 0.010 | |
| Planning | 0.45 | 0.21–0.68 | < 0.001 | |
| Acceptance | −0.36 | −0.63 to −0.09 | 0.010 | |
| Perceptions (no = reference group) | ||||
| Access to health care compromised? | Yes | 0.90 | 0.42–1.39 | <0.001 |
| Access to prescriptions? | Yes | 1.73 | 0.34–3.13 | 0.015 |
| Partially | 2.52 | 1.02–4.02 | 0.001 | |
| Susceptibility to infection with COVID‐19?* | −0.01 | −0.02 to −0.00 | 0.036 | |
| Knows how to protect self from COVID‐19? † | −0.02 | −0.03 to −0.00 | 0.009 | |
| Perception of probability of getting infected with COVID‐19? (extremely unlikely = reference group) | Extremely likely | 0.97 | 0.20–1.74 | 0.014 |
| Neither likely nor unlikely | 0.85 | 0.37–1.33 | 0.001 | |
| Confidence individuals and organizations can handle COVID‐19 well? | Doctor/GP | −0.01 | −0.02 to −0.01 | 0.000 |
| Department of health | −0.01 | −0.02 to −0.00 | 0.047 | |
| Government | −0.01 | −0.02 to −0.00 | 0.005 | |
0 = not susceptible, 100 = very susceptible.
0 = don’t know at all, 100 = know very well.
β coefficient rounded off to two decimal places.
Discussion
This large cross‐sectional study of unselected solid organ transplant recipients focused on identifying risk factors for poor health‐related quality of life during shielding for the COVID‐19 pandemic. A poorer self‐perception of health status in shielded SOT recipients was most significantly associated with a previous history of mental health illness, being overweight, reporting compromised access to health care and a coping strategy of behavioural disengagement. Increased uncertainty was also associated with poorer health status index scores, compromised access to health care, and several coping strategies (denial, substance abuse, behavioural disengagement and planning).
Overall, our study population showed resilience with low levels of uncertainty, the ability to use acceptance, self‐distraction and positive reframing as coping strategies and to adhere to protective behaviours.
The main concepts of illness uncertainty, coping strategies and quality of life, have previously been described to be interrelated and incorporated in a theoretical framework. This is the first study to report these in detail in a shielded transplant population during the COVID pandemic (Fig. 4) [43, 44, 45, 46, 47].
Figure 4.

COVID transplant survey infographic. Antecedents and outcomes of the COVID transplant survey, showing identified predictors of vulnerability (left pane), low levels of uncertainty in illness and appraisal of the context (middle pane), supportive and maladaptive coping strategies (right pane), and health‐related quality of life compared to a pre‐pandemic English population (far right pane). HRQOL, health‐related quality of life. Adapted from: Wright et al. Curr Pain Headache Rep 2009, and Mishel et al. Image J Nurs Sch 1990.
Solid organ transplant recipients perceived themselves to be at high risk of contracting COVID‐19 and experiencing a severe course of illness. Although, these perceptions may not be inappropriate, accurately quantifying risk, for example through methods such as cognitive re‐appraisal and pro‐active protective health behaviours, can reduce levels of fear and ensure it is proportional to the degree of threat [48, 49, 50, 51]. In accordance, adherence levels to shielding were high and responders generally felt they knew how to protect themselves from COVID very well. This was accompanied by low levels of uncertainty, comparable to previous pre‐pandemic transplant cohorts [52]. Previous studies suggest that structure providers, such as credible authorities, can decrease uncertainty directly by promoting interpretation and congruency of events [53]. The overarching public health recommendation of shielding for clinically extremely vulnerable individuals in England may have decreased uncertainty in our study population by promoting a clear interpretation of events. This was evidenced in our study by high levels of public trust, high adherence to shielding and high confidence in the effectiveness of shielding. However, no international comparison cohort of SOT recipients was available to compare uncertainty levels and health status under different public health approaches [19]. Survey respondents expressed the highest level of confidence in the transplant service healthcare professionals to manage issues with COVID‐19 well. These findings suggest that transplant units may be optimally positioned to promote interpretation of public health interventions aimed at improving the effectiveness and tolerability of shielding.
Acceptance was a frequent coping strategy in our patient cohort and is suggested to be beneficial in times of uncertainty and improve psychological flexibility [19, 54, 55]. Our data support previous findings in transplant recipients of strong protective coping strategies to reduce uncertainty and focus on opportunities. SOT recipients may have developed strong coping strategies of acceptance and reframing with positive associations by their earlier experiences surrounding their transplantation. Previous studies have suggested that this may point at probabilistic perspectives on life, accepting uncertainty as a natural part of it [56, 57]. In contrast, avoidance coping strategies (denial, behavioural disengagement and substance abuse), often associated with psychological rigidity, were significantly related to poorer health and increased uncertainty in our study. The identified risk factors for poor HRQoL and higher levels of uncertainty may help target interventions for individuals at higher risk (such as previous mental health illness and obesity), as well as at specific items or components of the healthcare system (such as psychological support). Techniques such as sign‐posting and encouraging activities balancing pleasure, mastery and social connection have been described to reduce behavioural disengagement [49, 50, 51]. Health providers could, for example, improve access to video rather than telephone consultations, to provide a greater sense of social interaction and engagement.
Unintended harm caused by shielding has previously been reported in shielded patients. The Office of National Statistics reported 785 000 (35%) of shielded patients experiencing worsening mental health and well‐being and 6% reporting much worse mental health [58]. The general public has similarly experienced reductions in social interaction through ‘lockdowns’, and population surveys report stress and anxiety ranging from 20 to 53% and depression rates of 2.7–37.8% [59, 60, 61, 62, 63, 64, 65]. The shielded population represent the most extreme end of this spectrum with regard to restrictions. While our data are unable to exactly quantify the impact of shielding on perceived health status in SOT, a cautious comparison of our cohort with data from a pre‐pandemic general population cohort suggested poorer health status index scores in shielded SOT recipients. However, comparisons of subgroups of shielded transplant recipients with pre‐pandemic transplant cohorts at equivalent stages post‐transplant were difficult to interpret. Previous studies show poorer HRQoL specifically in the first 6 months post‐transplant, while improving and stabilizing significantly after this period [34, 35, 36, 66]. Our results may suggest these changes in HRQoL are equivocal and mild in a majority, but an ideal comparison group of non‐shielded SOT recipients during the pandemic was lacking in our study population or nationally. International recruitment would have been hampered by major differences in policies and language barriers. The survey data were collected during the pandemic in a cross‐sectional manner, and therefore, a direct comparison to pre‐pandemic levels was not achievable. It is possible that associations of HRQoL and uncertainty were pre‐existing and unrelated to the COVID pandemic.
Reuken et al. surveyed 394 SOT recipients and included 112 wait‐list candidates, and 394 immediate household contacts during shielding for COVID‐19 as controls [67]. They identified high levels of fear of COVID‐19 infection in SOT recipients. This study was limited by its use of non‐validated tools, and therefore, interstudy comparison is not possible.
A limitation of the present study is the significantly higher proportion of non‐responders from black, Asian and minority ethnic (BAME) groups, potentially related to language barriers. Including multi‐lingual versions was, however, not possible due to our PRO tools not being validated in different languages [68, 69]. Subsequent studies need to ensure inclusion of at‐risk groups, such as BAME, by including translated or culturally validated measures. The survey response rate was 21.5% which is below the generally accepted 60% threshold for survey research, and the possibility responders do not accurately represent the target population exists [70]. However, the number of respondents in this study exceeded the threshold of 351 that has been previously reported to be representative of a population of approximately 4000 [70, 71]. Selection bias may have impacted the results of this study, an often unintended consequence of a cohort study design with an effect size and direction that is difficult to predict [72]. The method of delivering the survey by postal letters and invitation to participate online have likely contributed and may have imparted bias. However, alternate methods such as providing the survey at outpatient visits were not possible due to shielding requirements.
Conclusive evidence of which patients are most vulnerable to severe COVID‐19 disease and would benefit most from shielding is currently lacking. We relied on self‐reported COVID infection rates at a time that confirmatory testing was not implemented and our study obtained no responses from recipients with severe COVID infection. The national NHSBT registry data on showed similarly low COVID‐19 infection rates in SOT; however, our study carries a significant risk of responder bias for this parameter.
In conclusion, our findings demonstrate a self‐perceived health status that is below average in shielded SOT recipients, a subgroup of clinically extremely vulnerable individuals. Our study shows a resilient population reliant on acceptance and adherence to protective behaviour. Strategies to improve outcomes during shielding for the pandemic may be targeted at identified risk groups, reducing uncertainty and prevention of maladaptive coping strategies. Provision of continuity of care, information and clear guidance during different stages of the pandemic may increase public trust and address the specific concerns of individuals deemed clinically extremely vulnerable to COVID‐19.
Authorship
SM, HJ, AH, MA, JM, NI, AR, RB, JF, JI, MC, TP and HH: conceptualized the study. AH, HJ, SM, KN, LM, TDP, MC and HH: curated the data. AH, KO, HJ, SM, KN, BT, MC and HH: involved in formal analysis. SM, AH and HH: involved in funding acquisition. AH, SM, HJ, LM, MA, JM, NI, TDP, AR, RB, JF, JI, MC, TP and HH: investigated the study. SM, HJ, AH, KO, DS, LM, BT, MA, JM, TDP, AR, RB, JF, JI, MC, TP and HH: contributed to methodology. AH, SM, HJ, LM, NI, TDP, RB, JI, TP, MC and HH: contributed to project administration AH, SM, TDP, JI, TP and HH provided resources. HJ, SM and HH: provided software. KN, LM, TDP, JI, TP and HH: supervised the study. AH, SM, HJ, KO, KN and HH: validated the study. SM, AH and HH: visualized the study. SM, AH, HJ, KO, BT, MC and HH: wrote – original draft. AH, SM, HJ, JA, KO, NK, LM, BT, MA, JM, NI, TDP, AR, RB, JF, JI, MC, TP and HH: wrote – review & editing. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Funding
The study received funding from the RCS/Saven Research and Development Programme, Royal College of Surgeons of England.
Conflicts of interests
Angus Hann, Kelvin Okoth, Joy Anderton, Krishnarajah Nirantharakumar, Laura Magill, Barbara Torlinska, Matthew Armstrong, Jorge Mascaro, Nicholas Inston, Thomas Pinkney, Aaron Ranasinghe, Hanns Lembach Jahnsen, Richard Borrows, James Ferguson, John Isaac, M Thamara PR Perera and Hermien Hartog have no conflicts of interest to declare. Siobhan C McKay received a grant from The RCS/Saven Research and Development Programme, Royal College of Surgeons of England, to undertake the study. Melanie Calvert reports grants from Macmillan Cancer Support, PCORI, Innovate UK, HDRUK, GSK, UCB Pharma, the NIHR Birmingham Biomedical Research Centre, NIHR Surgical Reconstruction and Microbiology Research Centre (SRMRC) and NIHR Applied Research Collaboration at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, and personal fees from Astellas, Takeda, Merck, Glaukos, GSK, PCORI and Daiichi Sankyo outside the submitted work. Melanie Calvert is an NIHR Senior Investigator; no other relationships or activities that could appear to have influenced the submitted work.
Supporting information
Table S1. Comparison of characteristics of survey responders and non‐responders.
Table S2. Comparison of reported compliance with shielding advice between COVID transplant responders and National UK survey data.*
Table S3. Comparison of reported COVID infection rate in national and regional (midlands) areas*, compared to COVID transplant survey.
Acknowledgements
SM received a grant from The RCS/Saven Research and Development Programme, Royal College of Surgeons of England, to undertake the study. The funding source had no role in the conduct of the study.
Data availability statement
Data collected for this study, including individual participant data and the data dictionary, will be made available to others at publication. The data will be in anonymized form to protect participants’ privacy. The authorship agrees to provide access to all additional study documents.
References
- 1. Farsalinos K, Poulas K, Kouretas D, et al. Improved strategies to counter the COVID‐19 pandemic: lockdowns vs. primary and community healthcare. Toxicol Rep 2021; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bavli I, Sutton B, Galea S. Harms of public health interventions against covid‐19 must not be ignored. BMJ 2020; 371: m4074. [DOI] [PubMed] [Google Scholar]
- 3. Ebrahim SH, Ahmed QA, Gozzer E, Schlagenhauf P, Memish ZA. Covid‐19 and community mitigation strategies in a pandemic. BMJ 2020; 368: m1066. [DOI] [PubMed] [Google Scholar]
- 4. Wise J. Covid‐19: experts divide into two camps of action‐shielding versus blanket policies. BMJ 2020; 370: m3702. [DOI] [PubMed] [Google Scholar]
- 5. Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID‐19: a timeline of immunological insights. Nat Rev Immunol 2021; 21: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Price E, MacPhie E, Kay L, et al. Identifying rheumatic disease patients at high risk and requiring shielding during the COVID‐19 pandemic. Clin Med (Lond) 2020; 20: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michie S, West R, Rogers MB, Bonell C, Rubin GJ, Amlot R. Reducing SARS‐CoV‐2 transmission in the UK: a behavioural science approach to identifying options for increasing adherence to social distancing and shielding vulnerable people. Br J Health Psychol 2020; 25: 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Middle east respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis 2018; 18: e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant 2020; 26: 832. [DOI] [PubMed] [Google Scholar]
- 11. Tsuang WM, Budev MM. COVID‐19 and lung transplant patients. Clevel Clin J Med 2020; ccc004. [DOI] [PubMed] [Google Scholar]
- 12. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant 2020; 20: 1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohamed IH, Chowdary PB, Shetty S, et al. Outcomes of renal transplant recipients with SARS‐CoV‐2 infection in the eye of the storm: a comparative study with waitlisted patients. Transplantation 2021; 105: 115. [DOI] [PubMed] [Google Scholar]
- 14. Belli LS, Fondevila C, Cortesi PA, et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid‐19: results from the ELITA/ELTR multi‐center European study. Gastroenterology 2020; 160: 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westcott KA, Wilkins F, Chancellor A, et al. The impact of COVID‐19 shielding on the wellbeing, mental health and treatment adherence of adults with cystic fibrosis. Future Hosp J 2021; 8: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sloan M, Gordon C, Lever E, et al. COVID‐19 and shielding: experiences of UK patients with lupus and related diseases. Rheumatol Adv Pract 2021; 5: rkab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith SJ, Busby J, Heaney LG, et al. The impact of the first COVID‐19 surge on severe asthma patients in the UK. Which is worse: the virus or the lockdown? ERJ Open Res 2021; 7: 00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holmes EA, O'Connor RC, Perry VH, et al. Multidisciplinary research priorities for the COVID‐19 pandemic: a call for action for mental health science. Lancet Psychiat 2020; 7: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massey EK, Forsberg A. Dealing with uncertainty after transplantation in times of COVID‐19. Transpl Int 2020; 33: 1337. [DOI] [PubMed] [Google Scholar]
- 20. Calvert M, Kyte D, Mercieca‐Bebber R, et al. Guidelines for inclusion of patient‐reported outcomes in clinical trial protocols: the SPIRIT‐PRO extension. JAMA 2018; 319: 483. [DOI] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nazroo J, Becares L. Evidence for ethnic inequalities in mortality related to COVID‐19 infections: findings from an ecological analysis of England. BMJ Open 2020; 10: e041750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah N, Ahmed IM, Nazir T. Torn between caution and compassion: a dilemma for clinicians from black and minority ethnic groups during the COVID‐19 Pandemic. J Racial Ethn Health Disparities 2021; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. English indices of deprivation 2019: ministry of housing, communities and local government, 2019. Available from: http://imd‐by‐postcode.opendatacommunities.org/imd/2019.
- 25. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol Group. Ann Med 2001; 33: 337. [DOI] [PubMed] [Google Scholar]
- 26. Mishel MH. The measurement of uncertainty in illness. Nurs Res 1981; 30: 258. [PubMed] [Google Scholar]
- 27. Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med 1997; 4: 92. [DOI] [PubMed] [Google Scholar]
- 28. EuroQol Group . EuroQol – a new facility for the measurement of health‐related quality of life. Health Policy 1990; 16: 199. [DOI] [PubMed] [Google Scholar]
- 29. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res 2011; 20: 1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ‐5D‐5L compared to the EQ‐5D‐3L across eight patient groups: a multi‐country study. Qual Life Res 2013; 22: 1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagen KB, Aas T, Lode K, et al. Illness uncertainty in breast cancer patients: validation of the 5‐item short form of the Mishel Uncertainty in Illness Scale. Eur J Oncol Nurs 2015; 19: 113. [DOI] [PubMed] [Google Scholar]
- 32. Carver CS. Brief COPE [cited 2021 29th May]. Available from: https://local.psy.miami.edu/faculty/ccarver/sclBrCOPE.phtml.
- 33. Europe’ WROf . COVID‐19 Snapshot MOnitoring (COSMO Standard): monitoring knowledge, risk perceptions, preventive behaviours, and public trust in the current coronavirus outbreak – WHO standard protocol: PsychArchives, 2020. [updated WHO Regional Office for Europe. Available from: 10.23668/psycharchives.2782. [DOI]
- 34. Russell RT, Feurer ID, Wisawatapnimit P, Pinson CW. The validity of EQ‐5D US preference weights in liver transplant candidates and recipients. Liver Transpl 2009; 15: 88. [DOI] [PubMed] [Google Scholar]
- 35. Cleemput I, Kesteloot K, Moons P, et al. The construct and concurrent validity of the EQ‐5D in a renal transplant population. Value Health 2004; 7: 499. [DOI] [PubMed] [Google Scholar]
- 36. Ratcliffe J, Longworth L, Young T, et al. Assessing health‐related quality of life pre‐ and post‐liver transplantation: a prospective multicenter study. Liver Transpl 2002; 8: 263. [DOI] [PubMed] [Google Scholar]
- 37. NICE . Position statement on use of the EQ‐5D‐5L value set for England (updated October 2019), 2019. Available from: https://www.nice.org.uk/about/what‐we‐do/our‐programmes/nice‐guidance/technology‐appraisal‐guidance/eq‐5d‐5l.
- 38. Li B, Cairns JA, Draper H, et al. Estimating health‐state utility values in kidney transplant recipients and waiting‐list patients using the EQ‐5D‐5L. Value Health 2017; 20: 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M. Assessing health‐related quality of life pre‐ and post‐liver transplantation: a prospective multicenter study. Liver Transpl 2002; 8: 263. [DOI] [PubMed] [Google Scholar]
- 40. NHSBT . NHS blood and transplant: weekly report on SARS‐CoV‐2 positive patients in transplantation: report for 1 March 2020–22 July 2020.
- 41. ONS . Coronavirus and shielding of clinically extremely vulnerable people in England: 9 July to 16 July 2020. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronavirusandshieldingofclinicallyextremelyvulnerablepeopleinengland/9julyto16july2020.
- 42. Coronavirus and shielding of clinically extremely vulnerable people in England: 9 July to 16 July 2020: office for national statistics, 2020. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronavirusandshieldingofclinicallyextremelyvulnerablepeopleinengland/9julyto16july2020.
- 43. Eastwood JA, Doering L, Roper J, Hays RD. Uncertainty and health‐related quality of life 1 year after coronary angiography. Am J Crit Care 2008; 17: 232; quiz 43. [PubMed] [Google Scholar]
- 44. Somjaivong B, Thanasilp S, Preechawong S, Sloan R. The influence of symptoms, social support, uncertainty, and coping on health‐related quality of life among cholangiocarcinoma patients in northeast Thailand. Cancer Nurs 2011; 34: 434. [DOI] [PubMed] [Google Scholar]
- 45. Verduzco‐Aguirre HC, Babu D, Mohile SG, et al. Associations of uncertainty with psychological health and quality of life in older adults with advanced cancer. J Pain Symptom Manage 2020; 61: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson Wright L, Afari N, Zautra A. The illness uncertainty concept: a review. Curr Pain Headache Rep 2009; 13: 133. [DOI] [PubMed] [Google Scholar]
- 47. Mishel MH. Reconceptualization of the uncertainty in illness theory. Image J Nurs Scholarsh 1990; 22: 256. [DOI] [PubMed] [Google Scholar]
- 48. Montemurro N. The emotional impact of COVID‐19: from medical staff to common people. Brain Behav Immun 2020; 87: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lazarus RS, Alfert E. Short‐circuiting of threat by experimentally altering cognitive appraisal. J Abnorm Psychol 1964; 69: 195. [DOI] [PubMed] [Google Scholar]
- 50. Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well‐being. J Pers Soc Psychol 2003; 85: 348. [DOI] [PubMed] [Google Scholar]
- 51. Fiorillo A, Gorwood P. The consequences of the COVID‐19 pandemic on mental health and implications for clinical practice. Eur Psychiatry 2020; 63: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lasker JN, Sogolow ED, Olenik JM, Sass DA, Weinrieb RM. Uncertainty and liver transplantation: women with primary biliary cirrhosis before and after transplant. Women Health 2010; 50: 359. [DOI] [PubMed] [Google Scholar]
- 53. Mishel MH. Uncertainty in chronic illness. Annu Rev Nurs Res 1999; 17: 269. [PubMed] [Google Scholar]
- 54. Arch JJ, Wolitzky‐Taylor KB, Eifert GH, Craske MG. Longitudinal treatment mediation of traditional cognitive behavioral therapy and acceptance and commitment therapy for anxiety disorders. Behav Res Ther 2012; 50: 469. [DOI] [PubMed] [Google Scholar]
- 55. Gloster AT, Klotsche J, Chaker S, Hummel KV, Hoyer J. Assessing psychological flexibility: what does it add above and beyond existing constructs? Psychol Assess 2011; 23: 970. [DOI] [PubMed] [Google Scholar]
- 56. Almgren M, Lennerling A, Lundmark M, Forsberg A. Self‐efficacy in the context of heart transplantation – a new perspective. J Clin Nurs 2017; 26: 3007. [DOI] [PubMed] [Google Scholar]
- 57. Almgren M, Lennerling A, Lundmark M, Forsberg A. The meaning of being in uncertainty after heart transplantation – an unrevealed source to distress. Eur J Cardiovasc Nurs 2017; 16: 167. [DOI] [PubMed] [Google Scholar]
- 58. ONS . Coronavirus and shielding of clinically extremely vulnerable people in England: 28 may to 3 June 2020, 2020.
- 59. Newby JM, O'Moore K, Tang S, Christensen H, Faasse K. Acute mental health responses during the COVID‐19 pandemic in Australia. PLoS One 2020; 15: e0236562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parsons Leigh J, Fiest K, Brundin‐Mather R, et al. A national cross‐sectional survey of public perceptions of the COVID‐19 pandemic: self‐reported beliefs, knowledge, and behaviors. PLoS One 2020; 15: e0241259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shatla MM, Khafagy AA, Bulkhi AA, Aljahdali IA. Public concerns and mental health changes related to the COVID‐19 pandemic lockdown in Saudi Arabia. Clin Lab 2020; 66. [DOI] [PubMed] [Google Scholar]
- 62. Hyland P, Shevlin M, McBride O, et al. Anxiety and depression in the Republic of Ireland during the COVID‐19 pandemic. Acta Psychiatr Scand 2020; 142: 249. [DOI] [PubMed] [Google Scholar]
- 63. Stylianou N, Samouti G, Samoutis G. Mental health disorders during the COVID‐19 outbreak in Cyprus. J Med Life 2020; 13: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Twenge JM, Joiner TE. Mental distress among U.S. adults during the COVID‐19 pandemic. J Clin Psychol 2020; 76: 2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Talarowska M, Chodkiewicz J, Nawrocka N, Miniszewska J, Bilinski P. Mental health and the SARS‐COV‐2 epidemic‐polish research study. Int J Environ Res Public Health 2020; 17: 7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mikolasevic I, Milic S, Filipec‐Kanizaj T. Fatty liver allografts are associated with primary graft non‐function and high mortality after transplantation. Liver Int 2017; 37: 1113. [DOI] [PubMed] [Google Scholar]
- 67. Reuken PA, Rauchfuss F, Albers S, et al. Between fear and courage: attitudes, beliefs, and behavior of liver transplantation recipients and waiting list candidates during the COVID‐19 pandemic. Am J Transplant 2020; 20: 3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature 2020; 584: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cantani A, Genovese S, Tacconi ML, Benincori N, Picarazzi A, Bamonte G. Rare syndromes. II. Joubert syndrome: a review of the 43 cases published in the literature. Riv Eur Sci Med Farmacol 1987; 9: 19. [PubMed] [Google Scholar]
- 70. Draugalis JR, Plaza CM. Best practices for survey research reports revisited: implications of target population, probability sampling, and response rate. Am J Pharm Educ 2009; 73: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Krejcie RV, Morgan, DW . Determining sample size for research activities. Educ Psychol Measure 1970; 30: 607. [Google Scholar]
- 72. Catalogue of Bias Collaboration ND , Bankhead C, Aronson JK. Selection bias. Available from: https://catalogofbias.org/biases/selection‐bias/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of characteristics of survey responders and non‐responders.
Table S2. Comparison of reported compliance with shielding advice between COVID transplant responders and National UK survey data.*
Table S3. Comparison of reported COVID infection rate in national and regional (midlands) areas*, compared to COVID transplant survey.
Data Availability Statement
Data collected for this study, including individual participant data and the data dictionary, will be made available to others at publication. The data will be in anonymized form to protect participants’ privacy. The authorship agrees to provide access to all additional study documents.


