Abstract
Background
There is a scarcity of data comparing the consequences of first and second COVID‐19 waves on kidney transplant recipients (KTRs) in India.
Methods
We conducted a single‐centre retrospective study of 259 KTRs with COVID‐19 to compare first wave (March 15–December 31 2020, n = 157) and second wave (April 1–May 31 2021, n = 102).
Results
KTRs during second wave were younger (43 vs. 40 years; p‐value .04) and also included paediatric patients (0 vs. 5.9%; p‐value .003). Symptoms were milder during the second wave (45 vs. 62.7%; p‐value .007); COVID‐19 positive patients had less frequent cough (32 vs. 13.8%; p‐value .001), fever was less frequent (58 vs. 37%; p‐value .001), and we observed fewer co‐morbidities (11 vs. 20.6%; p‐value .04). The percentages of neutrophils (77 vs. 83%; p‐value .001) and serum ferritin (439 vs. 688; p‐value .0006) were higher during second wave, while lymphocyte counts were reduced (20 vs. 14%; p‐value .0001). Hydroxychloroquine (11 vs. 0%; p‐value .0001) and tocilizumab (7 vs. 0%; p‐value .004) were more frequently prescribed during first wave, while utilization of dexamethasone (6 vs. 27%; p‐value .0001) and remdesivir (47 vs. 65%; p‐value .03) increased during the second wave. Mucormycosis (1.3 vs. 10%; p‐value .01) and ICU admissions (20 vs. 37.2%; p‐value .002) were more frequent during second wave. The 28‐day mortality rate (9.6 vs. 10%; p‐value 1) was not different.
Conclusions
There has been a different clinical spectrum of COVID‐19 amongst KTR with similar mortality between the two waves at a large Indian transplant centre.
Keywords: COVID‐19, first wave, kidney transplant recipients, second wave
SUMMARY AT A GLANCE
A large single centre study from India reporting on the health outcomes of COVID‐19 infected kidney transplant recipients during the two waves of between 2020 and 2021. The rate of intensive care admission was more frequent during the second wave of infection although mortality rates were similar in both waves.
Abbreviations
- COVID‐19
coronavirus disease‐19
- KTR
kidney transplant recipients
- LDKT
living donor kidney transplant
- RT‐PCR
reverse real‐time polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The timeline of COVID‐19 waves varied amongst different geographic regions of the world and even in different geographic areas/states within the country. In India, the first wave commenced in March 2020 with cases started dropping in September 2020 till December 2020, India faced its COVID‐19 second wave from March to June 2021. 1 On May 1 2021, India reported more than 400 000 new daily COVID‐19 cases. 2 Several recent reports detail India's COVID‐19 emergency during the second wave 3 , 4 , 5 with COVID‐19 management depending on location, resources and disease burden under very limited healthcare resources. 6 , 7 , 8 , 9
Several differences have been reported between the first and second waves, with a lower proportion of severe cases and younger patients, including children 10 affected by the second wave. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Likewise, data comparing the characteristics of the infection in kidney transplant recipients (KTR) between two COVID‐19 waves are scarce. 19 , 20 , 21 There are reports on the effects of the first wave on KTR in India; 22 , 23 , 24 , 25 however, there are no data currently available on the effects of the second wave from India.
The second wave raises several unanswered questions. Is there any difference in demographics, immunosuppression regimen, clinical profile, treatment and outcomes in KTR with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection compared to the first wave? Is the mortality in KTRs with SARS‐CoV‐2 infection different during the second wave? What is the impact of the second wave on transplant activity and services, especially in public sector transplant hospitals where the care of patients with COVID‐19 has priority over transplant activities? What is the impact of vaccination on KTRs with SARS‐CoV‐2 infections during the second wave? Clearly, there is a need to evaluate the second wave of COVID‐19 in the KTR. Here, we try to shed light on some of the open questions with information gathered in 259 KTRs with SARS‐CoV2 infections during the first and second wave in India. We present our experience of SARS‐CoV‐2 infections in KTR during the second wave and show differences between the first wave (March 15–December 31 2020, n = 157) and second wave (April 1–May 31 2021, n = 102) in the largest public sector transplant hospital in India.
2. METHODS
We conducted a retrospective observational single‐centre study in India and identified 259 KTRs with real‐time reverse transcription polymerase chain reaction confirmed SARS‐CoV2 infection. Of these 157(60.6%) corresponded to the first wave and 102 (39.4%) to the second wave. Ethical approval for this study was obtained from the Ethics Committee of the Institute of Kidney Diseases and Research Centre, Dr HL Trivedi Institute of Transplantation Sciences, Ahmedabad, India. All transplants were performed based on local laws and regulations (Transplantation of Human Organ Act, India). The study was conducted according to the guidelines of the Declaration of Helsinki and the Declaration of Istanbul principles. We detailed the demographics, immunosuppression regimen, clinical profile, treatment and outcomes. KTRs diagnosed with COVID by a positive RT‐PCR assay of a specimen collected on a nasopharyngeal swab and oropharyngeal swab were included. All treatments were performed according to the National Organ and Tissue Transplant Organization (NOTTO), Government of India guidelines for COVID‐19. 26 , 27 Immunosuppressive regimens for KTR with COVID‐19 and management protocols have been reported in our previous publication of KTR with COVID‐19 in the first wave. 22 , 23
Policy change (or change to management/follow‐up off transplant recipients) between first and second waves:
Table 1 shows the differences in the management protocol of first and second wave. The second wave was less severe but more contagious and has been linked to a more pronounced burden on the healthcare system with a limited number of hospital beds and deficit in ICU beds and oxygen supply. It is worth noting that with experience learned during the first wave, the second wave has been encountered more prepared with more knowledge about the disease to trained health care workers who were mostly vaccinated and less concerned about acquiring COVID‐19. Hospitals had adequate availability of personal protective equipment, better drug availability (e.g., plasma therapy and remdesevir), more enhanced bed capacity, more ICU/ventilator beds and better telemedicine utilization. During the second wave, there was easy access to RT‐PCR laboratory testing with rapid turnaround time, home care medical teams, markedly reduced costs for testing, reduced treatment cost, better knowledge about COVID‐19, better access to health care due to mini‐lockdowns, night curfews, and micro containments instead of national lockdown, and access to vaccines, which led to lower death rates. These measures applied for better management of patients in the second wave compared to the first one. Social distancing, hand wash, mask/face cover and covid appropriate behaviour helped to prevent infection rate outside the hospital.
TABLE 1.
Differences in the management protocol of first wave and second wave
| First wave | Second wave | |
|---|---|---|
| National lock down, travel restrictions | Yes | No |
| Resource limitations | Yes | No |
| COVID‐19 vaccination of HCW | None | Majority received at least first dose of vaccine |
| HCW/hospital and ICU beds capacity | Less | More |
| PPE kits and training | Shortage | Adequate and no shortage |
| COVID PCR testing | Shortage and higher turnaround time | No shortage and results in 24 h |
| COVID‐19 medications | Shortage | Adequate and no shortage |
| COVID‐19 antibody test | Not used | Frequently done |
| Quarantine policy for HCW in COVID ward | Mandatory | Dissolved |
| Follow‐up tele‐consultation | In few | In majority |
| Dedicated mucormycosis ward | No, not required | Yes, required |
| COVID‐19 follow‐up OPD | No | Yes |
| COVID‐19 management | ||
| Home treatment/quarantine | Limited | More for asymptomatic and mild cases |
| Main investigational therapies | Hydroxychloroquine; azithromycin; convalescent plasma and tocilizumab | Remdesivir |
| Radiology | HRCT thorax was used in majority of the cases with shortage of PCR and higher turnaround time | HRCT in only moderate–severe cases |
| Criteria for hospital admission | All RT‐PCR positive irrespective of symptoms were admitted in March–May 2020 | Only symptomatic cases |
| Duration of hospital stay | Prolonged, some cases were admitted till a negative RT‐PCR report | Short; home isolation with clinical recovery discharged with positive RT‐PCR report in majority |
| Choice of steroids | Methyl prednisolone | Dexamethasone |
| Inflammatory markers | Repeated frequently | Not repeated in clinically responsive cases |
| Criteria for hospital discharge | Two Negative RT‐PCR test required in March–May 2020, later patient can be discharged after 10 days of symptom onset and no fever for 3 days | Clinical recovery, no need for RT‐PCR testing prior to discharge |
Abbreviations: COVID‐19, coronavirus disease; CNI, calcineurin inhibitors; HCW, health care workers; HRCT, high‐resolution computed tomography; MMF, mycophenolic acid; OPD, outpatient department; PPE, personnel protective equipment; RT‐PCR, real time polymerase test.
Statistical analysis: Statistical analysis was performed using the Statistical Package for Social Science (SPSS) version 17.0 (SPSS Inc., Chicago, IL). Continuous data are presented as median and interquartile ranges (IQR) and mean ± SD, and student's t tests were used to compare two groups. Categorical data were compared using the χ2 test or Fisher's exact test. Statistical significance was set at p < .05.
3. RESULTS
Of the 259 KTRs with COVID‐19 during both waves, 157 (60.6%) corresponded to the first wave and 102 (39.4%) to the second wave. We performed a comparative analysis of the profile of the first wave KTR (n = 157) with the second wave (n = 102).
3.1. Impact of the second wave of COVID‐19 on transplant activity
(Figure 1) Both waves had a drastic impact on transplant activity. During the first wave, DDKT was suspended between March 15 2020, and June 3 2020, and LDKT was suspended between March 15 2020, and July 10 2020. The rapid expansion of the second wave led to the collapse of healthcare systems, negatively affecting transplant programmes. Our hospital was converted to a dedicated COVID‐19 hospital in the last week of March 2021 due to overflow of cases in the nearby large dedicated COVID‐19 centres. All routine nephrology, urology services and living and deceased donor transplants were suspended from April 1 2021 to May 31 2021.COVID‐19 cases were in declining trend in Gujarat in last week of May 2021. Our hospital is the only one public sector transplant hospital in our State. Our hospital resumed its transplant and nephrology services in a phased manner from June 2021. The second wave in Gujarat ended in the second week of June 2021.
FIGURE 1.
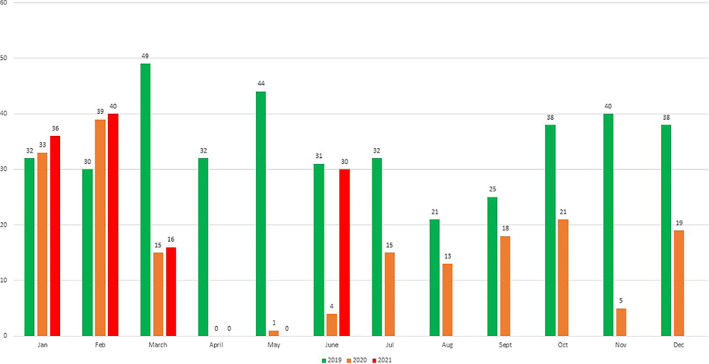
COVID‐19 impact on Kidney transplant numbers in 2019–2021
3.2. Demographic and baseline characteristics of COVID‐19 positive transplant patients
Table 2 shows baseline demographics, co‐morbidities and immunosuppression regimen of KTR with COVID‐19 during both COVID‐19 waves. The second wave cohort was younger (43 [32–50] vs. 40 [32–46] years; p‐value .04), and the age differences further increased amongst non‐survivors (53 [41–56) vs. 42 [35–47.5] years; p‐value .015). There were significantly more paediatric transplant cases who were infected in the second wave than in the first wave (0% [0/157,] vs. 5.9% [6/102]; p‐value .003). There were no sex‐specific differences in mortality between the two waves. Living–related transplants were more frequent during both waves (n = 39, 25% vs. n = 18, 17.7%). There were no significant differences in blood group or native kidney disease. Similarly, no difference was observed in the induction agents used. The COVID‐19 unimmunized vaccination status (93.4 vs. 70%; p‐value .04) was associated with an increased mortality in the second wave. We also identified three KTRs (2.9%) with repeated manifestations of SARS‐CoV‐2 during the second wave. Cytomegalovirus co‐infection was not associated with increased mortality and reportedly declined (n = 18, 11.4% vs. n = 2, 1.9%) during the second wave. Hypertension was the most common comorbidity during both waves; however, fewer hypertensive patients were seen during the second wave (n = 114, 72% vs. n = 61, 59.8%; p‐value .04). Additionally, there were more cases without any comorbid conditions (n = 17, 11% vs. n = 21, 20.6%; p‐value .04) during the second wave. The proportion of obese patients was similar in both waves (24 vs. 22.5%; p‐value .88), but mortality in obese KTR was higher during the first wave (21 vs. 54%; p = .01, vs. 20.7 vs. 40%; p‐value .22). Chronic allograft dysfunction was frequent (n = 68, 43% versus n = 37, 36.2%) and was associated with higher mortality (p‐value .003 vs. .033) in both waves. The median time from kidney transplantation to COVID‐19 infection was similar between the two waves.
TABLE 2.
Comparison of demographic and baseline features of kidney transplant recipients with COVID‐19 in the two waves
| Characteristics | 1st wave (overall) n = 157 | 2nd wave (overall) n = 102 | p‐value | 1st wave (survivors) n = 142 | 2nd wave (survivors) n = 92 | p‐value | 1st wave (non‐survivors) n = 15 | 2nd wave (non‐survivors) n = 10 | p‐value | Mortality difference within waves (p‐value) |
|---|---|---|---|---|---|---|---|---|---|---|
| Age in years, median (interquartile range) | 43 (32–50) | 40 (32–46) | .04 | 42 (32–49) | 40 (32–46) | .64 | 53 (41–56) | 42 (35–47.5) | .015 | .002 versus 1 |
| Age group in years, n (%) | ||||||||||
| <18 | 0 (0) | 6 (5.9) | .003 | 0 (0) | 6 (6.5) | .003 | 0 (0) | 0 (0) | N/A | |
| 18–50 | 114 (73) | 80 (78.4) | .30 | 108 (76) | 72 (78.2) | .75 | 6 (40) | 8 (80) | .09 | |
| 50–65 | 40 (25) | 15 (14.7) | .04 | 32 (23) | 14 (15.3) | .18 | 8 (54) | 1 (10) | .04 | |
| >65 | 3 (2) | 1 (1) | 1 | 2 (1) | 0 (0) | .52 | 1 (6) | 1 (10) | 1 | |
| Sex, n (%) | ||||||||||
| Female | 24 (15) | 18 (17.7) | 1 | 23 (16) | 17 (18.5) | .72 | 1 (6) | 1 (10) | 1 | .47 versus .68 |
| Male | 133 (85) | 84 (82.3) | .6 | 119 (84) | 75 (81.5) | .72 | 14 (94) | 9 (90) | 1 | .68 versus .68 |
| BMI > 30 kg/m2, n (%) | 38 (24) | 23 (22.5) | .88 | 30 (21) | 19 (20.7) | 1 | 8 (54) | 4 (40) | .68 | .01 versus .22 |
| Years from transplantation to COVID‐19, n (%) | ||||||||||
| <1 | 24 (15) | 17 (16.6) | .86 | 22 (16) | 15 (16.4) | .85 | 2 (13) | 2 (20) | 1 | 1 versus .62 |
| 1–5 | 78 (50) | 43 (42.2) | .25 | 72 (51) | 39 (42.3) | .22 | 6 (40) | 4 (40) | 1 | .58 versus 1 |
| 5–10 | 32 (20) | 19 (18.7) | .75 | 30 (20) | 17 (18.5) | .73 | 2 (13) | 2 (20) | 1 | .73 versus 1 |
| >10 | 23 (15) | 23 (22.5) | .13 | 18 (13) | 21 (22.8) | .04 | 5 (34) | 2 (20) | .65 | .047 versus 1 |
| Type of transplantation, n (%) | .52 versus 1 | |||||||||
| LDKT | 118 (75) | 84 (82.3) | .21 | 108 (76) | 75 (73.5) | .33 | 10 (66) | 9 (90) | .34 | |
| DDKT | 39 (25) | 18 (17.7) | .21 | 34 (24) | 17 (18.5) | .33 | 5 (34) | 1 (10) | .34 | |
| Blood group distribution, n (%) | ||||||||||
| A | 37 (24) | 20 (19.6) | .53 | 34 (24) | 19 (20.7) | .63 | 3 (20) | 1 (10) | .62 | 1 versus .68 |
| B | 61 (39) | 35 (34.3) | .51 | 55 (39) | 32 (34.7) | .58 | 6 (40) | 3 (30) | .69 | 1 versus 1 |
| AB | 7 (4) | 12 (11.8) | .04 | 6 (4) | 10 (10.9) | .06 | 1 (6) | 2 (20) | .54 | .51 versus .33 |
| O | 52 (33) | 35 (34.3) | .89 | 47 (33) | 31 (33.7) | 1 | 5 (34) | 4 (40) | 1 | 1 versus .73 |
| NKD, n (%) | ||||||||||
| HTN | 64 (41) | 38 (37.2) | .60 | 59 (42) | 34 (37) | .46 | 5 (34) | 4 (40) | 1 | .59 versus 1 |
| DM | 29 (18) | 21 (20.6) | .74 | 27 (19) | 19 (20.7) | .86 | 2 (13) | 2 (20) | 1 | .73 versus 1 |
| CGN | 26 (17) | 17 (16.7) | 1 | 23 (16) | 16 (17.3) | .85 | 3 (20) | 1 (10) | .62 | .71 versus 1 |
| Obstructive uropathy | 10 (6) | 6 (5.9) | 1 | 7 (5) | 6 (6.6) | .77 | 3 (20) | 0 (0) | .25 | .056 versus 1 |
| CKD UE | 14 (9) | 14 (13.7) | .22 | 13 (9) | 12 (13) | .38 | 1 (7.5) | 2 (20) | .54 | 1 versus .62 |
| Others | 14 (9) | 6 (5.9) | .47 | 13 (9) | 5 (5.4) | .32 | 1 (7.5) | 1 (10) | 1 | 1 versus .47 |
| Induction, n (%) | ||||||||||
| rATG | 129 (82) | 76 (74.5) | .15 | 117 (82) | 69 (75) | .18 | 12 (80) | 7 (70) | .65 | .73 versus .71 |
| Basiliximab | 25 (15) | 22 (21.5) | .25 | 22 (15) | 20 (21.7) | .22 | 3 (20) | 2 (20) | 1 | .7 versus 1 |
| No induction | 3 (2) | 4 (4) | .43 | 3 (2) | 3 (3.3) | .68 | 0 (0) | 1 (10) | .40 | .4 versus .34 |
| Baseline immunosuppression drugs, n (%) | ||||||||||
| Steroids | 157 (100) | 102 (100) | 1 | 142 (100) | 92 (100) | 1 | 15 (100) | 10 (100) | 1 | 1 versus 1 |
| CNI | 135 (86) | 77 (75.4) | .04 | 123 (86) | 69 (75) | .03 | 12 (60) | 8 (80) | 1 | .44 versus 1 |
| MMF | 142 (90.4) | 90 (88.2) | .67 | 132 (93) | 83 (90.2) | .47 | 12 (60) | 7 (70) | .65 | .11 versus .09 |
| AZA | 11 (7) | 6 (5.9) | .80 | 7 (5) | 5 (5.4) | 1 | 2 (13) | 1 (10) | 1 | .20 versus 1 |
| mTOR inhibitors | 14 (9) | 12 (11.8) | .52 | 12 (8) | 9 (9.8) | .81 | 2 (13) | 3 (30) | .35 | .65 versus .09 |
| H/O antirejection therapy | 26 (17) | 17 (16.7) | 1 | 23 (16) | 16 (17.3) | .85 | 3 (20) | 1 (10) | .62 | .71 versus 1 |
| Co‐morbid conditions, n (%) | ||||||||||
| None | 17 (11) | 21 (20.6) | .04 | 16 (11.2) | 19 (20.7) | .06 | 1 (6) | 2 (20) | .54 | 1 versus 1 |
| n ≥ 2 | 38 (24) | 25 (24.5) | 1 | 24 (17) | 22 (23.9) | .23 | 14 (93) | 3 (30) | .001 | .0001 versus .70 |
| HTN | 114 (72) | 61 (59.8) | .041 | 104 (73) | 55 (59.7) | .043 | 10 (66) | 6 (60) | 1 | .55 versus 1 |
| DM | 38 (24) | 24 (23.5) | 1 | 33 (23) | 21 (22.8) | 1 | 5 (33) | 3 (30) | 1 | .36 versus .15 |
| CVD | 17 (11) | 14 (13.7) | .55 | 14 (9.8) | 12 (13) | .54 | 3 (20) | 2 (20) | 1 | .2 versus .62 |
| Chronic allograft dysfunction | 68 (43) | 37 (36.2) | .30 | 56 (39) | 30 (32.6) | .33 | 12 (80) | 7 (70) | .65 | .003 versus .033 |
| Viral marker status | ||||||||||
| CMV positive | 18 (11.4) | 2 (1.9) | .004 | 14 (10) | 2 (2.2) | .03 | 4 (26) | 0 (0) | .12 | .07 versus 1 |
| HCV positive | 11 (7) | 14 (13.7) | .08 | 8 (5) | 12 (13) | .057 | 3 (20) | 2 (20) | 1 | .07 versus .62 |
| HBV positive | 8 (5) | 6 (5.9) | .78 | 6 (4) | 5 (5.4) | .75 | 2 (13) | 1 (10) | 1 | .17 versus .47 |
| H/O previous symptomatic COVID‐19 | 0 (0) | 3 (2.9) | .06 | 0 (0) | 3 (3.3) | .059 | 0 (0) | 0 (0) | N/A | N/A |
| H/O any recent hospitalization | 14 (9) | 7 (6.8) | .64 | 12 (8) | 5 (5.4) | .44 | 2 (13) | 2 (20) | 1 | .62 versus .65 |
| H/O pneumococcal vaccine | 10 (6) | 6 (5.9) | 1 | 8 (5%) | 6 (6.6) | .78 | 2 (13) | 0 (0) | .50 | .24 versus 1 |
| ChAdOx1 nCoV‐19 vaccine status | ||||||||||
| First dose | N/A | 7 (6.8) | N/A | N/A | 5 (5.4) | N/A | N/A | 2 (20) | N/A | N/A versus .13 |
| Second dose | N/A | 2 (1.9) | N/A | N/A | 1 (1) | N/A | N/A | 1 (10) | N/A | N/A versus 1 |
| Unimmunized | N/A | 93 (91.3) | N/A | N/A | 86 (93.4) | N/A | N/A | 7 (70) | N/A | N/A versus .0 |
Note: Bold indicates statistically significant value.
Abbreviations: AZA, azathioprine; BMI, body mass index; CGN, chronic glomerulonephritis; CKD UE, chronic kidney disease of unknown aetiology; CMV, cytomegalovirus; CNI, calcineurin inhibitors; CVD, cardiovascular disease; DDKT, deceased donor kidney transplant; DM, diabetes; HBV, hepatitis B virus; HCV, hepatitis C virus; HTN, hypertension; LDKT, living related kidney transplant; MMF, mycophenolate; mTOR, rapamycin; NKD, native kidney disease; rATG, rabbit thymoglobulin.
3.3. Clinical profile and laboratory analysis of the two waves
Table 3 shows comparison of clinical spectrum and peak laboratory details of KTR with COVID‐19 during the two waves. The spectrum of COVID‐19 exposure changed from unknown (n = 59, 38% versus n = 14, 13.8%; p‐value .0001) to community exposure in the second wave (n = 41, 26% vs. 62, 60.7%; p‐value .0001). Hospital acquired (10 vs. 5.9%; p‐value .26) COVID‐19 declined during the second wave, although differences did not reach significance. Milder symptoms were more frequent during the second wave (n = 71, 45% vs. 64, 62.7%; p‐value 0.007). The proportion of severe COVID‐19 cases was similar (20 vs. 16.2%; p‐value 1). Fever which was the prominent symptom during the first wave was reported less frequently during the second wave (n = 91, 58% vs. 38, 37.2%; p‐value .001). Cough as a symptom was more frequent in non‐survivors of the second wave (n = 8, 53% vs. n = 10 100%; p‐value .02). Expectoration (n = 50, 32% vs. n = 14, 13.8%; p‐value .001) was less frequent during the second wave.
TABLE 3.
Comparison of clinical spectrum and peak laboratory details of kidney transplant recipients with COVID‐19 in the two waves
| 1st wave (overall) n = 157 | 2nd wave (overall) n = 102 | p‐value | 1st wave (survivors) n = 142 | 2nd wave (survivors) n = 92 | p‐value | 1st wave (non‐survivors) n = 15 | 2nd wave (non‐survivors) n = 10 | p‐value | Mortality difference within waves (p‐value) | |
|---|---|---|---|---|---|---|---|---|---|---|
| COVID‐19 exposure | ||||||||||
| Community | 41 (26) | 62 (60.7) | .0001 | 38 (26.7) | 57 (62.1) | .0001 | 3 (20) | 5 (50) | .19 | .76 versus .5 |
| Family/peers cluster | 41 (26) | 20 (19.6) | .29 | 37 (26) | 18 (19.5) | .27 | 4 (27) | 2 (20) | 1 | 1 versus 1 |
| Nosocomial | 16 (10) | 6 (5.9) | .26 | 14 (9.9) | 5 (5.4) | .32 | 2 (13) | 1 (10) | 1 | .65 versus .47 |
| Unknown | 59 (38) | 14 (13.8) | .0001 | 53 (37.4) | 12 (13) | .0001 | 6 (40) | 2 (20) | .40 | 1 versus .62 |
| COVID‐19 severity | .0001 versus .0001 | |||||||||
| Asymptomatic | 7 (4) | 0 (0) | .04 | 7 (0) | 0 (0) | .04 | 0 (0) | 0 (0) | N/A | |
| Mild | 71 (45) | 64 (62.7) | .007 | 71 (50) | 64 (69.6) | .0043 | 0 (0) | 0 (0) | N/A | |
| Moderate | 48 (31) | 21 (20.6) | .08 | 46 (34) | 21 (22.8) | .13 | 2 (13) | 0 (0) | .50 | |
| Severe | 31 (20) | 17 (16.7) | .62 | 18 (12) | 7 (7.6) | .28 | 13 (87) | 10 (100) | .50 | |
| Clinical symptoms on admission | ||||||||||
| Subjective fever | 91 (58) | 38 (37.2) | .001 | 84 (59) | 34 (37) | .001 | 7 (46) | 4 (40) | 1 | .41 versus 1 |
| Dry cough | 77 (49) | 53 (52) | .70 | 69 (48) | 43 (46.7) | .79 | 8 (53) | 10 (100) | .02 | .79 versus .016 |
| Expectoration | 50 (32) | 14 (13.8) | .001 | 42 (30) | 12 (13) | .004 | 8 (53) | 2 (20) | .21 | .08 versus .62 |
| Difficulty of breathing | 46 (29) | 36 (35.3) | .33 | 33 (23) | 26 (28.3) | .44 | 13 (87) | 10 (100) | .50 | .0001 versus .0001 |
| Sore throat | 23 (14) | 14 (13.7) | 1 | 21 (15) | 12 (13) | .84 | 2 (13) | 2 (20) | 1 | 1 versus .62 |
| Running nose | 11 (7) | 6 (5.9) | .80 | 8 (5) | 5 (5.4) | 1 | 3 (20) | 1 (10) | .62 | .07 versus .47 |
| Fatigue | 46 (29) | 35 (34.3) | .41 | 33 (23.2) | 28 (30.4) | .22 | 13 (87) | 7 (70) | .35 | .0001 versus .029 |
| Malaise | 48 (31) | 37 (36.2) | .34 | 42 (30) | 31 (33.6) | .56 | 6 (40) | 6 (60) | .42 | .39 versus .16 |
| Anxiety | 63 (40.1) | 43 (42.1) | .79 | 56 (39.4) | 37 (40) | 1 | 7 (46) | 6 (60) | .68 | .59 versus .31 |
| Depression | 67 (42.7) | 44 (43.1) | 1 | 62 (43.6) | 38 (41.3) | .78 | 5 (33.3) | 6 (60) | .24 | .58 versus .32 |
| Head ache | 11 (7) | 13 (12.7) | .12 | 8 (5) | 11 (12) | .09 | 3 (20) | 2 (20) | 1 | .07 versus .61 |
| Diarrhoea | 37 (23) | 28 (27.4) | .55 | 35 (24) | 26 (28.2) | .54 | 2 (13) | 2 (20) | 1 | .52 versus 1 |
| Anosmia | 32 (20) | 18 (17.6) | .63 | 29 (20) | 14 (15.2) | .38 | 3 (20) | 4 (40) | .37 | 1 versus .07 |
| Ageusia | 39 (25) | 28 (27.4) | .66 | 36 (25) | 21 (22.8) | .75 | 3 (20) | 7 (70) | .03 | .76 versus .004 |
| Nausea/vomiting | 10 (6) | 6 (5.8) | 1 | 8 (5) | 6 (6.6) | .78 | 2 (13) | 0 (0) | .50 | .24 versus 1 |
| Radiological changes | 110 (70) | 81 (79.4) | .11 | 96 (68) | 71 (77.1) | .13 | 14 (93) | 10 (100) | 1 | .04 versus .11 |
| Laboratory parameters (normal) | ||||||||||
| Haemoglobin (10–14 g/dl) | 11.4 (10–13) | 12 (10.7–13.9) | .03 | 11.4 (10–13.1) | 12 (10.9–13.9) | .04 | 11 (8–9‐12) | 10.85 (9.375–12.00) | .80 | .2 versus .32 |
| TLC (4000–14 000 × 103 mm3) | 6000 (3800–8600) | 7030 (4924–10 255) | .10 | 5840 (3660–7987) | 6865 (4927–10 178) | .12 | 10 000 (6500–12 000) | 10 245 (5662.5–13965.00) | .88 | .003 versus .37 |
| Neutrophil (60–70%) | 77 (70–86) | 83 (74–88.5) | .001 | 76 (67–84) | 81.5 (74–88) | .006 | 86 (75–90) | 90 (87.5–90.5) | .20 | .013 versus .13 |
| Lymphocytes (25–33%) | 20 (12–26) | 14 (8–20.5) | .0001 | 20 (12–27) | 14.5 (8–22) | .0001 | 12 (8–20) | 8 (7.5–10.5) | .18 | .023 versus .13 |
| Platelet count (100–600 × 103 mm3) | 207 (160–252) | 194 (138.2–253) | .21 | 211 (163–251) | 198 (139–257) | .24 | 165 (124–252) | 178 (126.25–257.75) | .68 | .09 versus .57 |
| PCT (<0.05 pg/ml) | 0.15 (0.05–1.04) | 0.175 (0.05–1.16) | .98 | 0.11 (0.05–0.99) | 0.17 (0.05–0.8) | .96 | 0.96 (0.25–9.9) | 1.61 (0.07–5.62) | .92 | .005 versus .86 |
| hsCRP (<10 mg/dl) | 49 (19–109) | 55 (18.4–104) | .52 | 43 (16–85) | 51.16 (17.4–100) | .43 | 160 (87–178) | 74.67 (43.18–169) | .003 | .0001 versus .51 |
| IL‐6 (<10 pg/ml) | 25 (14–82) | 45 (20.5–127) | .81 | 24 (10–58) | 45 (19.1–119) | .72 | 65 (24–319) | 71.54(20.77–540.9) | .99 | .02 versus .0001 |
| S. Ferritin (<500 ng/ml) | 439 (196–998) | 688 (237–1024) | .0006 | 362 (185–994) | 650 (191–119) | .0001 | 996 (425–1276) | 860.50 (435.75–1065) | .55 | .02 versus .15 |
| D‐dimer (<600 micromol/L) | 1060 (540–2330) | 790 (445–1840) | .27 | 980 (490–2100) | 780 (425–1850) | .43 | 2200 (1247–4865) | 1010 (380–2385) | .13 | .011 versus .82 |
| LDH (<245 IU/L) | 308 (252–410) | 398 (280–446) | .19 | 302 (246–387) | 345 (281–428) | .056 | 598 (356–1129) | 379 (260–845) | .22 | .0001 versus .0001 |
| S. albumin (<3 g/dl) | 3.5 (3–3.8) | 3.5 (3.2–3.8) | 1 | 3.5 (3.1–3.8) | 3.5 (3.17–3.8) | 1 | 3.1 (2.8–3.3) | 3.35 (3.17–3.88) | .12 | .01 versus .54 |
| S.G.P.T. (<45 IU/L) | 24 (17–39) | 27 (20.2–42.75) | .48 | 24 (17–39) | 27 (19.7–44) | .49 | 31 (19–80) | 28 (22.25–40) | .85 | .21 versus .16 |
| Blood urea (>45 mg/dl) | 47 (42–70) | 46 (31.7–84) | .86 | 48 (31–65) | 43 (31–71.7) | .60 | 69 (44–135) | 77 (44.7–109.2) | .73 | .01 versus .8 |
Note: Bold indicates statistically significant value.
Abbreviations: hsCRP, high‐sensitive C reactive protein; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; PCT, procalcitonin; S.G.P.T.: aspartate transferase; TCL: total leucocyte count.
Ageusia (n = 3, 20% vs. n = 7, 70%; p‐value .03) was more common in the second wave amongst the non‐survivor cohort of the two waves. Mortality associated with radiological changes was more frequent during the first wave (p‐value: 0.04 versus 0.11. Amongst the laboratory parameters, neutrophil counts (77 [70–86] vs. 83 [74–88.5]; p‐value 0.001), serum ferritin (439 [196–998] vs. 688 [237–1024] ng/ml; p‐value .0006) were elevated during the second wave, whereas lymphocytes (20 [12–26] vs. 14 [8–20.5] %; p‐value .0001) had declined. Only high‐sensitive C‐reactive protein (160 [87–178] vs. 74.6 [43.18–169] mg/dl; p‐value .003) was higher amongst the non‐survivor group of the first wave. While there were many differences in laboratory parameters between survivors and non‐survivors of the first wave, only Interleukin‐6 (45 [19.1–119] versus 71.54 [20.77–540.9] pg/ml; p‐value .0001) and lactate dehydrogenase (345 [281–428] vs. 379 [260–845] IU/L; p‐value .0001) were higher amongst survivors of the second wave.
3.4. Management and outcome of the cohort in the two waves
Table 4 shows a detailed analysis of immune modulation, treatment and outcomes comparing waves one and two. Patients who did not require any oxygen support were more common in the second wave (n = 78, 54.9% vs. n = 64, 69% p‐value .02). Oxygen requirement (n = 48, 30.6% vs. n = 21, 20.6%; p‐value .08) was lower in COVID patients of the second wave, although the difference was not statistically significant. In the second wave, a significant proportion of cases were managed without any alteration of maintenance immunosuppression (n = 0, 0% vs. n = 25, 24.5%; p‐value .0001). Similarly, antimetabolite discontinuation (n = 122, 80% vs. n = 42, 41.2%) was less frequent during the second wave. Overall, the increase in steroids was less pronounced during the second wave (n = 79, 50% vs. 38, 37.2%; p‐value .04). The choice of steroid shifted from methylprednisolone (n = 70, 44.5% vs. 10, 9.8%; p‐value .0001) to dexamethasone (n = 9, 5.7% vs. n = 28, 27.4%; p‐value .0001) during the second wave. Remdesivir (n = 74, 47% vs. 67, 65.6%; p‐value .03) and anticoagulation (n = 79, 50.3% vs. n = 94, 92%; p‐value .0001) were more frequently used in the second wave. Various investigational therapies used in the first wave, such as IV immunoglobulin (n = 35, 22% vs. n = 5, 4.9%; p‐value .0001), hydroxychloroquine (n = 18, 11% vs. n = 0, 0%; p‐value .0001), favipiravir (n = 36, 22% vs. n = 12, 11.8%; p‐value .032), convalescent plasma (n = 21, 13% vs. n = 3, 2.9%; p‐value .004) and tocilizumab (n = 11, 7% vs. n = 0.0%; p‐value .004), were not used during the second wave with a lack of evidence. There is also a list of drugs that were more frequently utilized in the second wave, including azithromycin (n = 84.53% vs. n = 68, 66.7%), antifungals (n = 8.5% vs. n = 21 205%; p‐value .0002), tofacitinib (n = 8,7.9%), IV bevacizumab (n = 2,1.9%), IV itocilizumab (n = 2,1.9%), nitazoxanide (n = 14, 13.7%), and ivermectin (n = 18, 17.7%). Allograft dysfunction measured by serum creatinine (n = 79, 50% vs. n = 67, 65.6%; p‐value .015) in the second wave was higher than that in the first wave. The median follow‐up of the first wave was 7 (6–9) months and of second wave was 2 (2, 3) months. Out of nine breakthrough SARS‐CoV2 infections after Oxford vaccine; two fully vaccinated patients presented with COVID‐19 after 20 and 8 days respectively and the other seven patients those who had only one dose of vaccine presented with COVID‐19 at a median of 14 days (13–23). COVID‐19 IgG spike protein antibody was done in five cases and four had suboptimal response and three died due to COVID‐19. 28 We had around 400 chronic kidney disease patients on haemodialysis (CKD‐5D) on deceased donor waiting list. Fifty eight CKD‐5D patients with RT‐PCR confirmed COVID‐19 admitted to our COVID‐19 hospital during first wave.CKD‐5D patients with COVID‐19 were had higher mortality (37.9%) compared to KTR (10%).
TABLE 4.
Comparing COVID‐19 severity, treatment and outcome of kidney transplant recipients with COVID‐19 in the two waves
| 1st wave (Overall) n = 157 | 2nd wave (Overall) n = 102 | p‐value | 1st wave (Survivors) n = 142 | 2nd wave (Survivors) n = 92 | p‐value | 1st wave (non‐survivors) n = 15 | 2nd wave (non‐survivors) n = 10 | p‐value | Mortality difference within waves (p‐value) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Oxygen support | .0001 versus .0001 | |||||||||
| Ambient air | 78 (49.6) | 64 (62.7) | .23 | 78 (54.9) | 64 (69.6) | .02 | 0 (0) | 0 (0) | N/A | |
| Low‐flow oxygen | 48 (30.6) | 21 (20.6) | .08 | 48 (33.8) | 21 (22.8) | .07 | 0 (0) | 0 (0) | N/A | |
| NRBM/HFO | 10 (6.6) | 5 (4.9) | .78 | 10 (7) | 5 (5.4) | .78 | 0 (0) | 0 (0) | N/A | |
| Bi‐PAP | 6 (3.8) | 2 (1.9) | .48 | 6 (4.3) | 2 (2.2) | .48 | 0 (0) | 0 (0) | N/A | |
| Mechanical ventilation | 15 (9.4) | 10 (9.9) | 1 | 0 (0) | 0 (0) | N/A | 15 (100) | 10 (100) | 1 | |
| Immunosuppression changes | .0001 versus .0001 | |||||||||
| No change in maintenance drugs | 0 (0) | 25 (24.5) | .0001 | 0 (0) | 25 (27.1) | .0001 | 0 (0) | 0 (0) | N/A | |
| MMF/AZA tapered | 31 (20) | 35 (34.3) | .012 | 31 (22) | 35 (38) | .01 | 0 (0) | 0 (0) | N/A | |
| MMF/AZA stopped | 122 (80) | 42 (41.2) | .0001 | 108 (78) | 32 (34.7) | .0001 | 15 (100) | 10 (100) | 1 | |
| CNI stopped/tapered | 26 (19) | 18 (17.6) | .86 | 26 (21) | 8 (8.6) | .056 | 15 (100) | 10 (100) | 1 | |
| Steroids IV added | 79 (50.3) | 38 (37.2) | .04 | 64 (45) | 28 (30.4) | .02 | 15 (100) | 10 (100) | 1 | |
| Therapeutic regimen | ||||||||||
| IV Methyl prednisone | 70 (44.5) | 10 (9.8) | .0001 | 64 (45) | 21 (22.8) | .0005 | 6 (40) | 7 (70) | .22 | .78 versus .004 |
| IV Dexamethasone | 9 (5.7) | 28 (27.4) | .0001 | 6 (4.2) | 7 (7.6) | .38 | 3 (20) | 3 (30) | .65 | .04 versus .057 |
| I V Remdesivir | 74 (47) | 67 (65.6) | .03 | 63 (44) | 57 (56.5) | .01 | 11 (73) | 10 (100) | .12 | .056 versus .014 |
| I V Bevacizumab | 0 (0) | 2 (1.9) | .15 | 0 (0) | 0 (0) | N/A | 0 (0) | 2 (20) | .15 | N/A versus .008 |
| I V Itolizumab | 0 (0) | 2 (1.9) | .15 | 0 (0) | 0 (0) | N/A | 0 (0) | 2 (20) | .15 | N/A versus .008 |
| I V Tocilizumab | 11 (7) | 0 (0) | .004 | 5 (3.5) | 0 (0) | 0.15 | 6 (40) | 0 (0) | .05 | .0001 versus N/A |
| I V/SC UFH or LMWH | 79 (50.3) | 94 (92) | .0001 | 64 (45) | 84 (91.3) | .0001 | 15 (100) | 10 (100) | 1 | .0001 versus 1 |
| I V antibiotics | 32 (20.3) | 28 (27.4) | .22 | 22 (15.5) | 18 (19.5) | .47 | 10 (66.7) | 10 (100) | .06 | .0001 versus .0001 |
| I V/oral antifungal | 8 (5) | 21 (20.5) | .0002 | 5 (3.5) | 14 (15.2) | .002 | 3 (20) | 7 (70) | .03 | .02 versus .0005 |
| I V immunoglobulin | 35 (22) | 5 (4.9) | .0001 | 23 (16) | 3 (3.3) | .0023 | 12 (80) | 2 (20) | .005 | .0001 versus .07 |
| Azithromycin | 84 (53) | 68 (66.7) | .039 | 78 (55) | 69 (75) | .002 | 6 (40) | 7 (70) | .22 | .29 versus .71 |
| Ivermectin | 0 (0) | 18 (17.7) | .0001 | 0 (0) | 15 (16.3) | .0001 | 0 (0) | 3 (30) | .052 | N/A versus .37 |
| Nitazoxanide | 0 (0) | 14 (13.7) | .0001 | 0 (0) | 14 (15.2) | .0001 | 0 (0) | 0 (0) | N/A | N/A versus .34 |
| Favipiravir | 36 (22) | 12 (11.8) | .032 | 32 (22) | 10 (10.8) | .02 | 4 (27) | 2 (20) | 1 | .52 versus .33 |
| Tofacitinib | 0 (0) | 8 (7.9) | .0005 | 0 (0) | 5 (5.4) | .0001 | 0 (0) | 3 (30) | .052 | N/A versus .02 |
| Hydroxychloroquine | 18 (11) | 0 (0) | .0001 | 15 (10.5) | 0 (0) | .0006 | 3 (20) | 0 (0) | .25 | .38 versus N/A |
| Convalescent plasma | 21 (13) | 3 (2.9) | .004 | 18 (12) | 2 (2.2) | .003 | 3 (20) | 1 (10) | .62 | .41 versus .26 |
| Outcome | ||||||||||
| Allograft dysfunction | 79 (50) | 67 (65.6) | .015 | 64 (45) | 57 (56.5) | .015 | 15 (100) | 10 (100) | 1 | .001 versus .01 |
| HD requirement | 26 (16) | 8 (7.9) | .058 | 19 (13.4) | 5 (5.4) | .07 | 7 (47) | 3 (30) | .67 | .0014 versus .02 |
| Graft loss | 10 (6.3) | 5 (4.9) | .78 | 10 (7) | 3 (3.3) | .25 | 0 (0) | 2 (20) | .15 | N/A versus .07 |
| ARDS | 31 (20) | 17 (16.7) | .62 | 18 (12) | 7 (7.6) | .28 | 13 (87) | 10 (100) | .5 | .001 versus .0001 |
| ICU admissions | 31 (20) | 38 (37.2) | .002 | 16 (11) | 28 (30.4) | .0005 | 15 (100) | 10 (100) | 1 | .0001 versus .0001 |
| 28‐day mortality | 15 (9.6) | 10 (10) | 1 | 0 (0) | 0 (0) | N/A | 15 (100) | 10 (100) | 1 | N/A |
Note: Data expressed as median (interquartile range) or numbers and percentages. Bold indicates statistically significant value.
Abbreviations: ARDS, acute respiratory syndrome; AZA, azathioprine; Bi‐PAP: bi‐level positive pressure ventilation; CNI, calcineurin inhibitors; HFO, high‐flow oxygen; ICU, intensive care unit; LMWH, low‐molecular weight heparin; MMF, mycophenolate; NRBM, non‐rebreather mask; UFH: unfractionated heparin.
3.5. Similarities and differences in risk factors for mortality in the two waves
Overall, intensive care admissions were more frequent during the second wave (n = 38, 37.2% vs. n = 31, 20%; p‐value .002). The 28‐day mortality rates (n = 15, 9.6% vs. n = 10, 10%; p‐value 1) were comparable. Interestingly, the incidence of mucormycosis (n = 2, 1.3% vs. n = 10, 10%; p‐value .01) was disproportionately higher during the second. During the first wave, older age was associated with higher mortality (p‐value .002 vs. 1). Sex did not affect the mortality rate. The distribution of obese patients was comparable; however, obesity was a risk factor for mortality during the first wave (p‐value .01 vs. .22). Chronic allograft dysfunction was associated with augmented (p‐value .003 vs. .033) during both waves. The presence of more than two co‐morbidities (p‐value .0001 vs. .70) and chest imaging changes (p‐value .04 vs. .11) were associated with a higher mortality during the first wave. Cough was not a risk factor for mortality during the first wave but during the second wave (p‐value .79 vs. .016).Fatigue was linked to mortality during both waves (p‐value .0001 vs. .029). Amongst the laboratory features, total leukocyte count (p‐value .003 vs. .37), neutrophil count (p‐value .013 vs. .13), lymphocyte percentage (p‐value .023 vs. .13), procalcitonin (p‐value .005 vs. .86), high‐sensitive C‐reactive protein (p‐value .0001 vs. .51), ferritin (p‐value .02 vs. .15), and D dimer (p‐value .011 vs. .82) were risk factors for mortality during the first wave. In both waves, allograft dysfunction and the need for dialysis were associated with increased mortality (p‐value .001 vs. .01).
4. DISCUSSION
Here, we present, to our knowledge, the first study to compare the first and second waves of COVID‐19 in Indian KTRs. Mortality was not different (9.6 vs. 10%, p‐value 1) in the two waves despite more health resource limitations during the second wave. Our data concur with the data from the Indian Council of Medical Research, suggesting that there has been no significant change in the death rate in both COVID‐19 waves. The baseline characteristics and clinical presentations of COVID‐19 were comparable during both waves. COVID‐positive KTRs were generally younger and included paediatric patients. During the second wave, we observed milder cases, less expectoration, less fever, fewer co‐morbidities, and the impact on transplant activities was less pronounced during the second wave, possibly due to the less virulent mutant strain. 29 We have documented an important decrease in the use of hydroxychloroquine and tocilizumab, which were often prescribed during the first wave, while dexamethasone and remdesivir prescriptions increased in the second wave. A recent study from Madrid reported six blood tests (neutrophil‐to‐lymphocyte ratio, C‐reactive protein, LDH, IL‐6, urea and d‐dimer) as predictors of severe COVID‐19 in both waves. 16 In our study, we identified IL6 level, LDH and allograft dysfunction as predictors of mortality in both waves.
The overall patient mortality rates were 11.6 and 14.5% in hospitalized patients, 47% in intensive care unit patients, and 96.7% in patients requiring ventilation in our previous Indian cohort study of 250 KTR with RT‐PCR‐confirmed COVID‐19 during the first wave. 23 COVID related mortality was 1.2% in the general population in India as on June7 2021. 2 A single centre study from Belgium reported similar survival of hospitalized KTR during the first (n = 18) and second waves (n = 27), despite high rates of ICU admission and more comorbidities in second wave. Dexamethasone was commonly used during the second wave in India, while hydroxychloroquine was more frequently applied during the first wave. 19 The analysis of the Spanish Registry (n = 1011) reported advanced age, a recent kidney transplant, and pneumonia as predictors of mortality, whereas gastrointestinal symptoms were protective. KTRs were significantly younger, had less pneumonia and received less frequently anti‐COVID‐19 treatment in the second wave, and the overall mortality was lower but similar in critical patients. 20 The observed high‐case fatality in the elderly transplant recipients could be due to age‐associated comorbidities. 30 Our study had findings similar to this analysis of the Spanish Registry. 20 The interaction between age and time post‐kidney transplant should be considered when selecting recipients for transplantation in the COVID‐19 pandemic. Advanced age and a recent KT should foster strict protective measures, including vaccination. The recent study from European transplant centre described similar mortality rates in both waves, which is similar to our report. 31 In their report, there was no difference in clinical spectrum except the significant high number of cases being managed as outpatients in the second wave. Older age and chronic graft dysfunction was associated with mortality similar to our study.
Mucormycosis has emerged as an epidemic during the COVID‐19 pandemic in the general population, including the KTR in India. Possible contributing factors for significantly increased mucormycosis during the second wave in India are the improper use of steroids amongst diabetic COVID‐19 patients, uncontrolled diabetes, improper use of broad‐spectrum antibiotics, zinc supplements, iron tablets, failure to use sterile water in oxygen concentrators, genetic predilection, steam inhalation abuse, poor oro‐nasal hygiene in hospitalized patients, reuse of masks for prolonged periods, and use of steroids with other immunomodulators; 32 , 33 thus, there seems to be a need for urgent investigation to delineate contributing factors.
India began administration of COVID‐19 vaccines on 16 January 2021 in phased manner. In the initial first phase, COVID‐19 vaccine was provided to the priority group—Health Care and Front‐line workers. The second phase vaccinations, which started on March 1 2021 allowed for all Indians above the age of 60 and Indians between the age of 45 and 59 with comorbidities to be vaccinated. From April 1 2021, People above the age of 45 years are eligible to get the COVID‐19 vaccine. From May 1 2021, all eligible citizens above the age of 18 years can get the COVID‐19 vaccine. The median transplant age of our cohort was below 45 years, and was few had vaccination. 2 In our centre, very few post transplants patients had vaccination (around 100 of 5000 follow‐up patients) at the start of the second wave and till May 2021, as COVID‐19 surge further decreased the vaccination rate along with shortage of vaccine during the second wave.
Implications: This comprehensive analysis of the COVID‐19 first and second waves in India provides a better understanding of the nature of the pandemic in the KTR. KTRs with risk factors for mortality should foster strict protective measures; vaccination should be a priority for transplant recipients. COVID‐19 vaccination for children has not yet started in India, and more vigilance is needed to prevent a future COVID‐19 surge in this population.
Limitations: A single‐centre study was a limiting factor. The low rate of asymptomatic or mild patients is likely an under‐reported. There is no facility available for virological sequencing and testing of mutant strains of COVID‐19 in resource‐limited settings. We reported mainly hospitalized patients, and thus conclusions may not be broadly applicable to all asymptomatic patients and those diagnosed and managed in the outpatient setting and multicentre studied are required to validate our findings for the entire country.
5. CONCLUSIONS
There was no difference in the mortality between the two waves. Mortality in KTRs with COVID‐19 (10%) was higher than that in non‐immunosuppressed patients (1.2%) in our study.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
All authors participated equally in research design, the writing of the article, the performance of the research, the data collection, the data analysis, data interpretation and all authors approved the version for publication and agreed to be accountable for all aspects of the work.
ACKNOWLEDGEMENTS
The authors are grateful for the editing support that they have received from Stefan G. Tullius, MD, PhD, Harvard Medical School, Boston.
Kute VB, Meshram HS, Navadiya VV, et al. Consequences of the first and second COVID‐19 wave on kidney transplant recipients at a large Indian transplant centre. Nephrology. 2022;27(2):195‐207. doi: 10.1111/nep.13961
REFERENCES
- 1. Mallapaty S. India's massive COVID surge puzzles scientists. Nature. 2021;592(7856):667‐668. [DOI] [PubMed] [Google Scholar]
- 2. Government of India . https://www.mohfw.gov.in/(2021)
- 3. The Lancet . India's COVID‐19 emergency. Lancet. 2021;397(10286):1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuppalli K, Gala P, Cherabuddi K, et al. India's COVID‐19 crisis: a call for international action. Lancet. 2021;397(10290):2132–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson B, Pickrell J. Coronapod: the variant blamed for India's catastrophic second wave. Nature. 2021. 10.1038/d41586-021-01308-0 [DOI] [PubMed] [Google Scholar]
- 6. Stock PG, Wall A, Gardner J, et al. Ethical issues in the COVID era: doing the right thing depends on location, resources, and disease burden. Transplantation. 2020;104(7):1316‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wall AE, Pruett T, Stock P, Testa G. Coronavirus disease 2019: utilizing an ethical framework for rationing absolutely scarce health‐care resources in transplant allocation decisions. Am J Transplant. 2020;20(9):2332‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Syed SM, Gardner J, Roll G, et al. COVID‐19 and abdominal transplant: a stepwise approach to practice during pandemic conditions. Transplantation. 2020;104(11):2215‐2220. 10.1097/TP.0000000000003387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brian R, Stock P, Syed S, Hirose K, Reilly L, O'Sullivan P. How COVID‐19 inspired surgical residents to rethink educational programs. Am J Surg. 2021;221(5):923‐924. 10.1016/j.amjsurg.2020.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chua GT, Wong JSC, Lam I, et al. clinical characteristics and transmission of COVID‐19 in children and youths during 3 waves of outbreaks in Hong Kong. JAMA Netw Open. 2021;4(5):e218824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan G, Yang Z, Lin Q, et al. Decreased case fatality rate of COVID‐19 in the second wave: a study in 53 countries or regions. Transbound Emerg Dis. 2020;00:1‐3. 10.1111/tbed.13819 [DOI] [PubMed] [Google Scholar]
- 12. Vahidy FS, Drews AL, Masud FN, et al. Characteristics and outcomes of COVID‐19 patients during initial peak and resurgence in the Houston metropolitan area. JAMA. 2020;324(10):998‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saito S, Asai Y, Matsunaga N, et al. First and second COVID‐19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2020;2:30693‐30699. 10.1016/j.jinf.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain VK, Iyengar KP, Vaishya R. Differences between first wave and second wave of COVID‐19 in India. Diabetes Metab Syndr. 2021;15(3):1047–1048. 10.1016/j.dsx.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salyer SJ, Maeda J, Sembuche S, et al. The first and second waves of the COVID‐19 pandemic in Africa: a cross‐sectional study. Lancet. 2021;397(10281):1265‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soriano V, Ganado‐Pinilla P, Sanchez‐Santos M, et al. Main differences between the first and second waves of COVID‐19 in Madrid Spain. Int J Infect Dis. 2021;105:374‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dutch COVID, Coalition T, Kaptein FHJ, Stals MAM, et al. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID‐19 in the second and first wave. Thromb Res. 2021;199:143‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mollinedo‐Gajate I, Villar‐Álvarez F, Zambrano‐Chacón MLÁ, et al. First and second waves of coronavirus disease 2019 in Madrid, Spain: clinical characteristics and hematological risk factors associated with critical/fatal illness. Crit Care Explor. 2021;3(2):e0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Georgery H, Devresse A, Scohy A, et al. The second wave of COVID‐19 disease in a kidney transplant recipient cohort: a single‐center experience in Belgium. Transplantation. 2021;105(3):e41‐e42. [DOI] [PubMed] [Google Scholar]
- 20. Villanego F, Mazuecos A, Pérez‐Flores IM, et al. Predictors of severe COVID‐19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish registry. Am J Transplant. 2021;21(7):2573–2582. 10.1111/ajt.16579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Sandro S, Magistri P, Bagnardi V, et al. The COVID‐19 second wave risk and liver transplantation: lesson from the recent past and the unavoidable need of living donors. Transplant Int. 2021;34(3):585‐587. [DOI] [PubMed] [Google Scholar]
- 22. Meshram HS, Kute VB, Patel H, et al. Feasibility and safety of remdesivir in SARS‐CoV2 infected renal transplant recipients: A retrospective cohort from a developing nation. Transpl Infect Dis. 2021;e13629. 10.1111/tid.13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kute VB, Bhalla AK, Guleria S, et al. Clinical profile and outcome of COVID‐19 in 250 kidney transplant recipients: a multicenter cohort study from India. Transplantation. 2021;105(4):851‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kute V, Gupta A, Patel H, et al. The impact of COVID‐19 pandemic on nephrology and transplant services and clinical training in India. Exp Clin Transplant. 2021;7:651‐658. 10.6002/ect.2021.0018 [DOI] [PubMed] [Google Scholar]
- 25. Meshram HS, Kute VB, Chauhan S, Desai S. Mucormycosis in post‐COVID‐19 renal transplant patients: a lethal complication in follow‐up. Transplant Infect Dis. 2021;e13663. 10.1111/tid.13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clinical Management Protocol: COVID‐19, 2020. https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf.
- 27. Kute V, Guleria S, Prakash J, et al. NOTTO transplant specific guidelines with reference to COVID‐19. Indian J Nephrol. 2020;30:215‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meshram HS, Kute VB, Shah N, et al. Letter to editor: COVID‐19 in kidney transplant recipients vaccinated with Oxford‐AstraZeneca COVID‐19 vaccine (Covishield): a single center experience from India. Transplantation. 2021. 10.1097/TP.0000000000003835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yadav PD, Nyayanit DA, Majumdar T, et al. An epidemiological analysis of SARS‐CoV‐2 genomic sequences from different regions of India. Viruses. 2021;13(5):925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jayant K, Reccia I, Virdis F, et al. COVID‐19 in hospitalized liver transplant recipients: an early systematic review and meta‐analysis. Clin Transplant. 2021;35(4):e14246. 10.1111/ctr.14246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elec FI, Bolboacă SD, Muntean A, et al. Comparing the first and second wave of COVID‐19 in kidney transplant recipients: an east‐European perspective. Eur Surg Res. 2021;1‐8. 10.1159/000517559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arana C, Cuevas Ramírez RE, Xipell M, et al. Mucormycosis associated with covid19 in two kidney transplant patients. Transpl Infect Dis. 2021;e13652. 10.1111/tid.13652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nehara HR, Puri I, Singhal V, Ih S, Bishnoi BR, Sirohi P. Rhinocerebral mucormycosis in COVID‐19 patient with diabetes a deadly trio: case series from the north‐western part of India. Indian J Med Microbiol. 2021;39(3):380–383. 10.1016/j.ijmmb.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]


