Abstract
The enhancer of yellow 1 gene, e(y)1, of Drosophila melanogaster has been cloned and demonstrated to encode the TAFII40 protein. The e(y)1 gene is expressed in females much more strongly than in males due to the accumulation of e(y)1 mRNA in the ovaries. Two different e(y)1 mutations have been obtained. The e(y)1ul mutation, induced by the insertion of Stalker into the coding region, leads to the replacement of 25 carboxy-terminal amino acids by 17 amino acids encoded by the Stalker sequences and to a decrease of the e(y)1 transcription level. The latter is the main cause of dramatic underdevelopment of the ovaries and sterility of females bearing the e(y)1 mutation. This follows from the restoration of female fertility upon transformation of e(y)1u1 flies with a construction synthesizing the mutant protein. The e(y)1P1 mutation induced by P element insertion into the transcribed nontranslated region of the gene has almost no influence on the phenotype of flies. However, in combination with the phP1 mutation, which leads to a strong P element-mediated suppression of e(y)1 transcription, this mutation is lethal. Genetic studies of the e(y)1u1 mutation revealed a sensitivity of the yellow and white expression to the TAFII40/e(y)1 level. The su(Hw)-binding region, Drosophila insulator, stabilizes the expression of the white gene and makes it independent of the e(y)1u1 mutation.
Initiation of transcription by RNA polymerase II requires an ordered assembly of a multiprotein preinitiation complex at the core promoter of eucaryotic genes (56, 58). TAFs (TATA-binding protein-associated factors), which are highly conserved in all organisms from yeasts to mammals, are the components of the TFIID complex of the basal transcription machinery (11). TAFs are considered to perform important functions both in transcription and in core promoter recognition (46, 47). Some TAFs can function as coactivators and mediate activation signals from enhancer-bound regulatory proteins (7, 8, 14, 15, 28, 33, 59).
While TFIID has been extensively studied in vitro, very little is known about the function of individual TAFs in vivo. Studies with yeasts demonstrated that the absence of several TAFs did not influence the overall level of transcription but led to the death of cells, associated with specific cell cycle arrest phenotypes (2, 40, 61, 63). It has been determined that transcription of some yeast genes depends on TAFII145 (62). Results of studies of higher-eucaryotic TAFs are consistent with these results. Mutations in genes for two highly conserved TAFs, TAFII60 and TAFII110, reduced transcription of Bicoid-dependent target genes in Drosophila embryos (52) and led to lethality at the embryonic stage.
TAFII40 of Drosophila melanogaster (dTAFII40) is a member of the TAF family that has homologues in other higher eucaryotes (28). Several studies of the TAFII40 function in vitro were performed. A protein-protein interaction assay revealed direct binding between TAFII40 and the activation domains of VP16 (28) and p53 (57). A human homologue of dTAFII40, hTAFII31, was also identified as a critical protein required for p53 (38)- and VP16 (35)-dependent activation of transcription. The TAFII40 protein was postulated to mediate the activation by proteins with acidic domains. TAFII40 and TAFII60 were shown to contain histone folding motifs and to cocrystallize in a histone-like structure (30, 64). Although in vitro results suggest that TAFII40 plays an important role in transcription, no studies of TAFII40 function have been performed in vivo.
In our previous works, we identified mutations in the e(y)1, e(y)2, and e(y)3 genes (19, 20) that enhanced the phenotype of the y2 mutation. It was suggested that the protein products of these genes performed general and related functions in the regulation of transcription. They are involved in the activation of several genes and cooperate with the zeste protein in the control of white gene expression (21). Combinations of weak mutations of these genes are lethal.
In this study, we have cloned the e(y)1 gene and found that it encodes dTAFII40. Two e(y)1 mutations have been described. TAFII40 has been demonstrated to be indispensable. It accumulates in ovaries, and the inhibition of e(y)1 transcription severely suppresses oogenesis. The expression of at least a certain group of genes has been shown to be sensitive to a partial inhibition of e(y)1 transcription.
MATERIALS AND METHODS
Genetic crosses.
Flies were cultured at 25°C in standard Drosophila wheat meal-yeast-sugar-agar medium. All crosses were performed in standard glass vials with 5 to 10 males and 10 to 15 females per vial. The origin of e(y)1u1 and e(y)1P1 [e(y)4PI] alleles, mutations and constructions used in this work were described elsewhere (17, 19–21, 24–26, 37).
Strains with the SUPor-PM25 (also designated RR126) and RR97 constructions were obtained from P. Geyer’s lab. P(white) is the P-element transformation vector CaSpeR3 (48, 49). This vector carries a mini-white gene containing approximately 300 bp of 5′ and 630 bp of 3′ flanking DNA, while a major portion of the first intron is deleted (42).
Small-scale P-element mobilization experiments were carried out as described elsewhere (48). The number of insertion sites was determined by Southern blot analysis designed to identify the flanking restriction fragments. For further analysis, only single independent transpositions were selected. The CaSpeR3 transposon was mobilized in the same way as SUPor-P M25 (48).
Combinations of mutations located on the X chromosome (X*) and constructions with the marker white gene on an autosome were obtained according to the following scheme: ♀ X*/FM4 × ♂ P{white}/P{white}(autosome) or ♂P{white}−2/+; P{white}−3/+, where −2 and −3 denote the second and third chromosomes.
To combine the phP1 mutation (1-0.5) with the e(y)1P1 mutation, y phP1 females were crossed to f e(y)1P1 males. In F1, y phP1/f e(y)1P1 females were crossed to y f Bx2 males. In F2, y phP1 f e(y)1P1/y f Bx2 females were selected and mated to FM4 males. As a result, the y phP1 f e(y)1P1/FM4 strain was obtained.
Compound strains with su(Hw)2 and su(Hw)v mutations were obtained as described elsewhere (18a).
Eye color analysis was performed under a dissecting microscope with 3-day-old flies developing at 25°C. In each case, from 50 to 100 flies were scored to determine the eye color phenotype. Eye pigmentation was evaluated on the basis of pigmentation of the major part of its area. Analysis of pigmentation of flies with different allelic combinations was done as described previously (4, 5).
Preparation of the P{w+, e(y)1+} and P{w+, Δe(y)1} constructions and P-element-mediated transformation.
P{w+, e(y)1+} was created by insertion of the HindIII-XhoI region of e(y)1 into the CaSpeR3 vector. P{w+, Δe(y)1} is P{w+, e(y)1+} in which 80 nucleotides of e(y)1 corresponding to amino acids 255 to 278 (GGAGGAGGATCATCTGGCGTTGGAGTGGCCGTCAAGCGGGAACGTGAGGAGGAGGAGTTTGAGTTTGTGACCAACTAGCG) were replaced by 91 nucleotides of the Stalker long terminal repeat (LTR) starting from the 3′-terminal nucleotide of Stalker and followed by 6 nucleotides of the EcoRI site (TG TAATAGATG TAATAGAT T TGC T T TCCGAGC TCAGAACC TC TGCTCTGTTTGAATC TCT T TAT TCGAATGATCAAAG TGTGC TGAAGT TGGAATTC).
The P{w+, e(y)1+} or P{w+, Δe(y)1} construct and p25.7wc (34) were injected into y ac w67c preblastoderm embryos as described previously (50, 55). Chromosomal insertion of P{w+, e(y)1+} or P{w+, Δe(y)1} was tested by the reversion of the white phenotype, and the number of copies was determined by Southern blot analysis using P-element sequences as a probe.
Construction of libraries.
The cDNA library was constructed in the Uni-ZAP XR vector (Stratagene). The genomic library was constructed by cloning of DNA partially digested with endonuclease Sau3A in the λGEM11 vector. DNA and mRNA for the libraries were prepared from Oregon R adult flies.
RNA isolation and Northern blot analysis.
Total cellular RNA was isolated from Drosophila embryos, larvae, pupae, or adult flies as described elsewhere (39). Poly(A)+ RNA was selected on oligo(dT)-cellulose columns, and 1.5 μg of poly(A)+ RNA was loaded per lane of agarose gel. After electrophoresis, the RNA was transferred to Hybond-N membranes (Amersham). Hybridization was performed at 50°C in high-SDS–formamide buffer (7% sodium dodecyl sulfate [SDS], 50% formamide, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 2% blocking reagent [Boehringer Mannheim], 50 mM sodium phosphate [pH 7.0], 0.1% sarcosyl) overnight. 32P-labeled DNA probes were obtained in a random priming reaction. The membranes were washed two times in 0.1% SDS–1× SSC at room temperature for 10 min and for 20 min in 0.1% SDS–0.2× SSC at 65°C and then exposed to Kodak BioMax MS film with a Kodak BioMax MS intensifying screen for 2 to 4 h. Precise quantitation of the RNA in bands was done with a PhosphoImager (for Fig. 2) or with a photodensitometer (for Fig. 3).
FIG. 2.
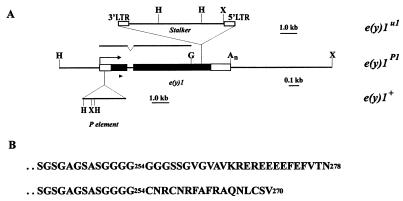
Structure of the e(y)1/TAFII40 gene. (A) Map of the e(y)1u1 mutation. Black boxes, the coding regions of e(y)1; open boxes, transcribed, nontranslated regions. The arrow indicates the direction of transcription. H, HindIII; X, XhoI; G, BglII. The region shown was used for wild-type phenotype rescue. The upper line indicates the region from the cDNA clone, which was used as a probe in Northern blot hybridization. The position of primers for RACE is indicated by a triangle. (B) Amino acid sequence of the carboxy terminus of wild-type (upper line) and mutant (lower line) TAFII40 protein.
FIG. 3.
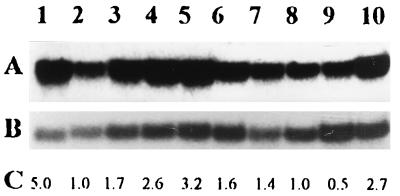
Transcription of the e(y)1/TAFII40 gene at different stages of development of D. melanogaster. (A) Northern blot hybridization of a fragment of e(y)1/TAFII40 cDNA (Fig. 2A) with mRNA from the Oregon R strain. Samples are from adult females (lane 1) and males (lane 2); late (lane 3), middle (lane 4), and early (lane 5) pupae; late third (lane 6)-, early third (lane 7)-, second (lane 8)-, and first (lane 9)-instar larvae; and embryos (lane 10). (B) The same blot, hybridized with the Ras2 probe. (C) Relative level of e(y)1/TAFII40 transcription. Signals were normalized according to the results of Ras2 hybridization. The level of e(y)1 transcription in males was taken as 1.
3′-RACE of e(y)1u1 mRNA.
For 3′-RACE (rapid amplification of 3′ cDNA ends), the first cDNA strand was synthesized by using 0.5 μg of mRNA from e(y)1u1 males with the (GA)10ACTAGTCTCGAG(T)18 primer and Superscript II reverse transcriptase (GibcoBRL). The product was purified in an agarose gel, and a two-step PCR was performed. For the first step, the following primers were used: GAGAGAGAGAACTAGTCTCGA and ATCCTGAAGGAGCTGAATG [sequences from the first exon of the e(y)1 gene (Fig. 2)]. Then, a nested PCR with the same first primer and with the nested second primer CGTGGTCAACCAACTGCT was performed (Fig. 2).
Protein expression and Western blot analysis.
The pQE-30 expression vector (Qiagen) and Escherichia coli XL1-Blue were used for His-tagged production of e(y)1 and e(y)1u1 proteins. Affinity-purified rabbit polyclonal antibodies against the His-tagged e(y)1 protein were used in immunoprecipitation, Western blot analysis, and immunodetection experiments. These antibodies were tested to give signals of the same rate on Western blots with the wild-type and mutant proteins.
Protein extracts were obtained from nuclei isolated from adult flies as described elsewhere (6) and lysed in a buffer containing 50 mM Tris HCl (pH 8.8), 150 mM NaCl, 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate, aprotinin (0.02 mg/ml), and leupeptin (0.1 mg/ml). Immunoprecipitation was performed as described elsewhere (51); the protein samples were subjected to electrophoresis in SDS–10% polyacrylamide gels (SDS-PAGE) and electroblotted to nitrocellulose membranes (Amersham). Western blotting was performed with an enhanced chemiluminescence system (Amersham) according to the manufacturer’s recommendations.
In situ hybridization to polytene chromosomes.
Drosophila polytene chromosome spreads were prepared from salivary glands of the third-instar larvae grown at 17°C. Preparation of spreads, fixation, denaturation, and hybridization were done as described in reference 16. Labeling was performed with [α-3H]dATP and [α-3H]dUTP in a random priming reaction.
Immunostaining of polytene chromosomes.
Fixation and squashing of salivary glands and antibody staining were performed as originally described by Platero et al. (45). Antibodies to TAFII40 were used at 1:10 dilution. Cy3-conjugated anti-rabbit antibodies (1:300; Sigma) were used as secondary antibodies.
In situ hybridization of tissue sections.
The flies were fixed in Carnoy’s solution for 1 h at room temperature. Paraffin embedding of the material and preparation of 7-μm sections were performed according to standard procedures (3). Digoxigenin (DIG) labeling of sense and antisense RNA and hybridization were performed according to the protocols for detection of mRNA with DIG-labeled RNA probes (Boehringer Mannheim).
Immunostaining of tissue sections.
Paraffin embedding, fixation, and sectioning were performed as described for in situ hybridization. Incubation with primary antibodies was performed as described for immunostaining of polytene chromosomes. Secondary horseradish peroxidase-conjugated anti-rabbit antibodies (1:1,000; Amersham) and diaminobenzidine (DAB) staining were used for visualization. The sections were counterstained with fast green.
RESULTS
The e(y)1 gene encodes the TAFII40 protein.
The e(y)1u1 mutation was induced by the insertion of the Stalker mobile element (19). Stalker is present in more than 50 copies in most D. melanogaster strains (20). Therefore, we have developed a special strategy based on preparing two sets of strains with the same genetic background differing in the location of a single Stalker copy responsible for the e(y)1u1 mutation (54). A clone containing Stalker and a flanking sequence of genomic DNA was obtained. The latter was used as a probe for screening the wild-type Oregon R library.
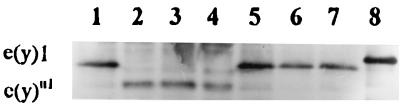
The 1.2-kb mRNA transcript changed in the e(y)1u1 strain (Fig. 1) was detected by Northern blot hybridization. A cDNA clone was obtained and sequenced. The result of a BLAST (1) search indicated that this sequence was identical to that of the gene encoding the TAFII40 protein (28).
FIG. 1.
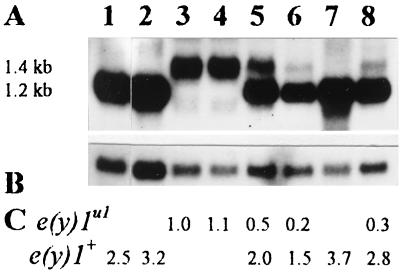
Transcription of the e(y)1u1 and e(y)1+ genes. (A) Northern blot hybridization of the fragment of e(y)1/TAFII40 cDNA (Fig. 2A) with mRNA from Oregon R males (lane 1), Oregon R embryos (lane 2), e(y)1u1/Y males (lane 3), e(y)1u1/e(y)1u1 females (lane 4), e(y)1u1/e(y)1+ females (lane 5), embryos from the ♀ e(y)1u1/e(y)1+ × ♂ e(y)1u1/Y cross (lane 6), C(1)RM,1yf females (lane 7), and embryos from the ♀ C(1)RM,yf × ♂ e(y)1u1/Y cross (lane 8). (B) The same blot hybridized with the Ras2 probe. (C) Relative level of e(y)1/TAFII40 transcription. The Northern blot was analyzed on a PhosphoImager; signals were normalized according to the results of Ras2 hybridization. The level of transcription in e(y)1u1/Y males was taken as 1.
To prove that the cloned gene was e(y)1, the genomic region of TAFII40 localization (Fig. 2A) was inserted into the CaSpeR3 vector and microinjected into embryos of the C(1)RM,yf/y2w e(y)1u1/Y strain. A complete reconstitution of the wild-type phenotype took place in five independent transgenic y2w e(y)1u1 P{w+, e(y)1+} lines of flies (Table 1), confirming that the cloned gene was indeed e(y)1. Thus, the e(y)1 gene encodes the TAFII40 protein.
TABLE 1.
Interactions between e(y)1 constructions and y2, e(y)1u1, and e(y)3u1 mutationsa
| Genotype | Pigmentation of bristlesb
|
Survivalc | |||
|---|---|---|---|---|---|
| Th | L | W | Ab | ||
| y2 e(y)1u1 | 1 | 2 | 5 | 5 | ND |
| y2 e(y)1u1;P{e(y)1+}-1-5/+ | 5 | 5 | 5 | 5 | ND |
| y2 e(y)1u1;P{Δe(y)1}-1/+ | 2 | 3 | 5 | 5 | ND |
| y2 e(y)1u1;P{Δe(y)1}-2/+ | 3 | 3 | 5 | 5 | ND |
| y2 e(y)1u1;P{Δe(y)1}-2/P{Δe(y)1}-2 | 4 | 5 | 5 | 5 | ND |
| y2 e(y)1u1;P{Δe(y)1}-3/+ | 4 | 4 | 5 | 5 | ND |
| y2 e(y)1u1;P{Δe(y)1}-2/P{Δe(y)1}-3 | 5 | 5 | 5 | 5 | ND |
| y2 e(y)1u1;P{Δe(y)1}-4/+ | 3 | 4 | 5 | 5 | ND |
| y2 e(y)1u1;P{Δe(y)1}-5/+ | 3 | 4 | 5 | 5 | ND |
| y2w e(y)3u1 | 3 | 3 | 5 | 5 | 54 |
| y2w e(y)1u1e(y)3u1 | 0 | ||||
| y2w e(y)1u1e(y)3u1;P{e(y)1+}-1-5/+ | 3 | 4 | 5 | 5 | 42–51 |
| y2w e(y)1u1e(y)3u1;P{Δe(y)1}-1/+ | 1 | 1 | 1 | 2 | 7 |
| y2w e(y)1u1e(y)3u1;P{Δe(y)1}-2/+ | 0 | 1 | 2 | 2 | 15 |
| y2w e(y)1u1e(y)3u1;P{Δe(y)1}-3/+ | 1 | 2 | 2 | 3 | 11 |
| y2w e(y)1u1e(y)3u1;P{Δe(y)1}-4/+ | 1 | 2 | 2 | 3 | 11 |
| y2w e(y)1u1e(y)3u1;P{Δe(y)1}-2/P{Δe(y)1}-3 | 2 | 2 | 4 | 5 | 17 |
Abbreviations: P{e(y)1+}-1-5 or P{Δe(y)1}-1-5, different single insertions of P{w+, e(y)1+} or P{w+, Δe(y)1} in five strains; P{Δe(y)1}-2, single insertion in the second chromosome; P{Δe(y)1}-3, single insertion in the third chromosome; Th, thoracal bristles; L, leg bristles; W, wing bristles; Ab, abdominal bristles.
Level of pigmentation in 3- to 5-day-old males developing at 25°C, ranked on a scale of 0 (pigmentation of y1 flies) to 5 (pigmentation of y+ flies). Flies with well-characterized y alleles were used to define the control level of pigmentation (4, 5). The effects of constructions with the e(y)1 gene on the y2 e(y)1u1, y2 e(y)3u1, and y2 e(y)1u1 e(y)3u1 mutation combinations was studied in the crosses of y2 e(y)1u1/FM4, y2 e(y)3u1/FM4, and y2 e(y)1u1e(y)3u1/FM4 females with y2w e(y)1u1;P{Δe(y)1}/P{Δe(y)1} and y2w e(y)1u1;P{e(y)1+}/P{e(y)1+} males. FM4 is an abbreviation for FM4,y31dsc8B, the balancer for the X chromosome.
Percentage of surviving males with a given phenotype, calculated as a ratio between given males and FM4 males. ND, not determined.
Expression of the e(y)1/TAFII40 gene during development.
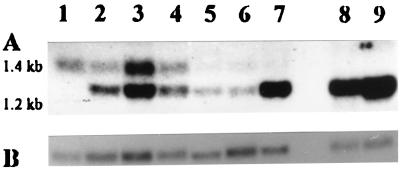
Northern blot hybridization was performed with mRNA isolated from the Oregon R strain at different developmental stages (Fig. 3). The transcription of the e(y)1 gene appeared to be stage dependent. An increased level of transcription was detected at the pupal and embryonic stages, but the highest level of e(y)1/TAFII40 mRNA—about five times higher than in adult males—was detected in adult females.
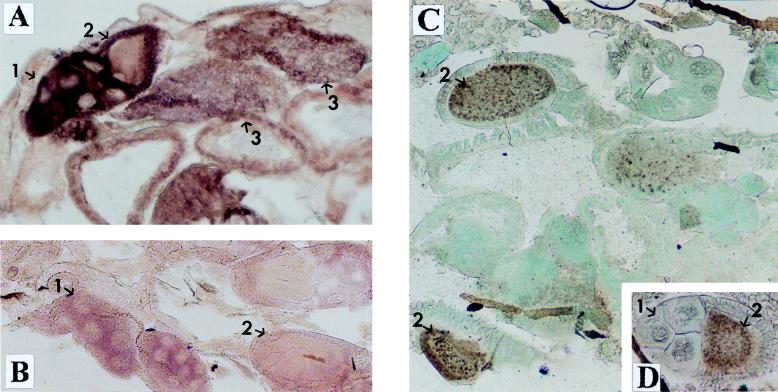
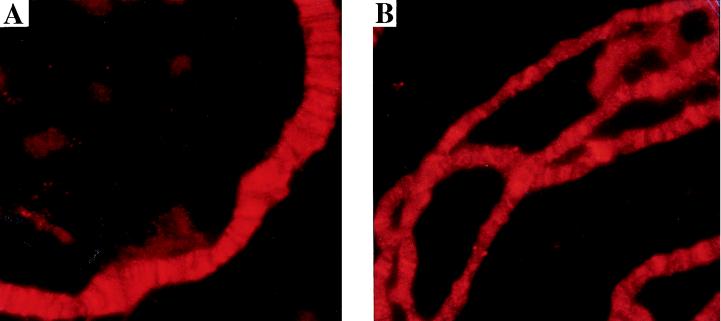
In situ hybridization on tissue sections of adult females demonstrated that the e(y)1/TAFII40 gene was highly expressed in trophocytes, follicular cells of gonads, and oocytes (Fig. 4A and B). The level of expression in all other tissues was much lower and did not significantly differ between tissues. Immunostaining with antibodies to the TAFII40 protein also showed a high content of the protein in oocytes (Fig. 4C and D). Thus, the high level of e(y)1/TAFII40 expression in ovaries explains the fivefold difference in the mRNA content between females and males.
FIG. 4.
Expression of e(y)1 in different tissues of Oregon R flies. (A and B) In situ hybridization of a frontal tissue section of female abdomen with the DIG-labeled e(y)1 antisense (A) and sense (B) RNA probes. (C and D) Immunostaining of a frontal tissue section of female abdomen with antibodies to e(y)1 protein. Horseradish peroxidase and DAB were used for visualization; the tissue was counterstained with fast green. One can see a high level of e(y)1 transcription and expression in ovaries: 1, in trophocytes; 2, in primary oocytes; 3, in mature oocytes. Note that while the level of e(y)1 mRNA content is high in trophocytes and mature oocytes and low in primary oocytes, the TAFII40 protein is predominantly detected in oocytes rather than in trophocytes (C). Magnification, ×130.
Molecular nature of the e(y)1u1 mutation: structural change of TAFII40.
Sequencing of the genomic copy of the e(y)1 gene showed that the latter consisted of two exons. The Stalker element in the e(y)1u1 mutation is inserted at the second exon in the direction opposite that of gene transcription (Fig. 2A). The insertion was located at a position corresponding to the 25th amino acid from the carboxy terminus of the protein (Fig. 2B). Therefore, the mutant e(y)1 protein can be assumed to represent a chimeric protein containing a foreign amino acid sequence at its carboxy terminus.
To check this, e(y)1u1 mRNA was studied. On Northern blots, it had an apparent size of ca. 1.4 kb, thus being about 0.2 kb longer than the wild-type mRNA (Fig. 1). The 3′ end of e(y)1u1 mRNA was cloned by reverse transcription-PCR with mRNA obtained from the mutant strain. Its sequence showed that e(y)1 mRNA terminated at different closely spaced sites within the 3′ LTR of Stalker. The chimeric protein was expected to be 270 amino acids in length, considering the location of the terminating codon within the Stalker sequence in all mRNAs (Fig. 2B). Thus, in the e(y)1u1 strain, 25 carboxy-terminal amino acids of TAFII40 are replaced by 17 amino acids encoded by Stalker sequence, and the change in the molecular mass of the protein should be 0.65 kDa.
On the other hand, the difference in molecular masses of normal and mutated proteins detected by Western blot analysis was 5 kDa (Fig. 5). This discrepancy can be explained by the anomalous mobility of TAFII40 in SDS-PAGE, because the wild-type and mutated proteins synthesized in the bacterial system had a similar difference in molecular mass (data not shown). Six glutamic amino acids were deleted in the e(y)1u1 protein, which may have greatly affected the mobility of the protein.
FIG. 5.
Western blot analysis of e(y)1 expression. Shown are results of immunoprecipitation of e(y)1 and e(y)1u1 proteins from nuclear extracts from adult flies of the Oregon R (lane 1), e(y)1u1 (lanes 2 to 4), and e(y)1+ strains (lanes 5 to 7) and the recombinant His-tagged protein (lane 8). The positions of e(y)1 and e(y)1u1 proteins are shown on the left.
Loss of the carboxy terminus does not affect the ability of TAFII40 to bind to chromatin. TAFII40 was detected in numerous sites on polytene chromosomes of the Oregon R strain (Fig. 6). The distribution of TAFII40 on chromosomes of the e(y)1u1 mutant was the same as on the wild-type chromosomes, although the content was decreased. However, the latter finding can be explained by a lower level of polytenization.
FIG. 6.
Immunostaining of polytene chromosomes from wild-type Oregon R (A) and e(y)1u1 (B) larvae with antibodies against e(y)1 and Cy3-conjugated secondary antibodies. Original magnification, ×1,000.
Inhibition of e(y)1 transcription in the e(y)1u1 flies.
The insertion of Stalker also interferes with e(y)1 transcription, possibly as a consequence of the activation of Stalker transcription from the 3′ LTR in the direction opposite that of the gene. A decrease of the e(y)1 mRNA content in mutated flies was detected at all stages of development (Fig. 1 and 7). In adult flies, the content of e(y)1+ mRNA was four times higher than that of e(y)1u1 mRNA in heterozygous e(y)1u1/e(y)1+ females (Fig. 1, lane 5) and 2.5 times higher in e(y)1+ males than in e(y)1u1 males (Fig. 1, lanes 1 and 3).
FIG. 7.
Effect of the e(y)1u1 mutation on e(y)1 transcription. (A) Northern blot hybridization of the fragment of e(y)1/TAFII40 cDNA (Fig. 2A) with mRNA isolated at different stages of development of the progeny of the ♀ C(1)RM, yf × ♂ e(y)1u1/Y cross: males (lane 1); late (lane 2), middle (lane 3), and early (lane 4) pupae; third (lane 5)- and first (lane 6)-instar larvae; embryos (lane 7); and adult females (lane 8). Lane 9, mRNA from females of the Oregon R strain. (B) The same blot, hybridized with the Ras2 probe.
The lowest ratio of e(y)1u1 mRNA to e(y)+ mRNA, equal to 1:9 to the progeny of the cross of C(1)RM,yf females to e(y)1u1/Y males, was found in embryos (Fig. 1, lane 8). This finding is not surprising, as the eggs were laid by females bearing only the wild-type copy of the gene and according to in situ hybridization, they should contain a large amount of maternal mRNA (see above). The presence of a weak 1.4-kb band (Fig. 1, lane 8) should represent e(y)1u1 mRNA synthesized in embryos. A more interesting finding was that the ratio of e(y)1u1 mRNA to e(y)+ mRNA was almost equally low in embryos from the cross of e(y)1u1/e(y)1+ females to e(y)1u1/Y males (Fig. 1, lane 6), revealing either the absence or an extremely low content of e(y)1u1 mRNA in the maternal mRNA of embryos. This means that homozygous e(y)1u1/e(y)u1 females may have difficulty supplying their oocytes with e(y)1u1 mRNA. On the other hand, immunohistochemistry detected the presence of the TAFII40 protein in the residual ovaries of e(y)1u1/e(y)1u1 homozygous females (Fig. 8).
FIG. 8.
Ovaries from wild-type (A) and e(y)1u1 (B) flies. Immunostaining of frontal tissue section of female abdomen with antibodies to the e(y)1 protein. Horseradish peroxidase and Sigma fast DAB with a metal enhancer were used for visualization. Magnification, ×70.
The main biological effects of the e(y)1u1 mutation depend on partial inhibition of e(y)1 transcription.
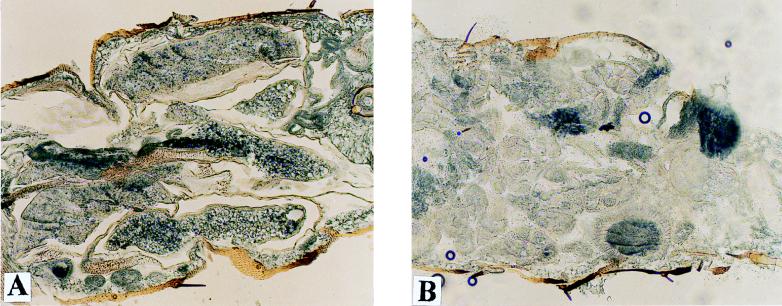
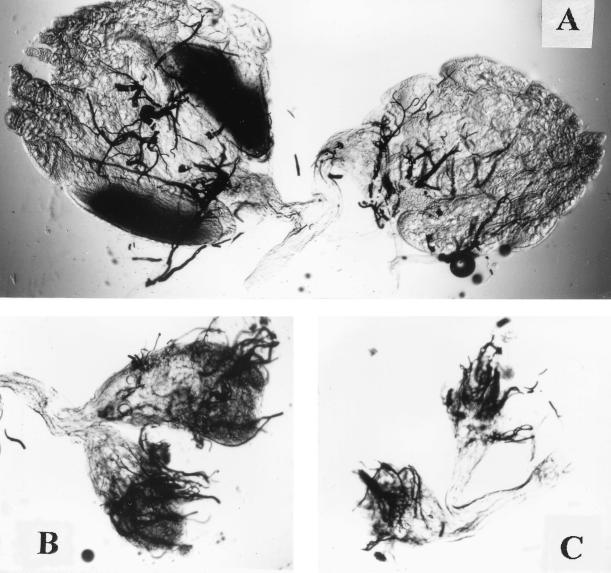
The e(y)1u1 mutation did not affect the viability of flies, and no visible morphological changes were detectable in adult mutant flies. However, females homozygous for the e(y)1u1 mutation were sterile. The ovaries of mutant flies were found to be dramatically underdeveloped. They were very small and did not contain mature oocytes (Fig. 8 and 9). Microinjection of a construction with the e(y)1+ gene restored normal fertility and ovary morphology in homozygous e(y)1u1 females, confirming the dependence of ovary development on the e(y)1 phenotype.
FIG. 9.
Ovaries from wild-type (A) and e(y)1u1 (B and C) flies (total preparation). Magnification, ×40.
There may be two possible explanations for female sterility. One is that the normal level of e(y)1 expression is important for oocyte development; the second is that the unchanged carboxy terminus of TAFII40 is essential for the expression of some genes involved in the maturation of oocytes. To test these two possibilities, we made an attempt to rescue the wild-type phenotype by microinjection of the P{w+, Δe(y)1} construction, which expressed exactly the same mutant TAFII40 protein with Stalker amino acids at the end as e(y)1u1 flies (Fig. 2). Five w+ revertants bearing the construction in different sites of autosomes were obtained. In all cases, the fertility of e(y)1u1 females was restored. Thus, it is the reduced transcription of the e(y)1 gene that leads to the sterility of e(y)1u1 females.
TAFII40 is indispensable.
We have also obtained another mutation of the e(y)1 gene induced by insertion of the P element. This allele was isolated in P-M hybrid expression dysgenesis as the e(y)4P1 mutation and had a milder phenotype in comparison to e(y)1u1: males had shortened and thin bristles, while females were morphologically normal and fertile. The mutation was genetically localized in approximately the same region of the X chromosome as e(y)1u1 (19). Southern blot analysis showed that insertion of the P element occurred in the e(y)1 gene. The exact site of the insertion was cloned by PCR using the P-element and e(y)1 sequences as primers (Fig. 2). As it is located in the e(y)1 gene, the designation e(y)1P1 will be used hereafter.
The e(y)1P1 mutation is induced by insertion of the P element into the transcribed noncoding 5′ region of the gene (Fig. 2). Thus, the coding region of the gene is not damaged, and we also did not detect any changes in the level of e(y)1 transcription by Northern blot hybridization (data not shown).
Recently we have developed a method to dramatically increase the effect of the P element on transcription of a target gene by introducing the phP1 mutation (5). The latter was induced by P-element insertion in the polyhomeotic (ph) gene, resulting in expression of the chimeric P-Ph protein consisting of the DNA-binding domain of P-element transposase and an almost complete Ph protein sequence. The P-Ph protein binds the P-element sequences and recruits to this site other members of the Pc-repressive complex. This leads to blocking of transcription from promoters located in close vicinity to the P-element insertion (5).
The combination of the e(y)1P1 mutation with phP1 led to the lethal phenotype. The wild-type phenotype could be restored by transformation of e(y)1P1 phP1 flies with the P{w+, e(y)1+} construction. To detect the stage of death, we crossed e(y)1P1 phP1/FM4 females to e(y)1P1 males. We found that embryos died at the middle and late embryonic stages (from stages 9 to 14). Thus, the TAFII40 protein is indispensable.
The e(y)1u1 mutation inhibits yellow expression in bristles but not in the body and wings.
The e(y)1u1 allele does not change the viability and phenotype of flies, suggesting that the transcription of most genes is not sensitive to a moderate decrease in the concentration of truncated TAFII40. However, the e(y)1u1 mutation was shown to inhibit the expression of several genes in the case of their partial inactivation as a result of insertion of foreign sequences, partial deletion of enhancer, or a mutation in trans-regulatory gene (17, 19, 21). These events probably make transcription more sensitive to the influence of e(y)1u1.
The yellow and white genes were further used to study some features of TAFII40 activity. The yellow gene contains different enhancers responsible for yellow expression in the wings, body, and bristles (22). The question was whether yellow expression driven by different enhancers was equally sensitive to e(y)1u1. The previously used y2 mutation was not suitable to clarify this question as the body and wing enhancers were blocked by an insulator, gypsy su(Hw)-binding region (27). Therefore, we checked the effect of the e(y)1u1 mutation on some other yellow alleles (Fig. 10).
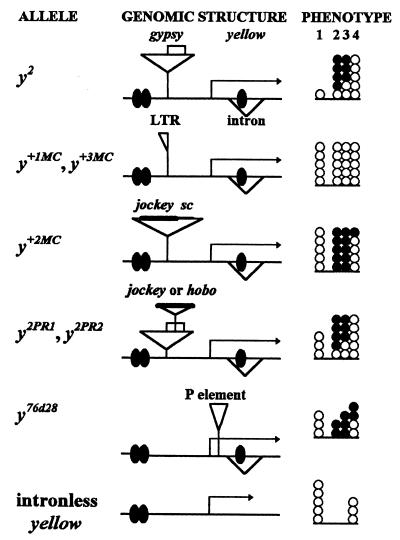
FIG. 10.
Genetic analysis of interaction of y alleles with e(y)1u1 mutation. The schemes for y alleles are not to scale. yellow transcripts are shown by arrows; transcriptional enhancers are indicated by shaded ovals. The enhancers that control yellow expression in the wings and body cuticle are located in the 5′-upstream region of the yellow gene, whereas enhancers controlling yellow expression in the bristles reside in the intron of the gene (22). The su(Hw)-binding region is indicated by empty boxes; insertions found in the various alleles are represented by triangles. The total number of circles in the phenotype column indicates the levels of pigmentation of the body and wings (column 1), thoracic bristles (column 2), leg bristles (column 3), and abdominal bristles (column 4). The number of black circles shows the inhibitory effect of the e(y)1u1 mutation on yellow expression for different y alleles. Each circle represents one point on the scale described in the footnote to Table 1.
The e(y)1u1 mutation interfered with yellow expression in revertants of y2 flies associated with rearrangements of gypsy but failed to affect the pigmentation of revertants which lacked the whole gypsy insertion except one LTR (y+IMC, y+3MC). The e(y)1u1 mutation had the strongest effect in combination with the y+2MC revertant, which was induced by the insertion of jockey and a deletion of the su(Hw)-binding region of gypsy (17, 25). y+2MCe(y)1u1 flies had the same color of the bristles as flies lacking the bristle enhancer, while the body and wings remained normally pigmented (Fig. 10). Similar results were obtained in experiments with two partial y2 revertants (24) which had the su(Hw)-binding region disrupted by the insertion of either jockey (y2PR1) or hobo (y2PR2). Flies from both strains have an intermediate coloration of the body and wings which was very sensitive to any modification of the transcription level. Again the e(y)1u1 mutation reduced the pigmentation of bristles in the same way as in the original y2 allele, but it did not change the level of body and wing pigmentation (Fig. 10).
We also tested the y76d28 mutation caused by the insertion of the P element into the 5′-transcribed, untranslated portion of the yellow gene (26). The color of all adult cuticular structures is tan in y76d28 flies, indicating that yellow gene expression decreases in all cell types to a level intermediate between that of wild-type flies and that of flies carrying a deficiency for the yellow gene. As in the previous cases, the e(y)1u1 mutation reduced the pigmentation of bristles but not of the body and wings in y76d28 flies.
Role of carboxy termini in TAFII40 protein function.
As shown above, the P{w+, Δe(y)1} construction expressing the truncated version of TAFII40 protein restores the fertility of e(y)1u1 females. However, fertility is a qualitative factor that does not allow for quantitative assessment of the role of the carboxy terminus in the TAFII40 function. Thus, it would be interesting to compare the effects of P{w+, Δe(y)1} and P{w+, e(y)1+} constructions on the bristle pigmentation of y2 w e(y)1u1 flies. Five strains with a single P{w+, Δe(y)1} construction and five strains with a single P{w+, e(y)1+} construction located on the second or third chromosome possessing orange or dark orange eyes were selected. The levels of e(y)1 expression in the constructions were comparable, as shown by Northern blot analysis of y2 w; P{w+, Δe(y)1}, and y2 w e(y)1u1;P{w+, e(y)1+} males (not shown).
All five tested P{w+, Δe(y)1} constructions in heterozygote (a single copy of the construction per genome) only partially restored the bristle pigmentation of y2 w e(y)1u1 flies. On the other hand, a single copy of any of five P{w+, e(y)1+} constructions completely suppressed the mutant phenotype of the e(y)1u1 allele (Table 1). Combination of two different P{w+, Δe(y)1} constructions in heterozygote or in homozygote [two copies of the same P{w+, Δe(y)1} construction] led to a stronger suppression of mutant bristle phenotype. This result suggests that more truncated protein is required for restoring the yellow expression in bristles.
Similar results were obtained in experiments with the e(y)1u1 e(y)3u1 combination of mutations. By itself, the e(y)3u1 mutation only mildly decreased the viability of flies. However, the combination of the e(y)1u1 mutation with e(y)3u1 is lethal at the late larval and early pupal stages of development (21). The viability and bristle pigmentation of flies carrying the e(y)1u1 e(y)3u1 combination were completely restored in three independent strains with the P{w+, e(y)1+} construction. The P{w+, Δe(y)1} constructions only partially rescued the viability of e(y)1u1 e(y)3u1 flies (Table 1).
Similarly, the surviving y2 e(y)1u1 e(y)3u1;P{w+, Δe(y)1}/+ flies still had a strong mutant bristle phenotype. Combination of two different P{w+, Δe(y)1} constructions in heterozygote led to more prominent suppression of mutant phenotype.
Effect of the e(y)1u1 mutation on white expression.
It was found previously that the e(y)1u1 mutation suppressed the enhancer-dependent transcription of the white gene in the absence of the zeste protein (21). The white gene has an enhancer element located in the 5′-upstream region (36, 44, 60). In the absence of the upstream enhancer, the eyes are yellow. The combination of zv77h and e(y)1u1 mutations, each of which does not significantly affect white expression, strongly and synergistically decreases the eye pigmentation almost to the level typical of enhancerless flies (21).
To further study the role of the e(y)1u1 mutation in activation of the white promoter by enhancers, we used the y−ac−w1118 strain with a mini-white CaSpeR3 construction which contained a mini-white gene without an eye enhancer (42). In general, y−ac−w1118 CaSpeR3 flies have yellow eyes, the residual color being maintained by the promoter-dependent transcription. The mini-white construction was mobilized by crosses with the Δ2-3(99B) strain, and 17 strains with a single insertion of the mini-white construction on the second or third chromosome that possessed eye color from dark orange to red were selected (Table 2). The activation of white expression in enhancerless constructions may be explained by the presence of a foreign enhancer element in the neighborhood of the white gene and by a local structure of chromatin. In 12 of 17 strains, the e(y)1u1 mutation strongly or moderately reduced the level of eye pigmentation (Tabel 2). This result suggests that white expression is sensitive to the e(y)1u1 mutation.
TABLE 2.
Influence of the e(y)1u1 mutation on white expression in different white constructions
| Constructiona | No. of strains | Genotype | Phenotypeb (no. of strains) |
|---|---|---|---|
| P(white) | 5 | + | Red |
| e(y)1u1 | Red (3), brown (1), yellow-orange (1) | ||
| 8 | + | Brown | |
| e(y)1u1 | Brown (2), orange (2), yellow-orange (3), yellow (1) | ||
| 4 | + | Orange | |
| e(y)1u1 | Yellow (4) | ||
| P(BR/Eye/white/BR) [SUPor-P transposon] | 5 | + | Red |
| su(Hw)2/su(Hw)v | Red | ||
| zv77h e(y)1u1 | Red | ||
| zv77h e(y)1u1;su(Hw)2/su(Hw)vv | Yellow-orange | ||
| 3 | + | Red | |
| su(Hw)2/su(Hw)v | Brown-orange | ||
| zv77h e(y)1u1 | Red | ||
| zv77h e(y)1u1;su(Hw)2/su(Hw)vv | Yellow | ||
| P(Eye/BR/white) [RR97 transposon] | 3 | + | Brown |
| zv77h e(y)1u1 | Yellow-orange |
P(BR/Eye/white/BR) (48, 49) has two su(Hw)-binding regions (BR) flanking the eye enhancer (Eye) and the CaSpeR mini-white gene. P(Eye/BR/white) (49) contains the su(Hw)-binding region inserted between the eye enhancer (Eye) and the CaSpeR mini-white gene.
A low level of white expression produces the yellow eye phenotype, whereas wild-type expression gives the red eye color. The number of strains showing the specified eye color is given in parentheses.
Insulation by the su(Hw)-binding region makes white transcription insensitive to the e(y)1u1 mutation.
The SUPor-P construction contains the mini-white gene and its eye enhancer framed by two su(Hw)-binding regions. The latter makes white transcription independent of the genomic position (48, 49). We obtained eight different strains carrying the SUPor-P construction in different sites of the second chromosome. All of them had the wild-type red-colored eyes (Table 2).
The combination of SUPor-P constructions with e(y)1u1 and zv77h mutations did not influence eye color. On the other hand, an additional introduction of su(Hw)2/su(Hw)v mutations inactivating the su(Hw) gene (29, 41) led to the inhibition of white expression in the presence of e(y)1u1 and zv77h mutations (Table 2). In three cases, su(Hw)2/su(Hw)v mutations alone induced a slight inhibition of white expression, but it was much weaker.
To test the possibility that the su(Hw) protein itself can activate white expression in the presence of e(y)1u1 and zv77h, we used the RR97 construction, obtained from P. Geyer, where the su(Hw)-binding region was inserted between the eye enhancer and the white promoter (49). Flies from three independent strains with a single insertion of RR97 had brown eyes. The introduction of e(y)1u1 and zv77h mutations enhanced the mutant white phenotype (Table 2), indicating that the su(Hw) protein could not directly activate white expression in the e(y)1u1 zv77h combination of alleles.
DISCUSSION
The e(y)1 gene encodes a TAFII40 protein.
The main result obtained is that one of abundant TAFs, i.e., dTAFII40, is encoded by the previously described e(y)1 gene. On the basis of some genetic data, the latter was suggested to be involved in the control of long-distance interactions, in particular between yellow and white enhancers and promoters (19, 21).
TAFII40 is incorporated into the TFIID multiprotein complex (47, 59). It has been proposed that various classes of gene-specific activators interact with one or more TAFs in order to provide transcription of their target genes. In vitro protein-protein interaction assay revealed a direct binding of dTAFII40 or its human homologue hTAFII31 with an activation domain of several transcription factors (28, 35, 38, 57). It has been postulated that the TAFII40 protein mediates the activation by proteins with acidic domains. Thus, TAFII40 possesses the features that can be expected for the protein product of the e(y)1 gene.
We have found that the wild-type TAFII40 protein seems to be involved in the organization of transcription from a large group of promoters, as it is present in practically every band of a polytene chromosome. In addition, TAFII40 expression was detected in all organs of adult flies. An elevated level of expression was detected at the embryonic and pupal stages of development, when the growth of new tissues is prominent.
A particularly high level of TAFII40/e(y)1 expression was found in female gonads, which leads to an approximately fivefold difference in the e(y)1 mRNA content between females and males. As a result, large amounts of mRNA and the protein accumulate in oocytes. This finding indicates that the TAFII40/e(y)1 gene may be a maternal gene and suggests an important role of the TAFII40 protein in gene activation during early embryogenesis.
In vivo consequences of e(y)1 mutations.
Here we have described for the first time mutations of the gene encoding the TAFII40 protein in higher eucaryotes. One of them, the e(y)1P1 mutation, is induced by P-element insertion and has almost no influence on e(y)1 expression. However, its effect can be significantly enhanced in combination with the phP1 mutation, known to repress transcription of genes with a P-element insertion in the neighborhood of the promoter element (5). The phP1 e(y)1P1 combination is lethal at the middle embryonic stage of development, indicating that TAFII40 is an indispensable protein.
Survival of embryos throughout stages 9 to 14 can be explained by a high concentration of TAFII40 in oocytes. Similar results were obtained for TAFII60 and TAFII110 (52). A large maternal contribution of wild-type TAFII60 and TAFII110 supported the first 15 to 16 stages of embryogenesis against the null-mutant background.
Another mutation, e(y)1u1, is induced by the Stalker mobile element insertion into the coding sequence of the e(y)1 gene. This insertion leads to two effects: (i) truncation of TAFII40 with replacement of 25 carboxy-terminal amino acids by 17 foreign amino acids and (ii) a decrease of the level of e(y)1 transcription. The mutation results in a dramatic underdevelopment of ovaries leading to female sterility and in a mild repression of transcription of several genes.
We found that female fertility could be restored by the synthesis of truncated TAFII40 protein and demonstrated that this major effect of e(y)1u1 mutation depended on reduced e(y)1 transcription rather than on TAFII40 structural changes. A specific effect on the development of ovaries may be explained either by a stronger inhibition of e(y)1 transcription by Stalker in ovaries or by a selective sensitivity of the expression of some genes critical for ovary development.
TATA-less promoters are sensitive to weak mutation in the TAFII40/e(y)1 gene.
It was found recently that the dTAFII60-dTAFII40 heterotetramer bound to the downstream promoter element (DPE), a distinct 7-nucleotide core promoter element located about 30 nucleotides downstream of the transcription start site of many TATA-box-deficient (TATA-less) promoters in Drosophila (9, 10). It was suggested that the dTAFII60-dTAFII40 heterotetramer plays a direct role in basal transcription of TATA-less DPE-containing genes.
Expression of the white gene was found to be sensitive to the combination of zv77h and e(y)1u1 mutations (21). Here we have demonstrated that white expression is frequently and strongly influenced by the e(y)1u1 mutation alone in enhancerless constructions putatively activated by different foreign enhancers. On the other hand, it is known that the white gene contains a TATA-less promoter with a DPE core sequence. This agrees with a strong dependence of white expression on the TAFII40 protein content.
Our data also demonstrate that the e(y)1u1 mutation moderately reduced yellow expression in the bristles but not in the body cuticle and wing blades. The yellow gene has a typical TATA box. However, we recently found that deletion of the TATA promoter affected only body and wing pigmentation, not yellow expression in bristles in the presence of a strong enhancer element (18). This finding suggests the presence of an internal promoter element interacting with the bristle enhancer and activating yellow expression in bristles. The sequence of the putative yellow promoter region has no homology to the DPE-containing promoters (9, 10), but the canonic DPE sequence is present in only 20% of TATA-less promoters. Thus, in vivo TATA-less promoters represent a group of promoters that are most sensitive to the reduction of the TAFII40 content.
A possible role of the TAFII40 carboxy-terminal domain in vivo.
As was shown, the mutant phenotype of the e(y)1u1 allele could be at least partially reversed by supplying an additional amount of truncated e(y)1/TAFII40 protein, in agreement with the results of in vitro experiments. It has been shown that 222 amino-terminal amino acids of TAFII40 harbor domains for interactions with basic factors, activators, and other TAFs (28). dTAFII40 and hTAFII31 have significant homology only in their amino termini (28). The carboxy-terminal portion of dTAFII40 bears similarity to many glycine-rich proteins (28), but in vitro experiments reveal no function of the carboxy terminus in protein-protein interactions.
However, any tested single copy of the P{w+, Δe(y)1} construction does not completely compensate for the effect of the e(y)1u1 mutation on the y2 phenotype or the lethal phenotype of the e(y)1u1 e(y)3u1 combination of mutations. Even the presence of two doses of the P{w+, Δe(y)1} construction fails to completely rescue the y+ phenotype or suppress the lethal phenotype of the e(y)1u1 e(y)3u1 combination of mutations. On the other hand, a single dose of the P{w+, e(y)1+} construction has a much stronger suppression effect.
Thus, deletion of the carboxy-terminal amino acids seems to make expression of tagged genes more sensitive to the concentration of TAFII40 protein. We speculate that the carboxy-terminal portion of TAFII40 promotes an effective binding of the protein to the DNA covered by nucleosomes. This may explain the compensation of its loss by an increase of the mutant protein concentration. It is worth noting that the deleted carboxy-terminal part of TAFII40 contains the only charged stretch of the protein (total charge is −6). As human TAFII31 also has a single charged stretch (total charge is −13) located at its carboxy terminus, this similarity may reflect some special function of this region. The negatively charged carboxy terminus is a characteristic feature of many transcription factors as well as the HMG-1 and HMG-2 families (12).
It is not clear why expression of the white gene flanked by the su(Hw)-binding regions is independent of the combination of the e(y)1u1 and zv77h mutations. The e(y)1u1 mutation in combination with the zv77h-null allele strongly reduces white expression (21). However, two su(Hw)-binding sites flanking the white gene stabilize white expression, making it independent of the e(y)1u1 and zv77h mutation combination. The su(Hw)-binding region in gypsy mobile element has the properties of an insulator: it interferes with expression of the gene in tissues where it is regulated by enhancers located distally from the su(Hw)-binding site with respect to the promoter (13, 23, 31, 32, 49, 53). Two su(Hw)-binding regions flanking a construction make the expression of a gene independent of the negative effect of a surrounding chromatin. It may be that su(Hw) insulators support an open chromatin structure in the promoter area of the mini-white gene that facilitates binding of the truncated TAFII40 protein to the white promoter. However, further experiments are necessary to support this proposition.
ACKNOWLEDGMENTS
We are greatly indebted to V. G. Corces and P. K. Geyer for providing the fly strains, A. Vasiljev for participation in antiserum purification, and S. Dzitoeva and Y. Schwartz for help with microinjections.
This work was supported by the Russian State Program “Frontiers in Genetics,” by the Russian Basic Research Fund, INTAS-94-3801 and HFSP grants, and by an International Research Scholar’s award from the Howard Hughes Medical Institute to P.G. The work of A. Soldatov and S. Georgieva was supported by a grant from the Centre for Medical Research, University of Oslo.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Apone L M, Virbasius C-M A, Reese J C, Green M R. Yeast TAFII90 is required for cell-cycle progression through G2/M but not for general transcription activation. Genes Dev. 1996;10:2368–2380. doi: 10.1101/gad.10.18.2368. [DOI] [PubMed] [Google Scholar]
- 3.Ashburner M. Drosophila: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 4.Belenkaya T, Barseguyan K, Hovhannisayn H, Biryukova I, Kochieva E Z, Georgiev P. P element sequences can compensate a deletion of the yellow regulatory region in Drosophila melanogaster. Mol Gen Genet. 1998;259:79–87. doi: 10.1007/s004380050791. [DOI] [PubMed] [Google Scholar]
- 5.Belenkaya T, Soldatov A, Nabirochkina E, Birjukova I, Georgieva S, Georgiev P. The allele of the polyhomeotic gene induced by P element insertion encodes a new chimeric protein, that negatively regulates the expression of P-induced alleles in the yellow locus of Drosophila melanogaster. Genetics. 1998;150:687–697. doi: 10.1093/genetics/150.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bickel S, Pirrotta V. Self-association of the Drosophila zeste protein is responsible for the transvection effects. EMBO J. 1990;9:2959–2967. doi: 10.1002/j.1460-2075.1990.tb07488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brou C, Chaudhary S, Davidson I, Lutz Y, Wu J, Egly J M, Tora L, Chambon P. Distinct TFIID complexes mediate the effect of different transcriptional activators. EMBO J. 1993;12:489–499. doi: 10.1002/j.1460-2075.1993.tb05681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brou C, Wu J, Ali S, Scheer E, Lang C, Davidson I, Chambon P, Tora L. Different TBP-associated factors are required for mediating the stimulation of transcription in vitro by the acidic transactivator GAL-VP16 and the two nonacidic activation functions of the estrogen receptor. Nucleic Acids Res. 1993;21:5–12. doi: 10.1093/nar/21.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke T W, Kadonaga J T. Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 1996;10:711–724. doi: 10.1101/gad.10.6.711. [DOI] [PubMed] [Google Scholar]
- 10.Burke T W, Kadonaga J T. The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev. 1997;11:3020–3031. doi: 10.1101/gad.11.22.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burley S K, Roeder R G. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 12.Bustin M, Lehn D A, Lendsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990;1094:231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- 13.Cai H, Levine M. Modulation of enhancer-promoter interactions by insulators in the Drosophila embryo. Nature. 1995;376:533–536. doi: 10.1038/376533a0. [DOI] [PubMed] [Google Scholar]
- 14.Chen J-L, Attardi L D, Verrijzer C P, Yokomori K, Tjian R. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell. 1994;79:93–105. doi: 10.1016/0092-8674(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 15.Dubrovskaya V, Lavigne A-C, Davidson I, Acker J, Staub A, Tora L. Distinct domains of hTAFII100 are required for functional interaction with transcription factor TFIIFb (RAP30) and incorporation into the TFIID complex. EMBO J. 1996;15:3702–3712. [PMC free article] [PubMed] [Google Scholar]
- 16.Fauvarque M-O, Dura J-M. polyhomeotic regulatory sequences induce developmental regulator-dependent variegation and targeted P-element insertions in Drosophila. Genes Dev. 1993;7:1508–1520. doi: 10.1101/gad.7.8.1508. [DOI] [PubMed] [Google Scholar]
- 17.Gause M, Georgieva S, Georgiev P. Phenotypic reversion of the gypsy-induced mutation scD1 of Drosophila melanogaster by replicative transposition of a sc enhancer to the yellow gene and by mutations in the enhancer of yellow and zeste loci. Mol Gen Genet. 1996;253:370–376. doi: 10.1007/pl00008603. [DOI] [PubMed] [Google Scholar]
- 18.Georgiev, P., and T. Kahn. Unpublished data.
- 18a.Georgiev P, Kozycina M. Interaction between mutations in the suppressor of Hairy wing and modifier of mdg4 genes of Drosophila melanogaster affecting the phenotype of gypsy-induced mutations. Genetics. 1996;142:425–436. doi: 10.1093/genetics/142.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgiev P G, Gerasimova T I. Novel genes influencing the expression of the yellow locus and mdg4 (gypsy) in Drosophila melanogaster. Mol Gen Genet. 1989;220:121–126. doi: 10.1007/BF00260865. [DOI] [PubMed] [Google Scholar]
- 20.Georgiev P G, Kiselev S L, Simonova O B, Gerasimova T I. A novel transposition system in Drosophila melanogaster depending on the Stalker mobile genetic element. EMBO J. 1990;9:2037–2044. doi: 10.1002/j.1460-2075.1990.tb07370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgiev P G. Identification of mutations in three genes that interact with zeste in the control of white gene expression in Drosophila melanogaster. Genetics. 1994;138:733–739. doi: 10.1093/genetics/138.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geyer P K, Corces V G. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1987;1:996–1004. doi: 10.1101/gad.1.9.996. [DOI] [PubMed] [Google Scholar]
- 23.Geyer P K, Corces V G. DNA position-specific repression of transcription by a Drosophila zinc finger protein. Genes Dev. 1992;6:1865–1873. doi: 10.1101/gad.6.10.1865. [DOI] [PubMed] [Google Scholar]
- 24.Geyer P K, Green M M, Corces V G. Mutant gene phenotypes mediated by a Drosophila melanogaster tetrotransposon require sequences homologous to mammalian enhancers. Proc Natl Acad Sci USA. 1988;85:8593–8597. doi: 10.1073/pnas.85.22.8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geyer P K, Green M M, Corces V G. Reversion of a gypsy-induced mutation at the yellow (y) locus of Drosophila melanogaster is associated with the insertion of a newly defined transposable element. Proc Natl Acad Sci USA. 1998;85:3938–3942. doi: 10.1073/pnas.85.11.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geyer P K, Richardson K L, Corces V G, Green M M. Genetic instability in Drosophila melanogaster: P-element mutagenesis by gene conversion. Proc Natl Acad Sci USA. 1988;85:6455–6459. doi: 10.1073/pnas.85.17.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geyer P K, Spana C, Corces V G. On the molecular mechanism of gypsy-induced mutations at the yellow locus of Drosophila melanogaster. EMBO J. 1986;5:2657–2662. doi: 10.1002/j.1460-2075.1986.tb04548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodrich J A, Hoey T, Thut C J, Admon A, Tjian R. Drosophila TAFII40 interacts with both VP16 activation domain and the basal transcription factor TFIIB. Cell. 1993;75:519–530. doi: 10.1016/0092-8674(93)90386-5. [DOI] [PubMed] [Google Scholar]
- 29.Harrison D A, Gdula D A, Coyne R S, Corces V G. A leucine zipper domain of the suppressor of Hairy-wing protein mediates its repressive effect on enhancer function. Genes Dev. 1993;7:1966–1978. doi: 10.1101/gad.7.10.1966. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman A, Chiang C-M, Oelgeschlager T, Xie X, Burley S K, Nakatani Y, Roeder R G. A histone octamer-like structure within TFIID. Nature. 1996;380:356–359. doi: 10.1038/380356a0. [DOI] [PubMed] [Google Scholar]
- 31.Holdridge C, Dorsett D. Repression of hsp70 heat shock gene transcription by the suppressor of Hairy-wing protein of Drosophila melanogaster. Mol Cell Biol. 1991;11:1894–1990. doi: 10.1128/mcb.11.4.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack J, Dorsett D, DeLotto Y, Liu S. Expression of the cut locus in the Drosophila wing margin is required for cell type specification and is regulated by a distant enhancer. Development. 1991;113:735–747. doi: 10.1242/dev.113.3.735. [DOI] [PubMed] [Google Scholar]
- 33.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 34.Kares R E, Rubin G M. Analysis of P transposable element functions in Drosophila. Cell. 1984;38:135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- 35.Klemm R D, Goodrich J A, Zhou S, Tjian R. Molecular cloning and expression of the 32-kDa subunit of human TFIID reveals interactions with Vp16 and TFIIB that mediate transcriptional activation. Proc Natl Acad Sci USA. 1995;92:5788–5792. doi: 10.1073/pnas.92.13.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levis R, Hazelrigg T, Rubin G M. Separable cis-acting control elements for expression of the white gene of Drosophila. EMBO J. 1985;4:3489–3499. doi: 10.1002/j.1460-2075.1985.tb04108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsley D L, Zimm G G. The genome of Drosophila melanogaster. New York, N.Y: Academic Press; 1992. [Google Scholar]
- 38.Lu H, Levine A. Human TAFII31 protein is a transcriptional coactivator of the p53 protein. Proc Natl Acad Sci USA. 1995;92:5154–5158. doi: 10.1073/pnas.92.11.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maes M, Messens E. Phenol as grinding material in RNA preparations. Nucleic Acids Res. 1992;20:4374. doi: 10.1093/nar/20.16.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moqtaderi Z, Bai Y, Poon D, Weil P A, Struhl K. TBP-associated factors are not generally required for transcriptional activation. Nature. 1996;382:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 41.Parkhurst S M, Harrison D A, Remington M P, Spane C, Kelley R L, Coyne R S, Corces V G. The Drosophila su(Hw) gene, which controls the phenotypic effect of the gypsy transposable element, encodes a putative DNA-binding protein. Genes Dev. 1988;2:1205–1215. doi: 10.1101/gad.2.10.1205. [DOI] [PubMed] [Google Scholar]
- 42.Pirrotta V. Vectors for P-mediated transformation in Drosophila. In: Rodriguez R L, Denhardt D T, editors. Vector: a survey of molecular cloning vectors and their uses. Boston, Mass: Butterworths; 1988. pp. 437–445. [Google Scholar]
- 43.Pirrotta V, Manet E, Harbon E, Bickel S E, Benson M. Structure and sequence of the Drosophila zeste gene. EMBO J. 1987;6:791–799. doi: 10.1002/j.1460-2075.1987.tb04821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pirrotta V, Steller H, Bozzetti M P. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 1985;4:3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platero J S, Sharp E J, Adler P N, Eissenberg J C. In vivo assay for protein-protein interactions using Drosophila chromosomes. Chromosoma. 1996;104:393–404. doi: 10.1007/BF00352263. [DOI] [PubMed] [Google Scholar]
- 46.Reese J C, Apone L, Walker S S, Griffin L A, Green M R. Yeast TAFIIs in a multisubunit complex required for activated transcription. Nature. 1994;371:523–527. doi: 10.1038/371523a0. [DOI] [PubMed] [Google Scholar]
- 47.Roeder R. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci. 1996;21:327–335. [PubMed] [Google Scholar]
- 48.Roseman R R, Johnson E A, Rodesch C K, Bjerke M, Nagoshi R N, Geyer P K. A P element containing suppressor of Hairy-wing binding regions has novel properties for mutagenesis in Drosophila melanogaster. Genetics. 1995;141:1061–1074. doi: 10.1093/genetics/141.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roseman R R, Pirrotta V, Geyer P K. The su(Hw) protein insulates expression of the Drosophila melanogaster white gene from chromosomal position-effects. EMBO J. 1993;12:435–442. doi: 10.1002/j.1460-2075.1993.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubin G M, Spradling A C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 52.Sauer F, Wassarman D A, Rubin J M, Tijan R. TAFIIs mediate activation of transcription in the Drosophila embryo. Cell. 1996;87:1271–1284. doi: 10.1016/s0092-8674(00)81822-x. [DOI] [PubMed] [Google Scholar]
- 53.Scott K S, Geyer P K. Effects of the su(Hw) insulator protein on the expression of the divergently transcribed Drosophila yolk protein genes. EMBO J. 1995;14:6258–6279. doi: 10.1002/j.1460-2075.1995.tb00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soldatov A V, Nabirochkina E N. A new method for cloning Drosophila melanogaster genes marked with highly copied mobile genetic element. Russ J Genet. 1996;32:1497–1500. [PubMed] [Google Scholar]
- 55.Spradling A C, Rubin G M. Transposition of cloned P elements into germlike chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 56.Tansey W P, Herr W. TAFs: guilt by association. Cell. 1997;88:729–732. doi: 10.1016/s0092-8674(00)81916-9. [DOI] [PubMed] [Google Scholar]
- 57.Thut C J, Chen J L, Klemm R, Tjian R. p53 transcriptional activation mediated by coactivators TAFII40 and TAFII60. Science. 1995;267:100–104. doi: 10.1126/science.7809597. [DOI] [PubMed] [Google Scholar]
- 58.Tjian R, Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994;77:5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 59.Verrijzer C P, Tjian R. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci. 1996;21:338–342. [PubMed] [Google Scholar]
- 60.Qian S, Varjavand B, Pirrotta V. Molecular analysis of the zeste-white interaction reveals a promoter-proximal element essential for distant enhancer-promoter communication. Genetics. 1992;131:79–90. doi: 10.1093/genetics/131.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker S S, Reese J C, Apone L M, Green M R. Transcription activation in cells lacking TAFIIs. Nature. 1996;382:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 62.Walker S S, Shen W-C, Reese J C, Apone L M, Green M R. Yeast TAFII145 required for transcription of G1/S cyclin genes and regulated by the cellular growth state. Cell. 1997;90:607–614. doi: 10.1016/s0092-8674(00)80522-x. [DOI] [PubMed] [Google Scholar]
- 63.Wang E H, Tjian R. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science. 1994;263:811–814. doi: 10.1126/science.8303298. [DOI] [PubMed] [Google Scholar]
- 64.Xie X, Kokubo T, Cohen S L, Mirza U A, Hoffmann A, Chait B T, Roeder R G, Nakatani Y, Burley S K. Structural similarity between TAFs and the heterotetrameric core of the histone octamer. Nature. 1996;380:316–322. doi: 10.1038/380316a0. [DOI] [PubMed] [Google Scholar]