To The Editor,
Despite growing evidence indicating that solid organ transplant (SOT) recipients often have a low humoral response to mRNA‐based coronavirus disease 2019 (COVID‐19) vaccines, the reports of severe COVID‐19 after vaccination among immunocompromised subjects are few. 1 We identified a kidney transplant recipient (KTR) unable to mount an adequate immune response after two doses of mRNA‐1273 vaccine (Moderna), and who subsequently developed severe COVID‐19.
A 73‐year‐old Asian American male presented to our emergency department (ED) in New York on May 29, 2021, with decreased oral intake, nausea, lethargy, and progressive shortness of breath and cough for two weeks. He had a medical history significant for receiving a deceased donor renal allograft on February 10, 2020, insulin‐dependent diabetes mellitus with a hemoglobin A1c level of 8.4%, and hypertension. His maintenance immunosuppressive therapy was prednisone 5 mg daily, mycophenolate mofetil (MMF) 500 mg twice daily and tacrolimus extended‐release tablet 16 mg daily. He completed the two‐dose series of mRNA‐1273 severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccines (Moderna) on February 11, 2021. On examination, the patient appeared to be tachypneic. His vital signs revealed temperature 36.9°C, blood pressure 97/55 mm Hg, pulse 100 beats per minute, respiratory rate 35 breaths per minute, the oxygen saturation 92% while breathing ambient air. He was started on supplemental oxygen, delivered by nasal cannula at 2 L/min, which improved his oxygen saturation values to 94%–99%. His nasopharyngeal swab tested positive for SARS‐CoV‐2 by real‐time reverse‐transcriptase–polymerase‐chain‐reaction (rRT‐PCR) assay. Admission laboratory tests noted leukocytosis (white blood cell count 11.74 k/µl, reference range: 3.80–10.50 k/µl), and lymphopenia (absolute lymphocyte count 0.11 k/µl, reference range: 1.00–3.30 k/µl). Recent blood work from April 2, 2021 (two months prior to admission) showed a white blood cell count of 5.62 k/µl and an absolute lymphocyte count of 1.01 k/µl. Antibody tests against the SARS‐CoV‐2 nucleocapsid and receptor‐binding domain (RBD) of the spike protein using the anti‐SARS‐CoV‐2 S enzyme immunoassay (Roche Elecsys) were both negative on May 31, 2021. The anti‐nucleocapsid total antibody cutoff index (COI) in this patient was 0.14 (COI ≤ 0.99 was determined negative by the manufacturer) while the anti‐spike RBD total antibody level was 0.40 U/ml (≤ 0.79 U/ml was determined negative). The initial chest computed tomography scan (Figure 1) showed extensive bilateral patchy interstitial and nodular ground‐glass opacities, consistent with the findings of COVID‐19.
FIGURE 1.
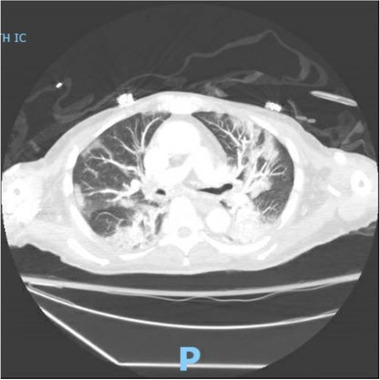
The chest computed tomography scan showed extensive bilateral patchy interstitial and nodular ground‐glass opacities, consistent with the findings of coronavirus disease 2019 (COVID‐19)
Prednisone was held while he was receiving dexamethasone in the hospital. MMF 500 mg twice daily was also held, and it was resumed at 500 mg daily after discharge given his fragility. Tacrolimus extended‐release tablet dosage was decreased from 16 mg to 10 mg daily in order to reduce immune suppression to target a level between 3.5 and 5.0 ng/ml. Inpatient daily tacrolimus levels ranged between 3.8 and 6.0 ng/ml. He received intravenous (IV) remdesivir for 4 days, but it was discontinued one day early due to acute kidney injury (serum creatine increased from baseline 1.25 to 1.96 mg/dl). On day 5 of hospitalization, the supplemental oxygen was discontinued while he was able to breathe comfortably on ambient air with oxygen saturation 97%–99%. One time T cell subset was checked on June 5, 2021 (one week after hospitalization), which showed absolute CD4+ T cell count of 384/µl (reference range: 325 – 1251/µl), CD4 percentage of 66% (reference range: 30%–56%), absolute CD8+ T cell count of 88/µl (reference range: 90–775/µl), CD8 percentage of 15% (reference range: 30%–56%), and the CD4/CD8 ratio of 4.38 (reference range: 0.86–4.14). After completing 10 days of IV dexamethasone 6 mg/day, he was discharged home on June 7, 2021. His serum creatine had normalized to his baseline at the time of discharge. Two weeks post‐discharge, his tacrolimus level had fallen to 2.2 ng/ml, and his tacrolimus dose was increased to 16 mg daily. On July 6, 2021 (a month after discharge), the SARS‐CoV‐2 nucleocapsid antibody remained negative (COI = 0.18, reference range ≤ 0.99), and the anti‐spike RBD protein antibody turned positive with a low titer of 2.31 U/ml (reference range ≤ 0.79 U/ml).
As of June 2021, two mRNA COVID‐19 vaccines (mRNA‐1273 and BNT162b2) which were under the United States Food and Drug Administration emergency use authorization showed more than 90% effectiveness across all age groups. However, individuals who were receiving chronic immunosuppressive drug therapy were excluded from the trials. 2 , 3 Current US Centers for Disease Control and Prevention interim clinical considerations for the use of mRNA vaccines do not recommend antibody testing to assess for immunity to SARS‐CoV‐2 following COVID‐19 vaccination. 4 In a study among 658 SOT recipients who received two doses of mRNA COVID‐19 vaccines, 301 (46%) of them did not have a detectable level of anti‐spike protein antibodies. 5 In this study, the subgroup analysis showed only 36 of 322 (11%) KTRs developed antibody response after the first dose of the mRNA vaccine, while 118 (37%) of them had detectable anti‐spike protein antibodies after the second dose of vaccine. Fifty‐two percent of the KTRs lacked antibody response after the two‐dose series. Persons who were taking antimetabolites (including mycophenolate mofetil, mycophenolic acid, or azathioprine) had a higher percentage of inadequate antibody response after two doses of the mRNA vaccine, compared to those who were not taking antimetabolites among SOT recipients (57% vs. 8%, respectively). 5 A similar result from Benotmane et al showed that only 98 (48%) individuals had positive anti‐spike IgG of 204 KTRs despite two doses of the Moderna vaccine. 6 Grupper et al reported that all 25 controls developed antibodies to spike protein, while only 51 of 136 KTRs (37.5%) had positive serology following full vaccination with the BNT162b2. 7
Markedly diminished generation of antigen‐specific B cells, especially plasmablasts and memory B cells, can explain the low level of spike‐antibody production by KTRs after vaccination. 8 Apart from the weak humoral response, KTRs also show impaired cellular response to mRNA vaccines. Spike‐specific CD8+ T cells were minimally detectable in KTRs, accompanied by impairment of the interleukin‐2 production and downregulation of other cytokine signaling pathways, while the CD4+ counterparts are comparable to healthy controls. 9 We hypothesized that COVID‐19 infection boosted the vaccine‐induced anti‐spike antibody response in our patient but was not able to induce a natural antibody response to nucleocapsid protein.
Additional information is needed to recommend re‐vaccination or additional doses of COVID‐19 vaccines. The beneficial effect of a third dose of SARS‐CoV‐2 vaccine in SOT recipients has been described in a case series of 30 patients. 10 Of the six patients who had low‐positive anti‐spike antibody titers before the third dose, all developed high‐positive antibody titers after receiving the third dose of vaccine. In contrast, of the 24 patients with negative antibody titers before the third dose, 16 (67%) remained negative. 10 Another study, involving 101 SOT recipients (including 78 KTRs), discovered that 26 of 59 (44%) seronegative patients became seropositive 4 weeks after receiving the third dose of BNT162b2 (Pfizer‐BioNTech) vaccine. 11
Despite vaccination, immunocompromised patients need to continue precautions against SARS‐CoV‐2 as breakthrough infections remain a threat.
AUTHOR CONTRIBUTIONS
All the authors have contributed equally for preparation of the manuscript
REFERENCES
- 1. Caillard S, Chavarot N, Bertrand D, et al. Occurrence of severe Covid‐19 in vaccinated transplant patients. Kidney Int. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Interim Clinical Considerations for Use of COVID‐19 Vaccines Currently Authorized in the United States. Centers for Disease Control and Prevention . Accessed June 11, 2021. https://www.cdc.gov/vaccines/covid‐19/clinical‐considerations/covid‐19‐vaccines‐us.html
- 5. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐Dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benotmane I, Gautier‐Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA‐1273 SARS‐CoV‐2 vaccine. Kidney Int. 2021;99(6):1498‐1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS‐Cov‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021. 10.1111/ajt.16615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rincon‐Arevalo H, Choi M, Stefanski A‐L, et al. Impaired humoral immunity to SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Science Immunology. 2021;6(60):eabj1031. [DOI] [PubMed] [Google Scholar]
- 9. Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS‐CoV2 BNT162b2 (Tozinameran) prime‐boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14):e150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS‐CoV‐2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021. 10.7326/L21-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamar N, Abravanel F, Marion O, Couat C, Izopet , Del Bello A. Three doses of an mRNA Covid‐19 vaccine in solid‐organ transplant recipients. N Engl J Med. 2021. 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]


