Abstract
Objective
SARS‐CoV‐2 has caused nearly 4 million confirmed cases of COVID‐19 worldwide in the approximately 4 months since it emerged in Wuhan, China in December 2019. Comorbidities increase morbidity and mortality in COVID‐19, and many laboratory parameters have been associated with mortality. The aim of the present study was to identify the relationship between endogenous carboxyhaemoglobin (COHb) level and the clinical course and prognosis of COVID‐19.
Methods
The study included 48 non‐smokers or ex‐smokers aged 18 years or older who presented to the emergency department, were diagnosed with COVID‐19 by real‐time PCR analysis of nasopharyngeal swab sample and were treated in the pulmonary diseases ward of the Atatürk University hospital after 24 March 2020 and 15 April 2020. The patients’ laboratory parameters and demographic data were analysed retrospectively.
Results
Prothrombin time and C‐reactive protein (CRP), troponin‐I, and D‐dimer levels decreased in COVID‐19 patients during follow‐up (P = .024, P = .001, P = .001, P = .001), while PaO2/FiO2 ratio and COHb increased (P = .002, P = .001). COHb level at admission was significantly lower in patients who developed macrophage activation syndrome (MAS), acute respiratory distress syndrome (ARDS), and those who died compared with the other patients (P = .002, P = .001). COHb level on day 5 of treatment was significantly higher in patients with ARDS and patients who died (P = .001, P = .001). Significant correlations were detected between COHb level and CRP (r=−0.425, P = .001), ferritin (r = −.395, P = .001) and PaO2/FiO2 ratio (r = .431, P = .001).
Conclusions
COHb level may be an easily accessible biomarker that guides early follow‐up and treatment planning to avoid ARDS, MAS and mortality in COVID‐19.
What’s known
Carbon monoxide (CO) is naturally synthesised in the body and plays an important role in the regulation of physiological functions such as vasodilation, angiogenesis, vascular remodelling, protection against tissue damage and modulation of the inflammatory response.
Approximately 85% of CO is produced by haeme oxygenase and is excreted from the body through the respiratory system.
In critical diseases such as acute respiratory failure, chronic obstructive pulmonary disease, acute pulmonary embolism, and acute myocardial infarction, low initial endogenous carboxyhaemoglobin (COHb) level has been associated with high mortality and poor prognosis.
The leading causes of morbidity and mortality in COVID‐19 are acute respiratory failure, microthombi and cardiac involvement.
What’s new
Low COHb level at admission in COVID‐19 patients may be an easily accessible biomarker that guides early follow‐up and treatment planning to avoid ARDS, MAS and mortality.
1. INTRODUCTION
After appearing in Wuhan, China in December 2019, coronavirus disease (COVID‐19) spread rapidly around the world, with over 3 million confirmed cases by the end of April 2020. In the majority of infected patients, COVID‐19 is either asymptomatic or presents with mild symptoms such as loss of taste and smell, sore throat, fatigue and joint pain. However, it can have a much more severe course in older people, patients with hypertension (HT), and conditions that can impair immunity such as diabetes mellitus (DM), HIV, long‐term immunosuppressive therapy and pregnancy. 1
While many comorbidities have been associated with COVID‐19 mortality, there are also laboratory diagnostic tests associated with early poor prognosis. Of these, the most frequently used parameters are D‐dimer, ferritin, leukopenia, fibrinogen, prothrombin time and IL‐6 level. These parameters alone are not effective in directing treatment, but evaluation of correlation with clinical condition revealed a relationship with macrophage activation syndrome (MAS), which is among the most important causes of mortality. This led to the investigation of parameters that can be associated with mortality in this emerging disease. 2 , 3
Carbon monoxide (CO) is naturally synthesised in the body and plays an important role in the regulation of physiological functions such as vasodilation, angiogenesis, vascular remodelling, protection against tissue damage and modulation of the inflammatory response. Approximately 85% of CO is produced by haeme oxygenase and is excreted from the body through the respiratory system. 4 , 5 In critical diseases such as acute respiratory failure, chronic obstructive pulmonary disease, acute pulmonary embolism and acute myocardial infarction, low initial endogenous carboxyhaemoglobin (COHb) level has been associated with high mortality and poor prognosis. The leading causes of morbidity and mortality in COVID‐19 are acute respiratory failure, microthombi and cardiac involvement. 5 , 6 , 7 , 8
COVID‐19 is a new disease that does not have specific laboratory findings as in other known diseases, and as such is the focus of intense research. The aim of the present study was to determine the value of COHb levels measured at admission and follow‐up in the prediction of clinical course and prognosis in COVID‐19 patients who develop MAS and acute respiratory failure.
2. METHODS
2.1. Study design
The study included patients aged 18 years or older who presented to the Atatürk University emergency department from 24 March 2020 (the date of admission of the first COVID‐19‐positive patient to our centre) to 15 April 2020 with fever, cough, dyspnoea, fatigue and/or sudden attenuation of taste and smell and had contact with a suspected COVID‐19 patient in the past 14 days. Local ethics committee approval was obtained to use patients' records for our retrospective study.
As standard procedure, high‐resolution computed tomography (HRCT) was performed for high‐risk patients with COVID‐19. Patients with typical HRCT findings (bilateral ground‐glass opacity with primarily peripheral distribution, subsegmental consolidation or linear opacities, cobblestone pattern and inverse halo sign) and patients with atypical radiological findings but consistent clinical presentation were hospitalised. 9 Nasopharyngeal swab samples were obtained from the patients and COVID‐19 diagnosis was established using real‐time PCR analysis. Patients in the study who did not develop acute respiratory distress syndrome (ARDS) and/or MAS were followed in the respiratory ward, while those who developed MAS and/or ARDS were followed in the respiratory intensive care unit. Haematological parameters, biochemical parameters including liver and kidney function tests, coagulation parameters, ferritin, D‐dimer, troponin‐I, C‐reactive protein (CRP) and arterial blood gas parameters were analysed at admission and daily thereafter.
2.2. Study group
The 48 patients in the study were divided into three groups: patients without ARDS and MAS at admission and during follow‐up (n = 22), patients who had ARDS at admission or developed ARDS during follow‐up (n = 26), and patients who developed MAS during follow‐up (all patients who developed MAS also had ARDS) (n = 13).
2.3. Exclusion criteria
Patients with chronic or clinically significant infectious or inflammatory conditions in the last month, current smoking, uncontrolled asthma, chronic obstructive pulmonary disease (COPD), malignancy, invasive surgery in the last month, uncontrolled hypertension, high fasting blood glucose and newly developed cerebrovascular disease, kidney disease and coronary artery disease were excluded. History and laboratory parameters obtained at admission were used to evaluate patients in terms of the exclusion criteria. The presence of coronary artery disease, asthma, COPD and diabetes was determined through consultation with the cardiology, pulmonology and internal medicine departments.
2.4. Definitions and treatment
Fever was defined as an axillary temperature of 37.3°C or higher. Positive endotracheal aspirate or lower respiratory tract sputum culture with signs and symptoms of bacteraemia or pneumonia was considered a secondary bacterial infection. Treatment of patients diagnosed as having ventilator‐associated or hospital‐acquired pneumonia was planned based on available guidelines. Diagnosis and grading of ARDS were done according to Berlin 2015 diagnostic criteria. If the patients’ daily cardiac‐specific troponin level was above normal, echocardiography was performed to evaluate for the development of new cardiac pathologies. Coagulopathy was defined as a prothrombin time more than 3 seconds higher than normal and partial thromboplastin time 5 seconds higher than normal. Treatment strategies were implemented according to the Turkish Ministry of Health COVID‐19 Adult Diagnosis and Treatment guidelines based on the patients’ disease severity. Patients were monitored for MAS in the presence of signs such as persistent fever, persistently high or increasing CRP and ferritin levels, elevated D‐dimer levels, lymphopenia/thrombocytopenia, deterioration in liver function tests, hypofibrinogenaemia and increasing triglyceride levels despite treatment. Patients with successive increases in daily measurements of these parameters which could not be explained by secondary bacterial infections were administered 400 mg of tocilizumab for MAS if they had no contraindications. In patients who showed appropriate clinical and laboratory response after 24 hours, treatment was not continued. However, if an appropriate clinical and laboratory response was not observed, treatment was repeated at the same dosage.
2.5. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY). Between‐group comparisons were performed using Pearson's chi‐square test for parametric data and Mann‐Whitney U test for non‐normally distributed numerical data. The groups’ demographic data and laboratory parameters were compared using independent‐samples t test. Wilcoxon analysis was performed to compare repeated measures for laboratory parameters within groups. Pearson correlation analysis was used to evaluate the correlation between COHb and CRP, ferritin, D‐dimer level, lymphocyte count and the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2). A P‐value <.05 was considered statistically significant.
3. RESULTS
The mean age of the 48 patients included in the study was 57.6 ± 17.6 years. Twenty‐two of the patients were women (mean age 55.8 ± 18.3 years) and 26 were men (mean age 59.1 ± 17.3 years). Twenty‐eight of the patients had HT, 12 had DM, 4 had asthma, and 1 had epilepsy. Body mass index (BMI) was >30 kg/m2 in 15 of the patients. Of 26 patients who developed ARDS, 20 had HT, 8 had DM, 1 had epilepsy, and 6 had BMI >30 kg/m2. Of the patients who developed MAS, 10 had HT, 4 had DM, and 1 had epilepsy.
In the physical examination performed at hospital admission, mean respiratory rate (breaths/minute) was 21.4 ± 12.3 among patients who later developed MAS, 24.6 ± 13.4 among patients who developed ARDS, and 15.4 ± 4.8 in patients without ARDS and MAS. Comparison of patients with and without ARDS and MAS showed that respiratory rate was statistically significantly higher in the ARDS and MAS groups (P = .001, P = .001). On day 5 of treatment, the mean respiratory rate was 18.4 ± 4.5 in the MAS group, 20.1 ± 5.6 in the ARDS group, and 14.4 ± 3.6 in the group of patients without MAS or ARDS.
A comparative analysis of the patients’ laboratory parameters at hospital admission and on day 5 of treatment is shown in Table 1. When patients with and without MAS were compared in terms of their COHb levels at admission and on day 5 of treatment, it was found that patients who developed MAS had lower COHb level at admission (P =.002). Comparison of the changes in COHb levels over the course of 5 days showed a statistically significant increase in patients who developed MAS (Figure 1) (P = .001). When patients with and without ARDS were compared in terms of COHb levels at admission, it was found that COHb level was significantly lower at admission but higher on day 5 (P =.001, P =.001). As in the patients who developed MAS, COHb levels increased further over the course of 5 days in patients who developed ARDS (Figure 1) (P = .001). Changes in other laboratory parameters in patients who developed MAS and ARDS are shown in Tables 2 and 3. Comparison of COHb levels between deceased (n = 4) and surviving patients (n = 44) showed that in deceased patients, COHb levels were significantly lower at admission and higher on day 5 of treatment (P = .04, P = .001).
TABLE 1.
Comparison of laboratory parameters of COVID‐19 patients at admission and on day 5 of treatment
| Admission (n = 48) | Day 5 of treatment (n = 48) | P | |
|---|---|---|---|
| WBC (/µL) | 6911.5 ± 2839.6 | 6833.1 ± 3170.5 | .7 |
| Lymphocytes (/µL) | 1218 ± 560.1 | 1410.1 ± 828.1 | .09 |
| Neutrophils (/µL) | 5021.3 ± 2810.2 | 4812.3 ± 3037.8 | .925 |
| NLR | 7.1 ± 11.1 | 6.1 ± 10.9 | .371 |
| AST (U/L) | 38.6 ± 20.4 | 29.9 ± 16.5 | .004 |
| ALT (U/L) | 30.7 ± 25.3 | 27.6 ± 15.3 | .682 |
| LDH (U/L) | 369.1 ± 144.2 | 329.3 ± 151.4 | .024 |
| GGT (U/L) | 43.3 ± 37.3 | 39.4 ± 35.9 | .633 |
| ALP (U/L) | 84.6 ± 48.3 | 71.4 ± 28.3 | .009 |
| Sodium (mmol/L) | 137.3 ± 3.6 | 139.2 ± 3.3 | .122 |
| Potassium (mmol/L) | 4.1 ± 0.4 | 4.2 ± 0.6 | .652 |
| Creatine (mg/dL) | 1.2 ± 1.3 | 1.2 ± 1.4 | .241 |
| Prothrombin time (s) | 17.3 ± 8.9 | 16.3 ± 6.1 | .024 |
| CRP (mg/dL) | 84.7 ± 82.7 | 27.5 ± 27.4 | .001 |
| Troponin I (ng/dL) | 28.1 ± 61.1 | 22.1 ± 70.4 | .001 |
| PaO2/FiO2 | 284.6 ± 73.4 | 309.5 ± 49.2 | .02 |
| D‐dimer (ng/mL) | 1297.8 ± 1628.6 | 751.6 ± 844.5 | .001 |
| COHb (%) | 0.41 ± 0.34 | 1.75 ± 0.93 | .001 |
Statistically significant values are shown in bold.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COHb, carboxyhaemoglobin; GGT, gamma‐glutamyl transferase; LDH, lactate dehydrogenase; NLR, neutrophil/lymphocyte ratio; WBC, white blood cells.
FIGURE 1.
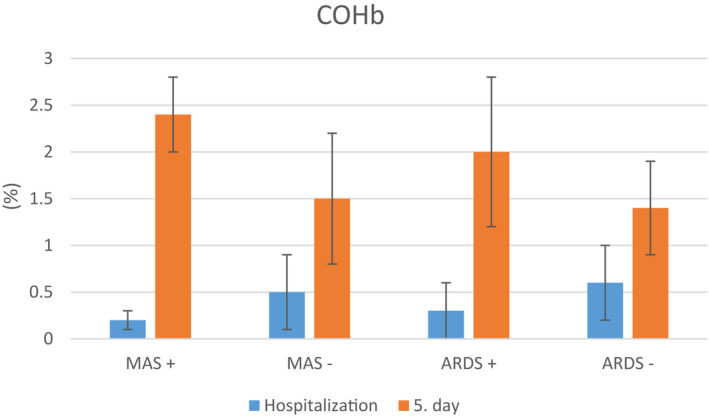
Comparison of COHb levels at admission and on day 5 of treatment in COVID‐19 patients who did and did not develop macrophage activation syndrome (MAS) and acute respiratory distress syndrome (ARDS)
TABLE 2.
Comparison of laboratory parameters at admission and on day 5 among COVID‐19 patients who did and did not develop macrophage activation syndrome (MAS)
| MAS patients (n = 13) | Non‐MAS patients (n = 35) | P | |||
|---|---|---|---|---|---|
| Admission | Day 5 of treatment | Admission | Day 5 of treatment | ||
| WBC (/µL) | 7813.1 ± 3449.4 | 8142.1 ± 3938.7 | 6576.6 ± 2553.5 | 6396.6 ± 2813.5 | .3/.2 |
| Lymphocytes (/µL) | 767.8 ± 373.6 | 694.1 ± 253.1 | 1386.1 ± 527.1 | 1648.7 ± 816.5 | .001/.001 |
| Neutrophils (/µL) | 6469.2 ± 3587.6 | 6993.1 ± 3939.2 | 4483.4 ± 2296.7 | 4085.3 ± 2325.5 | .08/.007 |
| NLR | 15.7 ± 18.6 | 15.1 ± 19.5 | 3.9 ± 3.2 | 3.1 ± 2.7 | .001/.002 |
| AST (U/L) | 40.4 ± 12.9 | 43.2 ± 26.2 | 37.9 ± 22.6 | 25.5 ± 8.4 | .71/.002 |
| ALT (U/L) | 32.2 ± 24.8 | 32.7 ± 15.9 | 30.2 ± 25.8 | 25.9 ± 14.9 | .8/.3 |
| LDH (U/L) | 456.5 ± 168.3 | 496.8 ± 144.5 | 336.6 ± 121.5 | 273.4 ± 107.1 | .009/.001 |
| GGT (U/L) | 61.9 ± 55.4 | 69.2 ± 61.2 | 36.3 ± 25.7 | 29.5 ± 12.8 | .03/.002 |
| ALP (U/L) | 79.1 ± 30.2 | 61.9 ± 26.5 | 86.7 ± 53.8 | 74.5 ± 28.6 | .5/.2 |
| Sodium (mmol/L) | 136.9 ± 3.7 | 141.1 ± 4.9 | 137.4 ± 3.7 | 138.3 ± 2.4 | .6/.02 |
| Potassium (mmol/L) | 4.2 ± 0.4 | 4.6 ± 0.8 | 3.9 ± 0.4 | 4.1 ± 0.4 | .08/.002 |
| Creatine (mg/dL) | 2.1 ± 2.2 | 2.6 ± 2.5 | 0.9 ± 0.6 | 0.8 ± 0.2 | .008/.001 |
| Prothrombin time (s) | 21.8 ± 14.9 | 21.1 ± 10.8 | 15.6 ± 4.5 | 14.7 ± 1.9 | .03/.003 |
| CRP (mg/dL) | 188.2 ± 54.4 | 50.8 ± 21.1 | 46.3 ± 52.9 | 18.9 ± 23.8 | .001/.001 |
| Troponin‐I (ng/dL) | 75.9 ± 100.9 | 67.2 ± 125.5 | 10.4 ± 18.9 | 4.8 ± 8.3 | .001/.005 |
| PaO2/FiO2 | 207.5 ± 57.4 | 304.7 ± 29.4 | 313.3 ± 56.3 | 310.1 ± 56.1 | .001/.7 |
| PaCO2 (mm_Hg) | 30,1 ± 12,8 | 36,2 ± 7,7 | 36,2 ± 8,1 | 36,1 ± 7,4 | .001/.8 |
| D‐Dimer (ng/mL) | 2134.2 ± 2173.1 | 1093.8 ± 757.3 | 987.2 ± 1277.9 | 616.8 ± 849.4 | .03/.02 |
| COHb (%) | 0.2 ± 0.1 | 2.4 ± 0.4 | 0.5 ± 0.4 | 1.5 ± 0.7 | .002/.07 |
| Ferritin (ng/mL) | 683.8 ± 82.5 | 490.3 ± 75.6 | 235.2 ± 89.8 | 134.6 ± 76.4 | .001/.001 |
Statistically significant values are shown in bold.
p: Comparison of parameters at the time of admission and day 5 of treatment between groups.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COHb, carboxyhaemoglobin; GGT, gamma‐glutamyl transferase; LDH, lactate dehydrogenase; MAS, macrophage activation syndrome; NLR, neutrophil/lymphocyte ratio; WBC, white blood cells.
TABLE 3.
Comparison of laboratory parameters at admission and on day 5 among COVID‐19 patients who did and did not develop acute respiratory distress syndrome (ARDS)
| ARDS patients (n = 26) | Non‐ARDS patients (n = 22) | P | |||
|---|---|---|---|---|---|
| Admission | Day 5 of treatment | Admission | Day 5 of treatment | ||
| WBC (/µL) | 7628.5 ± 3123.6 | 7741.9 ± 3022.1 | 6064.1 ± 2245.5 | 5828.4 ± 3098.7 | .05/.05 |
| Lymphocytes (/µL) | 942.7 ± 459.4 | 934.7 ± 463.3 | 1544.5 ± 495.3 | 1935.3 ± 832.2 | .001/.001 |
| Neutrophils (/µL) | 6090.4 ± 3039.9 | 6203.3 ± 3061.4 | 3757.7 ± 1893.7 | 3274.7 ± 2197.3 | .002/.001 |
| NLR | 11.1 ± 14.1 | 10.1 ± 14.1 | 2.5 ± 1.1 | 1.7 ± 0.8 | .005/.01 |
| AST (U/L) | 44.5 ± 22.3 | 34.4 ± 20.9 | 31.5 ± 8.6 | 25.1 ± 7.1 | .02/.07 |
| ALT (U/L) | 33.4 ± 31.9 | 30.1 ± 17.9 | 27.5 ± 14.1 | 24.9 ± 11.5 | .4/.3 |
| LDH (U/L) | 437.8 ± 163.1 | 420.9 ± 151.8 | 287.7 ± 45.1 | 227.9 ± 58.1 | .001/.001 |
| GGT (U/L) | 49.7 ± 45.1 | 47.8 ± 46.3 | 35.6 ± 23.9 | 30.2 ± 15.6 | .2/.1 |
| ALP (U/L) | 86.6 ± 59.6 | 63.1 ± 26.2 | 82.3 ± 31.5 | 80.6 ± 28.3 | .7/.05 |
| Sodium (mmol/L) | 136.8 ± 3.4 | 139.7 ± 4.1 | 137.7 ± 3.9 | 138.3 ± 2.3 | .4/.1 |
| Potassium (mmol/L) | 4.1 ± 0.5 | 4.3 ± 0.7 | 4.1 ± 0.3 | 4.1 ± 0.3 | .4/.3 |
| Creatine (mg/dL) | 1.4 ± 1.6 | 1.6 ± 1.9 | 0.9 ± 0.8 | 0.8 ± 0.2 | .2/.06 |
| Prothrombin time (s) | 20.1 ± 11.2 | 17.8 ± 8.1 | 14.1 ± 2.9 | 14.6 ± 1.5 | .02/.1 |
| CRP (mg/dl) | 130.1 ± 77.2 | 42.3 ± 26.3 | 30.7 ± 50.8 | 10.1 ± 16.3 | .001/.001 |
| Troponin‐I (ng/dL) | 47.8 ± 77.6 | 38.5 ± 92.1 | 4.9 ± 10.8 | 1.7 ± 1.7 | .01/.07 |
| PaO2/FiO2 | 229.8 ± 56.1 | 298.8 ± 41.8 | 349.3 ± 15.3 | 350.3 ± 47.2 | .001/.03 |
| PaCO2 (mm_Hg) | 28,2 ± 9,9 | 31,2 ± 6,7 | 37,4 ± 6,6 | 38,1 ± 5,8 | .001/.001 |
| D‐Dimer (ng/mL) | 1915.4 ± 2015.8 | 1083.5 ± 997.8 | 568.1 ± 263.1 | 320.1 ± 179.5 | .003/.002 |
| COHb (%) | 0.3 ± 0.3 | 2.0 ± 0.8 | 0.6 ± 0.4 | 1.4 ± 0.5 | .001/.001 |
| Ferritin (ng/mL) | 466.1 ± 137.1 | 301.6 ± 47.3 | 227.4 ± 92.4 | 180.6 ± 36.7 | .001/.001Note |
Statistically significant values are shown in bold.
p: Comparison of parameters at the time of admission and day 5 of treatment between groups.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; COHb, carboxyhaemoglobin; GGT, gamma‐glutamyl transferase; LDH, lactate dehydrogenase; NLR, neutrophil/lymphocyte ratio; WBC, white blood cells.
In the correlation analysis between COHb level and laboratory parameters and demographic data, moderate negative correlations were detected between COHb level and CRP (r = =−.425, P = .001), ferritin (r = −.395, P = .001) (Figure 2) and age (r = −.314, P = .001). Moderate positive correlations were detected between COHb and lymphocyte count (r = .43, P = .001) (Figure 2) and PaO2/FiO2 (r = .431, P = .001). There was also a weak negative correlation between COHb and troponin I level (r = −.287, P = .05).
FIGURE 2.
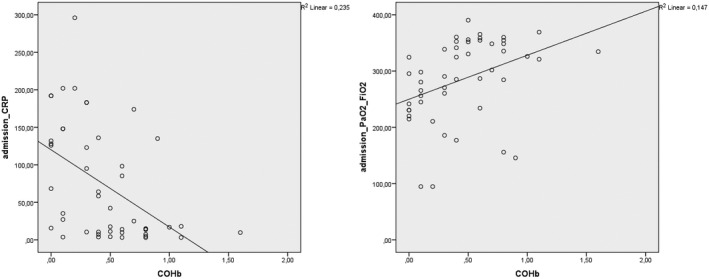
Correlation analysis of COHb level with CRP and PaO2/FiO2 levels in COVID‐19 patients [Correction added on 4 February 2022, after first online publication: Figure 2 was corrected in this version.]
In correlation analysis between COHb level on day 5 of treatment and laboratory parameters, a moderate positive correlation was detected between COHb level and CRP (r = .55, P = .001), prothrombin time (r = .387, P = .001) and creatinine (P = .408, P =.001), but no significant difference was detected in other parameters at the time of admission.
4. DISCUSSION
In the present study, we determined that the COHb levels of COVID‐19 patients treated in our centre were low at admission and increased with treatment. In particular, we observed that low COHb level is an important risk factor for the development of MAS and ARDS. In our study, non‐surviving COVID‐19 patients had lower COHb levels than surviving patients. COHb levels increased significantly during 5‐day follow‐up in patients who developed ARDS and MAS compared with patients who did not develop ARDS and MAS. Correlation analysis between COHb levels and parameters associated with mortality in the literature revealed negative correlations between COHb levels and CRP, ferritin and troponin and positive correlations between COHb levels and lymphocyte and PaO2/FiO2 ratio.
The novel coronavirus detected in Wuhan, China at the end of 2019 was named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) by the International Committee on Taxonomy of Viruses because it is closely related to SARS‐CoV and MERS‐CoV, viruses responsible for past epidemics that caused high morbidity and mortality. Because of the rapid spread of COVID‐19, the epidemic in China quickly escalated to a global pandemic, with the number of confirmed COVID‐19 cases worldwide approaching 4 million at the time of this writing. 10
Although most patients with COVID‐19 present with fever, coughing and shortness of breath, a smaller proportion of patients may present to emergency departments with confusion, diarrhoea, sore throat, chest pain, nausea and vomiting. Less common presenting symptoms also include loss or attenuation of smell and taste, and palpitations because of myocardial wall involvement as a complication of viral respiratory tract infections. COVID‐19 causes high rates of morbidity and mortality, especially in older patients and those with comorbidities such as HT, DM, immunosuppressive therapy, and obesity. MAS‐related multiorgan failure and acute respiratory failure are among the primary causes of mortality in COVID‐19. 3 , 11
Lymphopenia is the most common abnormality in laboratory tests, which suggests that leukocytes, especially T‐lymphocytes, are affected in COVID‐19 patients. Viral particles that spread from the respiratory tract and infect other cells create a cytokine storm. In a cytokine storm, myriad proinflammatory cytokines are released, primarily TNF‐alpha, IL‐1, IL‐2, IL‐6 and nitric oxide. These cytokines play an important role in increasing vascular permeability, endothelial damage, and microthrombus formation. 12 Progressive increases in CRP, ferritin, D‐dimer levels, and if measurable, IL‐6 levels in patients’ clinical follow‐up have become predictive biomarkers of cytokine storm syndrome and MAS for clinicians. 13 , 14 However, while there is no definite cut‐off value for these parameters, it indicates that clinical follow‐up plays an important role in the use of IL‐1 antagonist canakinumab and IL‐6 antagonist tocilizumab. 3 , 15
The level of CO naturally synthesised in the body can be measured using COHb, the product of its high‐affinity binding to haemoglobin. In addition to its anti‐inflammatory activity, it also plays an important role in vascular remodelling and prevention of tissue damage. Endogenous COHb is generated in the body when haeme oxygenase‐1 (HOX‐1) converts haeme to biliverdin. COHb released as a result of HOX‐1 activation is eliminated by the respiratory system and can be measured in exhaled breath. HOX‐1 has an important role in the reduction of reactive oxygen radicals and induction of enzymes that are cytoprotective for many organ and tissue epithelia, primarily the respiratory tract epithelium. 16 Low COHb level was found to be associated with high mortality in studies of intensive care patients. In addition, low COHb levels were shown to be correlated with poor prognosis in patients presenting with community‐acquired pneumonia, myocardial infarction, stroke and acute pulmonary thromboembolism. 5 , 6 , 7 , 8 , 17 In this study, we observed that COVID‐19 patients’ COHb levels were low at admission and progressively increased with treatment. Furthermore, COHb levels were even lower in patients who developed MAS and ARDS, for which early treatment is of great importance. In non‐surviving patients who developed ARDS, COHb levels on day 5 of treatment were higher than in surviving patients who did not develop ARDS. Patients who developed MAS exhibited greater changes in COHb on day 5 of treatment relative to patients who did not. In addition, we observed in our study that patients who developed ARDS and MAS had a higher respiratory rate at the time of hospital admission compared with those who did not. The higher respiratory rate in these patients may have been responsible for their initially low levels of CO, which is removed while breathing. The low PaCO2 values measured in these patients’ arterial blood gas analysis also showed that their ventilation capacity was not impaired at admission and that conditions were suitable for CO excretion. A study of patients who developed ARDS because of acute pulmonary thromboembolism showed that CO level was correlated with oxygen saturation, providing further evidence that CO level may fall because of an increase in respiratory work. In addition, although there has been no previous study on this subject in the literature, a decrease in HOX‐1 system activation associated with clinical worsening of COVID‐19 may also have reduced COHb production. Improved immune response and decreased respiratory workload with treatment may have resulted in an increase in COHb, which is known to have anti‐inflammatory activity. CRP, ferritin, troponin and PaO2/FiO2 ratio have been shown to be closely associated with MAS and mortality, and the correlations observed between these parameters and COHb support previous studies as well as our present findings.
The main limitation of this study was that the number of non‐surviving patients in our sample was too small to establish an association between COHb level and mortality. However, our finding that COHb levels in patients who developed ARDS and MAS were consistent with those of non‐surviving patients suggests that our results can be generalised.
In conclusion, low COHb level at admission in COVID‐19 patients may be an easily accessible biomarker that guides early follow‐up and treatment planning to avoid ARDS, MAS and mortality.
DISCLOSURE
The authors received no financial support for the research and/or authorship of this article. The authors declare that they have no conflict of interest in the publication of this article.
Kerget B, Kerget F, Koçak AO, et al. Is endogenous carboxyhaemoglobin level a useful biomarker of clinical course and prognosis in COVID‐19 patients? Int J Clin Pract. 2021;75:e14680. 10.1111/ijcp.14680
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eyi YE, Yetim M, Tekindur S. Endogenous and exogenous factors affecting the levels of carboxyhemoglobin. Am J Emergency Med. 2015;33:1310‐1311. [DOI] [PubMed] [Google Scholar]
- 5. Owens EO. Endogenous carbon monoxide production in disease. Clin Biochem. 2010;43:1183‐1188. [DOI] [PubMed] [Google Scholar]
- 6. Kakavas S, Papanikolaou A, Ballis E, Tatsis N, Goga C, Tatsis G. Carboxyhemoglobin and methemoglobin levels as prognostic markers in acute pulmonary embolism. Am J Emergency Med. 2015;33:563‐568. [DOI] [PubMed] [Google Scholar]
- 7. Yasuda H, Yamaya M, Nakayama K, et al. Increased arterial carboxyhemoglobin concentrations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:1246‐1251. [DOI] [PubMed] [Google Scholar]
- 8. Zegdi R, Perrin D, Burdin M, Boiteau R, Tenaillon A. Increased endogenous carbon monoxide production in severe sepsis. Intensive Care Med. 2002;28:793‐796. [DOI] [PubMed] [Google Scholar]
- 9. Akçay Ş, Özlü T, Yilmaz A. Radiological approaches to COVID‐19 pneumonia. Turk J Med Sci. 2020;50:604‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunningham L, Kimber I, Basketter DA, McFadden JP. Why judiciously timed anti‐IL 6 therapy may be of benefit in severe COVID‐19 infection. Autoimmun Rev. 2020;19:102563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368:473‐474. [DOI] [PubMed] [Google Scholar]
- 13. Kerget B, Kerget F, Aksakal A, Aşkın S, Sağlam L, Akgün M. Evaluation of alpha defensin, IL‐1 receptor antagonist, and IL‐18 levels in COVID‐19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J Med Virol. 2021;93:2090‐2098. 10.1002/jmv.26589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerget B, Kerget F, Aksakal A, Aşkın S, Uçar EY, Sağlam L. Evaluation of the relationship between KIM‐1 and suPAR levels and clinical severity in COVID‐19 patients: a different perspective on suPAR. J Med Virol. 2021;93:5568‐5573. 10.1002/jmv.27099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang C, Wu Z, Li J‐W, Zhao H, Wang G‐Q. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase‐1 and carbon monoxide in the respiratory system. Antioxid Redox Signal. 2007;9:2157‐2174. [DOI] [PubMed] [Google Scholar]
- 17. Kocak AO, Katipoglu B, Akbas I, Evrin T, Kasali K. Can carboxyhemoglobin and total bilirubin be new prognostic indicators for determining mortality in ischemic stroke? Kuwait Med J. 2019;51:356‐361. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


