Dear editor,
A 21‐year‐old male health care worker presented with an acute onset itchy rash over the trunk and limbs of 3 days' duration. Patient had received his first dose of vaccine against Covid‐19 (Covishield™, ChAdOx1 nCoV‐ 19 Corona Virus Vaccine [Recombinant], Serum Institute of India Pvt Ltd, Pune, India) 4 days prior to the onset of rash. Patient had a mild febrile episode 2 days prior to the rash associated with myalgia. He did not have any past history of, or symptoms suggestive of Covid‐19 prior to vaccination. He was also not on any medications prior to vaccination. Clinical examination revealed discrete and coalescent papulovesicular lesions involving the trunk and proximal extremities, typically distributed along the lines of cleavage on the back, reminiscent of pityriasis rosea (Figure 1A, B). Some of the lesions exhibited a targetoid aspect with central hemorrhagic crust (Figure 1C). Dermoscopy revealed red dots in an irregular distribution over a pink‐red structureless background. The targetoid lesions additionally showed central hemorrhagic crusting with collarette scaling (Figure 2, supplementary figure). Based on the clinical and dermoscopic observations, vaccination associated papulovesicular pityriasis rosea‐like eruption was diagnosed. Histopathology revealed epidermal spongiosis, perivascular lymphocytic infiltrate in the papillary dermis and extravasated red blood cells in the papillary and reticular dermis (Figure 3, supplementary figure), thus confirming the clinical diagnosis.
FIGURE 1.
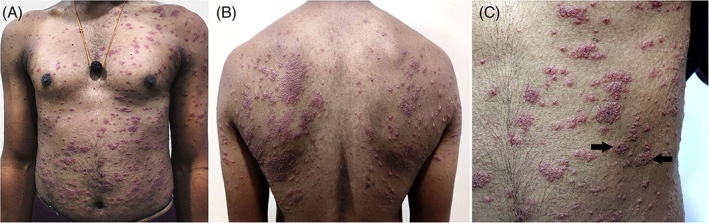
Erythematous discrete and coalescent papulovesicular rash involving the trunk and proximal extremities (A) and (B), typically along the lines of cleavage (B) with some of the lesions showing central hemorrhagic crust [(C), black arrows)]
Pityriasis rosea is an acute self‐limiting papulosquamous diseases characterized by round‐oval erythematous papules and plaques with a pathognomonic peripheral collarette of scales typically involving the trunk and proximal extremities and distributed along the lines of cleavage. These lesions may be preceded by a solitary larger lesion ('mother' or 'herald' patch). There are several clinical variants, designated as 'atypical' pityriasis rosea including the papulovesicular and erythema multiforme‐like forms. The exact etiopathogenesis of pityriasis rosea is not known. A viral etiology is proposed in which the human herpesvirus (HHV) 6 and 7 have been implicated in the pathogenesis. Upper respiratory tract infections, stress, and drugs like non‐steroidal anti‐inflammatory drugs, barbiturates, heavy metals, angiotensin converting enzyme inhibitors as well as vaccines are known precipitating factors. 1
Vaccine induced pityriasis rosea is rather well‐known and has been described with several vaccines including those against influenza, H1N1, diphtheria, pneumococcus, small pox, hepatitis B and human papilloma virus. Although the exact pathophysiology is not understood, post‐vaccination pityriasis rosea has been attributed to the reactivation of HHV 6 and 7 due to immune stimulation following vaccination. Cell mediated immune response related to molecular mimicry with a viral epitope is another hypothesis put forward. 2 , 3
The most frequent post‐vaccination effects associated with Covishield™ as per the manufacturers include mild to moderate fever, myalgia, and injection site pain which are usually transient and self‐resolving. The possible cutaneous manifestations include hyperhidrosis, pruritus, and rash which have been described as 'uncommon' and categorized as 'unsolicited' adverse reactions. 4 Other documented adverse cutaneous effects associated with Covid‐19 vaccination include herpes zoster and lichenoid eruptions in health care workers who received BNT162b2 mRNA COVID‐19 vaccine. 5
The diagnosis of pityriasis rosea in our case was established based on the clinical picture, dermoscopic findings and histopathological features. 1 , 6 Although there are clinical diagnostic criteria put forth for pityriasis rosea and have also been found applicable in Indian population, the atypical forms usually do not fit into these criteria. 1 , 7 In such atypical presentations (as in our case), we strongly believe the diagnosis of pityriasis rosea be established by factoring in the clinical aspects such as the history, symptomatology, lesional morphology and evolution complemented by histological features. Dermoscopy may further assist in the diagnosis.
Pityriasis rosea as a manifestation of Covid‐19 although has been reported, 8 , 9 our patient did not have any history of established Covid‐19 or symptoms suggestive of the same prior to immunization. Furthermore, appearance of the rash within a very short duration after vaccination makes the causal relationship between the two appear quite plausible. Although post vaccination pityriasis rosea is a relatively well‐known entity, this case, we believe, is the first report of such an occurrence following a recombinant vaccine against Covid‐19, possibly due to the same reasons as proposed previously.
We hereby declare that a written informed consent has been obtained from the patient. Approval from institutional review board has not been obtained as this submission is a case report and not a clinical trial/study involving participants.
AUTHOR CONTRIBUTIONS
Data collection for manuscript preparation: Keshavmurthy A. Adya and Warood Albadri; Analysis of data collected for manuscript preparation: Keshavmurthy A. Adya, Arun C. Inamadar, and Warood Albadri; Review of literature for manuscript preparation: Keshavmurthy A. Adya, Arun C. Inamadar, and Warood Albadri; Manuscript preparation: Keshavmurthy A. Adya; Manuscript review: Keshavmurthy A. Adya, Arun C. Inamadar, and Warood Albadri.
Supporting information
Figure 2 Dermoscopy shows irregularly scattered red dots (black arrows), hemorrhagic crusts (white stars) with collarette of white scales (white arrows) on a pink‐red structureless background. [a and b, Polarized dermoscopy, x10]
Figure 3 Photomicrograph showing spongiotic epidermis, upper dermal perivascular lymphocytic infiltrate (a, H&E x10) and clumps of extravasated red blood cells (b, black circle, H&E x40).
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Mahajan K, Relhan V, Relhan AK, Garg VK. Pityriasis rosea: an update on etiopathogenesis and management of difficult aspects. Indian J Dermatol. 2016;61:375‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papakostas D, Stavropoulos PG, Papafragkaki D, Grigoraki E, Avgerinou G, Antoniou C. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen JF, Chiang CP, Chen YF, Wang WM. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280‐282. [DOI] [PubMed] [Google Scholar]
- 4. ChAdOx1 nCoV‐ 19 Corona Virus Vaccine (Recombinant). [Last accesses 29 February. Available from: https//:www.seruminstitute.com/pdf/covisheild_ChAdOx1_nCoV19_corona_virus_vaccine_insert.pdf 2021]
- 5. Burlando M, Russo R, Cozzani E, Parodi A. COVID‐19 "second wave" and vaccines: the dermatologists' perspective. Int J Dermatol. 2021;60:889‐890. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elmas ÖF, Kilitçi A, Acar EM. Dermoscopic profile of pityriasis rosea. Dermatol Sin. 2019;37:199‐204. [Google Scholar]
- 7. Zawar V, Chuh A. Applicability of proposed diagnostic criteria of pityriasis rosea: results of a prospective case‐control study in India. Indian J Dermatol. 2013;58:439‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johansen M, Chisolm SS, Aspey LD, Brahmbhatt M. Pityriasis rosea in otherwise asymptomatic confirmed COVID‐19‐positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:e436‐e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 2 Dermoscopy shows irregularly scattered red dots (black arrows), hemorrhagic crusts (white stars) with collarette of white scales (white arrows) on a pink‐red structureless background. [a and b, Polarized dermoscopy, x10]
Figure 3 Photomicrograph showing spongiotic epidermis, upper dermal perivascular lymphocytic infiltrate (a, H&E x10) and clumps of extravasated red blood cells (b, black circle, H&E x40).
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


