Summary
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is causing an ongoing pandemic of coronavirus disease 2019 (Covid‐19). Effective therapies are required for the treatment of patients with severe stages of the disease. Mesenchymal stem cells (MSCs) have been evaluated in numerous clinical trials, but present challenges, such as carcinogenic risk and special storage conditions, coupled with insufficient data about their mechanism of action. The majority of unique properties of MSCs are related to their paracrine activity and especially to their exosomes. The impact of MSCs‐derived exosomes (MSC‐Es) on complications of Covid‐19 has been investigated in several studies. MSC‐Es may improve some complications of Covid‐19 such as cytokine storm, acute respiratory distress syndrome (ARDS) and acute lung injury (ALI). Additionally, these exosomes can be evaluated as an applicable nano‐size carrier for antiviral therapeutic agents. Herein, we consider several potential applications of MSCs and their derived exosomes in the treatment of Covid‐19.
Keywords: ALI, ARDS, Covid‐19, exosomes, mesenchymal stem cell, SARS‐CoV‐2
Abbreviations
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- COVID‐19
Coronavirus Disease of 2019
- MSCs
mesenchymal stromal cells
- MSC‐E
Mesenchymal stem cell derived exosomes
- ARDS
acute respiratory distress syndrome
- ALI
acute lung injury
- WHO
World health organization
- MERS
middle east respiratory syndrome
- SARS
severe acute respiratory syndrome
- NSP
non‐structural proteins
- S
spike
- E
envelope
- N
nucleocapsid
- M
membrane
- ACE2
Angiotensin‐converting enzyme 2
- CD209L
C‐type 209 lecithin
- EMMPRIN
extracellular matrix metalloproteinase inhibitor
- TMPRSS2
transmembrane protease serine 2
- IFN
interferons
- RAS
Renin‐angiotensin system
- IL
interleukin
- TNF
tumor necrosis factor
- GCSF
granulocyte‐colony stimulating factor
- CXCL‐10
interferon‐gamma‐induced protein‐10
- AKI
acute kidney injury
- SLE
systemic lupus erythematous
- GVHD
graft‐versus‐host disease
- DCs
dendritic cells
- NK
natural killer
- TLRs
toll‐like receptors
- PGE‐2
Prostaglandin E2
- PD‐L1
programmed death‐ligand 1
- CD73
Cluster of Differentiation 73
- ISGs
Interferon‐stimulated genes
- IFITM
Interferon Induced Transmembrane Family
- SAT1
Spermidine/Spermine N1‐Acetyltransferase 1
- IFI6
Interferon Alpha Inducible Protein 6
- AMPs
antimicrobial peptides
- VEGF
Vascular endothelial growth factor
- BBB
blood‐brain‐barrier
- Ev
Extracellular vesicles
- HSP
Heat Shock Protein
- TGF
Transforming growth factor
- NF‐κB
Nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- PBMC
Peripheral blood mononuclear cells
- LPS
Lipopolysaccharide
- MLE
Murine lung epithelial
- NrF‐2
Nuclear factor erythroid 2‐related factor 2
- ARE
Antioxidant response elements
- IKKβ
inhibitor of nuclear factor kappa‐B kinase subunit beta
- EMT
Epithelial‐Mesenchymal Transition
- AEC
alveolar epithelial cells
- MSC‐NTF
Neurotrophic and immunomodulatory factor secreting mesenchymal stem cell
- MSC‐NTF‐E
Neurotrophic and immunomodulatory factor secreting mesenchymal stem cell derived exosomes
- RANTES
regulated on activation, normal T cell expressed and secreted
- Usp
Ubiquitin specific peptidase
- DAMPs
damage‐associated molecular patterns
- miRNAs
microRNAs
- SCS
hstem cell secretome
1. INTRODUCTION
Covid‐19, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was first reported in Wuhan, China in December 2019 and quickly spread worldwide. 1 Due to the high and rapid spread of this viral disease, the World Health Organization (WHO) declared the current outbreak a pandemic on March 11, 2020. Until 20 July 2021, more than 190 million confirmed cases and 4 million deaths have been reported. 2 The virus gene encodes 16 non‐structural proteins (NSP1‐16), four structural proteins (spike (S), envelope (E), nucleocapsid (N), and membrane (M)), and about nine accessory proteins. 3 , 4 Population‐based vaccination strategies represent our current principal defense against this coronavirus. With the onset of mutations and their potential ability to significantly inhibit vaccine efficacy, the development of complementary anti‐ Covid‐19 strategies remains a global priority. 5 , 6 , 7 Stem cells and their derived exosomes have been shown as effective agents to repair, regenerate, and protect human organs against various body injuries with minimal side effects. 8 , 9
The stem cell secretome (SCS) has been demonstrated to have significant anti‐fibrotic, anti‐inflammatory, immunomodulatory, and angiogenic biological activities 10 , 11 , 12 Exosomes are nano‐sized extracellular vesicles with a diameter of 40‐150 nm that are produced by cells in their interior, more precisely in the endosomal compartments of most eukaryotic cells. 13 It was found that MSCs produce exosomes with significant immunomodulatory capacity for tissue restoration. These properties of MSC‐ES have been considered the basis for current on‐going clinical trials. 14 Many of these MSC‐Es contains microRNAs (miRNAs) that regulate crucial cellular functions including cell growth, apoptosis, and host immune responses. 15 , 16 It has been proposed that MSC‐Es represent an ideal vector for the delivery of targeted anti‐viral drugs for the treatment of Covid‐19. 17
2. PATHOGENICITY OF SARS‐CoV‐2
The cell entry mechanism of SARS‐CoV‐2 is a subject of interest of recent studies. Angiotensin‐converting enzyme 2 (ACE‐2) and C‐type 209 lecithin (CD209L) act as the main host receptors for binding the S protein of the virus, becoming the first stage of the pathogenesis of Covid‐19. The virus enters the host cell either via infusion with the host plasma membrane or via clathrin‐dependent and independent endocytosis. 18 , 19 It has also been reported that transmembrane protease serine 2 (TMPRSS2) facilitates virus entry into the host cell. 20 The RNA virus itself, after entering into the cell, promotes disruption in host cell immunity. 21 It has been shown that all three types of viral proteins (structural, non‐structural, and accessory) have various regulatory impacts on the host cell, 22 Structural and functional proteins of SARS‐CoV‐2 independently reduce the synthesis of host cell proteins and subsequently decrease the production of interferons (IFN) which significantly impairs the host cell adaptive immune response. Current evidence suggests this is via three mechanisms;
-
(1)
Non‐structural protein 16 (NSP16) suppresses mRNA splicing and diminishes recognition of viral RNA by intracellular helicase receptors,
-
(2)
NSP1 acts as a ribosome gatekeeper to impair cellular translation and specifically promotes viral translation,
-
(3)
NSP8, and NSP9 interfere with protein trafficking to the cell membrane. 23 , 24
ACE‐2, the SARS‐CoV‐2 receptor, is widely expressed in human organs such as the heart, kidney, gut, liver, and brain which can become involved in systemic infection. 25 , 26 , 27 The lungs are one of the most commonly affected organ in Covid‐19 infection and this is partly explained by the high level of expression of ACE‐2, in alveolar type II and capillary endothelial cells. Proposed mechanisms for viral‐induced lung injury include ‐ direct injury to the vascular bed and in particular, the capillary endothelial cells that also involves disruption to the renin‐angiotensin system (RAS) and a fulminant inflammatory response that results in the breakdown of capillary integrity in targeted organs and the progression to the acute respiratory distress syndrome (ARDS), acute lung injury (ALI), fibrosis, and multiple organ failure. 20 , 28 , 29 , 30 In addition to the lung, other organs involved include the heart and presentations with Kawasaki syndrome, acute coronary syndrome, coagulopathies, myocarditis, the brain and cerebrovascular accidents (CVA’s) and focused areas of neuro‐inflammation and acute kidney and renal failure, the gut and gastrointestinal alterations, the liver and acute liver injury and hepatic dysfunction. 31 , 32 , 33 , 34 , 35 During infection, neutrophils, monocytes, and T cells are recruited to the site of infection by following the chemoattractant gradient of inflammatory chemokines and chemokines produced by infected cells. 36 Under severe conditions, lymphocyte and leukocyte counts decrease, whereas inflammation marker (C‐reactive protein), lactic dehydrogenase, proinflammatory cytokines and chemokines (interleukin (IL) 1β, IL‐6, IL‐7, IL‐2, tumor necrosis factor (TNF)α, granulocyte‐colony stimulating factor (GCSF), interferon gamma‐induced protein‐10 (CXCL10), and monocyte chemoattractant protein‐1) are elevated dramatically. 37 , 38 , 39 The production of large amounts of proinflammatory cytokines by inflammatory cells leads to cytokine storm resulting in hyperinflammation and subsequently serious lung damage. 37 , 40
3. COMBATING COVID‐19
Currently, a small number of therapeutics such as remdesivir, dexamethasone and bamlanivimab plus etesevimab have already been authorized based on the results of randomised controlled trials (RTCs). Further RCTs of therapeutic candidates, including inhaled interferon‐beta, baricitinib, tocilizumab, sarilumab, casirivimab plus imdevimab, and tofacitinib all report clinical benefit. 41 , 42 It is now well recognised that patients recovering from acute Covid‐19 infection are susceptible to develop what is termed long‐Covid syndrome. It is estimated that 1:10 individuals are at‐risk of persistent disabling symptoms, particularly related to the brain and the lungs, long after the acute infective episode has subsided. 43 It has been postulated that in response to this overwhelming viral systemic infection that there is a dysregulation in our normal reparative pathways. 44 Mesenchymal stem cells (MSC’s) with their immunomodulatory and regenerative capacity, make them promising candidates in the treating of both acute infection but also restoring normal organ repair pathways. 45 , 46
3.1. Stem cell therapy
In recent years, MSCs and their therapeutic potential have received much attention. MSCs are non‐specialised, multipotent cells, self‐renewable cells, and capable of differentiating into specific cell types. MSCs are easily accessible cells that are derived from a variety of sources, including bone marrow, adipose tissues, lung, umbilical cord tissue, dental pulp, placenta, Wharton jelly, fetal liver, and menstrual blood. 47 They can expand to clinical volume and can be stored for repetitive therapeutic usage. 48 MSCs can also modulate the immune system through direct interaction with host immune cells or indirectly by secreting paracrine cytokines. 10 Their paracrine activity is through the secretion of molecules, termed secretome which includes transcription factors, growth factors, cytokines, chemokines, hormones, extracellular vesicles (e.g. exosomes), angiogenic molecules, nucleic acids, and lipid mediators which their biological functions and properties such as migration, angiogenesis, homing, metabolic regulation, anti‐inflammation, anti‐fibrotic and anti‐apoptosis help to regenerate tissues and improve end‐organ function. 26 , 49
3.2. Therapeutic effects of MSCs
MSCs therapy has attracted attention due to their potential immunomodulatory properties through interaction with immune cells, including B and T cells, dendritic cells (DCs), macrophages, neutrophils, and natural killer (NK). For instance, inflammatory factors in the host body modify the properties of MSCs to modulate the immune system. 25 , 36 The release of inflammatory mediators in the lung is regulated by the differential activation of damage‐associated molecular patterns (DAMPs) presented on the MSCs surface. Toll‐like receptors (TLRs) are activated by viral RNA (TLR3) and viral unmethylated CpG‐DNA (TLR9) leading to substantial cellular signaling pathways and activation of MSCs. 50 MSCs can play an important role in Immunomodulation by downregulating expression of anti‐inflammatory factors such as prostaglandin E2 (PGE‐2) and programmed death‐ligand 1(PD‐L1). 25 Their paracrine activity can restore the metabolic capacity of alveolar macrophages through direct transfer of their functional mitochondria and switch of inflammatory phenotype (M1) to anti‐inflammatory (M2). 51 , 52 They also have a protective effect against oxidative stress associated with lung inflammation by releasing antioxidant enzymes such as; catalase, superoxide dismutase, and glutathione peroxidase. 20 MSCs treatment has shown a significant inhibition in virus‐induced proinflammatory cytokines. 53 The immune system homeostasis and immunosuppression activities of MSCs are depend on (1) regulating metabolism; (2) expression of CD73, an ATPase with ectonucleotidase property which involves in cell proliferation; (3) induction of mature Dendritic cells (DCs) into novel Jagged‐2 dependent regulatory DCs; (4) regulation of immune cell function. 10 , 25 , 45 , 54 Moreover, MSCs have anti‐virus activity by expressing Interferon‐stimulated genes such as interferon induced transmembrane family (IFITM), spermine N1‐acetyltransferase 1(SAT1), and interferon alpha inducible protein 6 (IFI6). 50 MSCs repair and regenerate damaged lung tissue by increasing the expression of IL‐10 and vascular endothelial growth factor (VEGF) which benefits for ARDS patients as an inflammatory disease. 55 MSCs also have therapeutic effects on other organs. They reduce encephalitis and recover the blood‐brain barrier (BBB) in the brain. 56 In gut, they can regulate inflammation, remodel tissue, and promote the eradication of infections (Figure 1). 20
FIGURE 1.
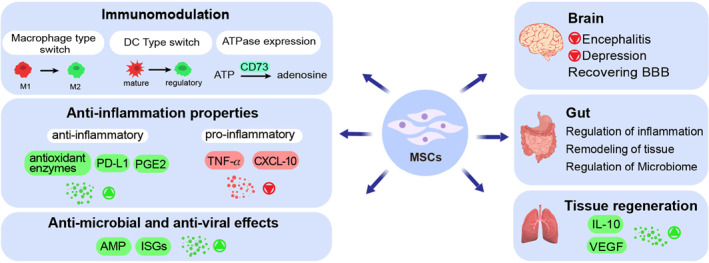
Therapeutic effects of mesenchymal stem cells (MSCs) in combating COVID‐19. MSCs can regulate the immune system by changing cell characteristics and releasing chemical compounds. MSCs also have anti‐inflammation, anti‐microbial, and anti‐viral properties by regulating different mechanisms and pathways. SARS‐CoV‐2 is a multi‐organ disease and using MSCs is beneficial for relieving the complications that this virus causes in organs such as the brain, gut, and lung
3.3. Clinical trials and challenges
MSCs have for Covid‐19 treatment due to previous clinical outcomes in the treatment of blood, heart, kidney, and lung diseases such as ARDS and fibrosis. 19 , 25 In addition, the successful outcome of previous investigations in the treatment of immune‐mediated inflammatory diseases such as systemic lupus erythematosus (SLE), graft‐versus‐host disease (GVHD), and viral infections (e.g. H5N1 and H7N9) have provided promising hope in the fight against COVID‐19. 57 , 58 , 59 , 60 , 61 Based on previous clinical trials, the required dose of 1‐10 × 106 MSCs/kg of body weight with an average of three booster doses has been suggested for the treatment of Covid‐19 patients, the exact dose may vary depending on the patient's immune system and clinical symptoms. 20 In a clinical trial, an increase in the number of peripheral lymphocytes and IL‐10 was observed, while over‐secretion of cytokines by immune cells decreased significantly, resulting in symptoms improvement after treatment without side effects. 57 A case study using human umbilical cord mesenchymal stem cells (HUCMSCs) aimed to assess the safety, feasibility, and tolerability of a high dose of prenatal MSCs. This study demonstrated that the injection of high doses of the MSCs can be beneficial for ARDS patients without serious adverse events. A significant reduction in inflammatory biomarkers (i.e. IL‐8, TNF‐α, and C‐reactive protein) and a remarkable increase in the level of anti‐inflammatory cytokines including IL‐4 and IL‐10 was observed. 64 Table 1 represents clinical trials operated with stem cells for the treatment of Covid‐19 patients.
TABLE 1.
Clinical trials performed with stem cells in COVID‐19 patients (www.clinicaltrials.gov)
| Trail identification | Official TITLE | Source of stem cell | Dose delivery | Actual or estimated enrollment | Phase | Status |
|---|---|---|---|---|---|---|
| NCT04473170 | Adaptive open‐label study evaluating the safety and efficacy of autologous non‐ hematopoietic peripheral Blood Stem Cells Therapy in COVID‐19 Outbreak in abu dhabi, 2020 (SENTAD‐COVID Study) | Non‐hematopoietic peripheral Blood Stem Cells (NHPBSC) | Inhalation | 146, randomized | 1 | Completed |
| NCT04713878 | A 8‐week trial of Mesenchymal Stem Cells Therapy in patients With COVID‐19 Pneumonia | Mesenchymal stem cells (MSCs) | 1 × 106 cell/kg, day 0, 2, and 4 Intravenously | 21, randomized | Not applicable | Completed |
| NCT04288102 | A phase II, multicenter, randomized, double‐blind, placebo‐controlled trial to evaluate the efficacy and safety of human umbilical cord‐derived Mesenchymal Stem Cells in the treatment of Severe COVID‐19 Patients | Umbilical cord mesenchymal stem cell (UC‐MSCs) | 4 × 107 cells Day 0, 3, and 6 Intravenously | 100, randomized | 2 | Completed |
| NCT04573270 | A pilot phase study evaluating the effects of a single Mesenchymal Stem Cell Injection in patients with suspected or Confirmed COVID‐19 Infection and healthcare providers exposed to coronavirus patients | Human umbilical‐cord‐derived mesenchymal stem cells (hUC‐MSCs) | Intravenously | 40, randomized | 1 | Completed |
| NCT04355728 | Umbilical cord‐derived Mesenchymal Stem Cells for COVID‐19 Patients with acute respiratory distress syndrome (ARDS) | hUC‐MSCs | 106 cells intravenously | 24, randomized | 1 | Completed |
| NCT04522986 | An exploratory study of ADR‐001 in patients with severe pneumonia caused by SARS‐CoV‐2 infection | Adipose‐derived mesenchymal stem cells (AD‐MSCs) | 1 × 108 cells once a week, four times intravenously | 6, N/A | 1 | Completed |
| NCT04535856 | Therapeutic study to evaluate the safety and efficacy of DW‐MSC in COVID‐19 Patients: Randomized, double‐blind, and placebo‐controlled | DW‐MSC | Low‐dose group (5 × 107 cells) 2 vials High‐dose group (1 × 108 cells)4 vials | 9, randomized | 1 | Completed |
| NCT04492501 | Role of investigational therapies alone or in combination to treat moderate, severe and Critical COVID‐19 | Bone marrow stem cells (BM.MSCs) | Single dose of 2 1 × 106 cells/kg | 600, non‐randomized | Not applicable | Completed |
| NCT04345601 | Single donor banked bone marrow mesenchymal Stromal Cells for the treatment of COVID‐19 Induced ARDS: A non‐blinded randomized, controlled study | MSCs | 1 × 108 cells intravenously | 30, randomized | 1 | Recruiting |
| NCT04486001 | COVID‐19 Stem Cell Therapy: A phase I study of intravenous administration of allogeneic Adipose Stem cells | AD‐MSCs | Intravenously | 20, N/A | 1 | Recruiting |
| NCT04625738 | Efficacy of infusions of Mesenchymal Stem Cells From wharton jelly in the moderate to severe SARS‐cov‐2 related acute respiratory distress syndrome (COVID‐19): A phase IIa double‐blind randomized controlled trial | Wharton's jelly derived MSCs | day 0: 1 × 106 cells/kg day 3: 0.5 × 106 cells/kg day 5: 0.5 × 106 cells/kg | 30, randomized | 2 | Not yet recruiting |
| NCT04416139 | Mesenchymal Stem Cells for the treatment of severe acute respiratory distress syndrome due to COVID‐19. Pilot study | MSCs | 1 × 106 cells a single dose Intravenously | 5, non‐randomized | 2 | Recruiting |
| NCT04494386 | Phase 1/2a Study of umbilical cord Lining Stem Cells (ULSC) in patients with ARDS due to COVID‐19 | Umbilical cord Lining Stem Cells (ULSC) | 1 × 108 cells/dose intravenously | 60 estimated, randomized | 2 | Recruiting |
| NCT04366063 | Mesenchymal Stem Cell Therapy for acute respiratory distress syndrome in coronavirus infection: A phase 2‐3 clinical trial | MSCs | Two doses of 1 × 108 (±10%) day 0 and day 2 Intravenously | 60, randomized | 2 and 3 | Recruiting |
| NCT04339660 | Clinical research of human Mesenchymal Stem Cells in the treatment of COVID‐19 Pneumonia | UC‐MSCs | 1×106 cells/kilogram of weight Intravenously | 30, randomized | 1 | Recruiting |
| NCT04400032 | Cellular Immuno‐therapy for COVID‐19 ARDS (CIRCA‐19) | MSCs | 75 ×106 (panel 1) 150 × 106 (panel 2) and 270 × 106 cells intravenously | 21, non‐randomized | 1 and 2 | Recruiting |
| NCT04361942 | Double blind, placebo‐controlled, phase II trial to evaluate safety and efficacy of allogenic mesenchymal Stromal Cells MSV_allo for treatment of acute respiratory failure in patients With COVID‐19 Pneumonia (COVID_MSV) | MSCs | 1× 106 cells/Kg intravenously | 24, randomized | 2 | Recruiting |
| NCT04269525 | Clinical research regarding the availability and safety of UC‐MSCs treatment for serious pneumonia and critical pneumonia caused by the 2019‐nCOV Infection | UC‐MSCs | 3.3 × 107 cell, 3 dose each time on day 1, day 3, day 5, and day 7 Intravenously | 16, N/A | 2 | Recruiting |
| NCT04367077 | A phase 2/3 study to assess the safety and efficacy of MultiStem® therapy in subjects with acute respiratory distress syndrome (ARDS) due to coronavirus disease (COVID‐19) | MultiStem | Intravenously | 400, randomized | 2 | Recruiting |
| NCT03042143 | Repair of acute respiratory distress syndrome by Stromal Cell Administration (REALIST): An open label dose escalation phase 1 trial followed by a randomized, double‐blind, placebo‐controlled phase 2 trial (COVID‐19) | hUC‐MSCs | 4 × 108 cells Intravenously | 75, randomized | 1 | Recruiting |
| NCT04445220 | A multi‐center, randomized, case controlled, double‐blind, ascending‐dose study of extracorporeal mesenchymal Stromal Cell Therapy (SBI‐101 therapy) in COVID‐19 Subjects with acute kidney injury receiving renal replacement therapy | SBI‐101 using allogeneic MSCs | 250 × 106 cells (low dose) and 750 × 106 cells (high dose) | 22, randomized | 1 and 2 | Recruiting |
| NCT04336254 | Safety and efficacy study of allogeneic human dental pulp Mesenchymal Stem Cells to treat severe pneumonia of COVID‐19: a single‐center, prospective, randomised clinical trial | Allogeneic human dental pulp stem cells | 3.0 × 106 cells day 1, day 4 and day 7 intravenously | 20, randomized | 1 | Recruiting |
| NCT04392778 | What is the effect of Mesenchymal Stem Cell Therapy on seriously Ill patients With Covid 19 in intensive Care? | hUC‐MSCs intravenously | 3 × 106 cells/kg day 0, 3 and 6 Intravenously | 30, randomized | 1 | Recruiting |
| NCT04390152 | Safety and efficacy of intravenous infusion of Wharton's jelly derived Mesenchymal Stem Cell Plus standard therapy for the treatment of patients with acute respiratory distress syndrome diagnosis due to COVID 19: A randomized controlled trial | Wharton's jelly derived MSCs | 50 × 106 cells, two doses intravenously | 40, randomized | 1 | Recruiting |
| NCT04461925 | Treatment of Coronavirus COVID‐19 Pneumonia (pathogen SARS‐CoV‐2) with cryopreserved allogeneic multipotent Mesenchymal Stem Cells of the placenta and umbilical cord | Placenta‐derived MSCs | 1 × 106 cells/kg body weight time on day 1, 4 and 7 Intravenously | 30, non‐randomized | 1 | Recruiting |
| NCT04565665 | Emergency use pilot study of cord blood derived Mesenchymal Stem Cells for treatment of COVID‐19 Related acute respiratory distress syndrome | Cord blood‐derived MSCs | Single dose Intravenously | 70, randomized | 1 | Recruiting |
| NCT04252118 | Safety and efficiency of Mesenchymal Stem Cell in treating pneumonia patients infected With COVID‐19 | MSCs | 3.0 × 106 intravenously day 0, day 3, day 6 | 20, non‐randomized | 1 | Recruiting |
| NCT04611256 | Adjuvant therapy with Mesenchymal Stem Cells in patients diagnosed With COVID‐19 in critical condition | AD‐MSCs | 1 × 106/kg body weight Intravenously | 20, randomized | 1 | Recruiting |
| NCT04457609 | Application of umbilical cord Mesenchymal Stem Cells as adjuvant therapy for critically‐Ill COVID‐19 patients | UC‐MSCs | 1 × 106 cells/kg body weight intravenously | 40, randomized | 1 | Recruiting |
| NCT04397796 | Phase 1b Randomized, double‐blind, placebo‐controlled study of the safety of therapeutic treatment with immunomodulatory Mesenchymal Stem Cells In adults With COVID‐19 Infection requiring mechanical ventilation | BM‐Allo.MSC | ‐ | 45, randomized | 1 | Recruiting |
4. Mesenchymal stem cells‐derived exosomes (MSC‐E) in combating Covid‐19
Although cell therapy provides numerous advantages, four main challenges still remain; (1) potential tumorigenesis of cytokines secreted by MSCs such as VEGF; (2) Low survival of transplanted MSCs in the body due to their high sensitivity to harsh environmental conditions (e. g. inflammation); (3) risk of obstruction of small‐diameter pulmonary arteries as cells pass through; (4) special requirements of storage at a temperature of −80°C. 37 , 62 To address these challenges, exosome therapy is a promising strategy due to its lower tumorigenicity, lack of transmission of secondary infections, easy manipulation, simple storage requirements, and availability. 20
In multicellular organisms, most of the physiological processes occur through intracellular signaling. 63 extracellular vesicles (EVs) are lipid‐bilayer vesicles that have been identified as signaling organelles for their role in mediating intercellular communication by serving as carriers of different biomolecules, including RNAs, proteins, and lipids. 64 EVs are categorized into three groups, based on their biogenesis and size. (1) exosomes; the small size EVs (40–150 nm in diameter) secreted by the fusion of multivesicular bodies with the cell membrane; (2) microvesicles; the medium size EVs (150–1000 nm in diameter) released by the direct budding of the cell membrane, and (3) the apoptotic bodies; random size EVs (50–2000 nm in diameter) produced during the programmed cell death–apoptosis. 65 , 66
Many types of cells, including immune cells, 64 and MSCs release exosomes. Although most of the exosomes share an evolutionarily conserved composition of the proteins regardless of their cellular origins, each one contains some tissue‐specific proteins dependent on their source (Figure 2). 67 MSC‐Es proteome contains about 2000 types of proteins which can be divided into two categories. First, membrane proteins; such as GPI‐anchored proteins and tetraspanins; second, soluble proteins encapsulated inside the vesicles; such as heat shock proteins, chaperones, signaling proteins, cytokines, and interleukins. 68 , 69 In addition to the proteins, mRNAs, and micRNAs are other bioactive molecules presenting in exosomes. 70 Depending on the origin of cells' microenvironmental stresses and conditions, exosomes can be wildly diverse in their composition. 71 It has been established that exosomes contain a similar cargo composition to that of the cells that secrete them, indicating that MSC‐Es are functionally in accordance with the function of MSCs, 72 while they have more advantages, including blood barrier penetration, lower immunogenicity, no tumor genesis risk, 67 large scale manufacture, and lower cost compared to MSCs, all of which make the MSC‐Es a potentially promising treatment for Covid‐19 infection. 73 , 74
FIGURE 2.
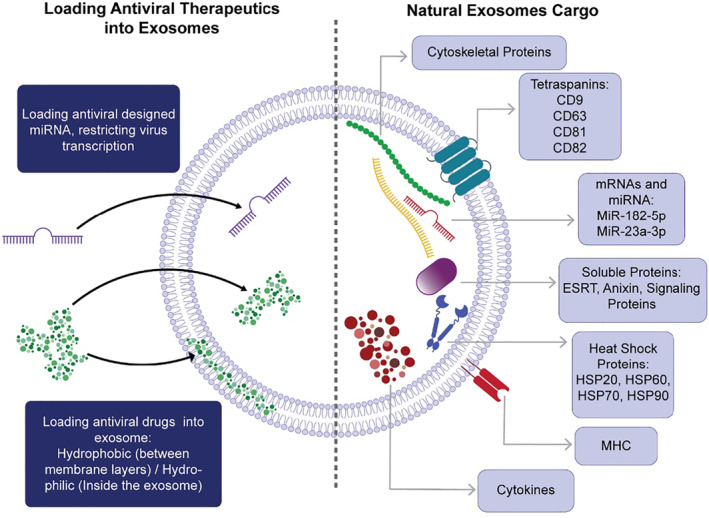
MSC‐Es have a cargo composition highly similar to other exosomes, consisting of proteins, mRNAs, and miRNAs. Besides, MSC‐Es are capable of delivering other therapeutic agents to achieve a combination therapy strategy
4.1. Natural MSC‐Es inherent therapeutic effects on Covid‐19
Therapeutic properties of MSC‐Es are divided into anti‐inflammatory, immunomodulatory and tissue regeneration effects, altogether resulting in inhibition of the cytokine storm and reduce tissue injury conditions including ARDS, ALI, and fibrosis (Figure 3). MSC‐Es modulate the immune microenvironment by increasing the secretion of anti‐inflammatory cytokines and reducing the pro‐inflammatory factors in peripheral blood mononuclear cells (PBMCs). In the presence of MSC‐Es, a remarkable reduction in pro‐inflammatory cytokines such as IL‐1β, TNF‐α, and IL‐17 can be archived, whereas anti‐inflammatory cytokines (e.g. TGF‐β and IL‐10) are increased significantely. 75 MSC‐Es therapy can reduce the proliferation and apoptosis of CD4+ T‐cells, but increase the ratio of regulatory T‐cells to effector T‐cells. These activities in the immune microenvironment of alveolar can inhibit the cytokine storm caused by infections. 76 , 77
FIGURE 3.
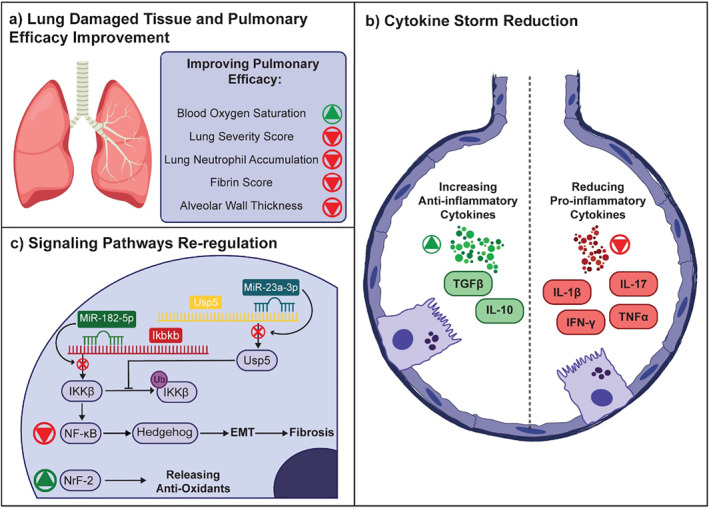
Natural MSC‐Es have inherent therapeutic effects caused by their cargoes. They can modulate immune responses and reverse tissue damages. (a) MSC‐Es can increase blood oxygen saturation and decrease lung severity score, neutrophil accumulation, fibrin score, and alveolar wall thickness by reversing ALI and ARDS and repairing lung tissue. (b) In addition, they can inhibit cytokine storms by reducing pro‐inflammatory factors and increasing anti‐inflammatory factors. (c) Also, MSC‐E miRNAs re‐regulate signaling pathways. The miR‐182‐5p and miR‐23a‐3p both reduce the IKKβ level that is the NF‐κB pathway initiator by decreasing its synthesis and increasing its ubiquitination respectively. The up‐regulation of the Nrf‐2 signaling pathway increases anti‐oxidant release on the other hand
Recently numerous investigations have focus on animal models of LPS (lipopolysaccharides)‐induced ARDS and ALI to understand the mechanism of MSC‐Es in reversing established fibrosis, ARDS, and ALI. 78 , 79 , 80 A previous cell model of ALI has shown that MSC‐Es can reverse the ALI through downregulation of nuclear factor erythroid 2‐related factor 2 (NrF‐2) and antioxidant response elements (ARE) factors. MSC‐Es also could upregulate significantly NF‐κB signaling pathways leading to treatment of ALI. 81
In another study on the LPS‐treated MLE‐12 ALI model, the mechanism of MSC‐Es for lung injury treatment has been demonstrated in more detail. There is a positive correlation between the NF‐κB pathway activation and the trigger of nuclear factor kappa‐B kinase subunit beta (IKKβ), thus Xiao and colleagues focused on the impact of MSC‐E on IKKβ activity. This study suggested that MSC‐Es reduce IKKβ and also increase its ubiquitination, resulting in inhibition of the NF‐κB and the Hedgehog pathways, both playing a key role in the Epithelial‐Mesenchymal Transition (EMT) process of alveolar epithelial cells (AECs) that is closely related to pulmonary fibrosis. 82 Investigation on a mouse ARDS model treated by MSC‐Es, which was differentiated to produce neurotrophic and immunomodulatory factors (MSC‐NTF) showed a substantial reduction in IFNγ, TNFα, IL‐6, and RANTES (regulated on activation, normal T cell expressed and secreted). The oxygen saturation level also was improved significantly after 72 h post‐treatment. Histological assessments indicated that this strategy efficiently improved LPS‐induced physical damage by reducing the total severity score and neutrophil accumulation in the lung. 83
Although the mechanism of action of MSC‐E have not been fully understood, these outcomes are thought to be related to exosome miRNA cargo. 84 miRNAs are a subclass of small RNAs that can inhibit translation process, cause mRNA decapping and deadenylation. 85 In‐silico analysis has shown massive amount of various miRNA cargo in MSC‐Es, which includes 258 miRNAs related to cytokines and chemokines and 266 miRNAs for cell death (pyroptosis, apoptosis, and necrosis) genes. 86 Xiao and colleagues have demonstrated the downregulation of the Ikbkb gene results in a significant decrease in the gene expression of IKKβ, leading to downregulation of ubiquitin‐specific peptidase (Usp) 5, a deubiquitinase that blocks IKKβ ubiquitination. 82 There are a limited number of clinical trials ongoing to assess the safety and efficacy of MSC‐E for the treatment of Covid‐19, despite promising results in preclinical studies (Table 2).
TABLE 2.
Clinical trials performed with mesenchymal stem cell‐derived exosomes on COVID‐19 patients (www.clinicaltrials.gov)
| Trial identification | Official title | Exosome source | Administration route | Dosage | Phase | Status | Enrolment and allocation (Estimated/Actual) |
|---|---|---|---|---|---|---|---|
| NCT04276987 | A pilot clinical study on aerosol inhalation of the exosomes derived from allogenic adipose mesenchymal stem cells in the treatment of severe patients with novel coronavirus pneumonia | Allogenic adipose mesenchymal stem cells | Inhalation | 2.0× of nano particle/3ml For the first five days | 1 | Completed | 24 |
| NCT04491240 | The protocol of evaluation of safety and efficiency of method of exosome inhalation in SARS‐CoV‐2 associated two‐sided pneumonia | MSC | Inhalation | Exo 1: Twice a day inhalation of 3ml solution containing 0.5‐2×10^10 nanoparticles of type 1 exosome for 10 days Exo 2: Twice a day inhalation of 3mL solution containing 0.5‐2× nanoparticles of type 2 exosome for 10 days Placebo: Twice a day inhalation of 3mL solution free of nanoparticle | 1 and 2 | Completed | 30, randomized |
| NCT04602442 | Safety and efficiency of method of exosome inhalation in COVID‐19 associated pneumonia (COVID‐19EXO2) | MSC | Inhalation | Exo 1: Twice a day inhalation of 3ml solution containing 0.5‐2×10^10 nanoparticles of type 1 exosome for 10 days Exo 2: Twice a day inhalation of 3mL solution containing 0.5‐2× nanoparticles of type 2 exosome for 10 days Placebo: Twice a day inhalation of 3mL solution free of nanoparticle | 2 | Enrolling by invitation | 90, randomized |
| NCT04798716 | Mesenchymal stem cell exosomes for the treatment of COVID‐19 positive patients with acute respiratory distress syndrome and/or novel coronavirus pneumonia | MSC | Intravenous | First cohort: Intervention of ardoxso for five days on an escalating dose 2×, 4×, 8× mL, with a minimum of 24 h between doses recorded Second cohort: Intervention of ardoxso for five days on an escalating dose 8×, 4×, 8×mL, with a minimum of 24 h between doses recorded Third cohort: Intervention of ardoxso for five days on an escalating dose 8×, 8×, 8× mL, with a minimum of 24 h between doses recorded Fourth cohort: Randomized control ratio 1:3 25% of patients will receive 3 doses of placebo over five days75% of patients will three doses of 8× mL exosome over five days | 1 and 2 | Not yet recruiting | 55, randomized |
A nonrandomized clinical trial study on 24 PCR positive Covid‐19 patients has conducted to evaluate the safety and efficacy of bone marrow mesenchymal stem cell‐derived exosomes. A single dose of MSC‐E, administered through the intravenous route, has shown a significant reduction in cytokine storm with substantial improvement in oxygenation patients without adverse effect, all in line with previous experiments. 87 Preclinical and clinical studies confirm the ability of MSC‐E as a promising, safe, and efficient strategy for combating Covid‐19.
4.2. Administration of MSC‐Es as a combined therapy in combating Covid‐19
Exosomes have attracted increasing attention from drug delivery systems in the past decade owing to their intrinsic properties such as high stability, biodegradability, biocompatibility, prolonged circulation time, low immunogenicity, and the ability to pass blood barriers, all of which represent exosomes an ideal carrier for therapeutic agents such as anti‐viral drugs and miRNAs. 88 , 89 , 90 Exosomes injected intravenously accumulate in the damaged tissues and inflammation sites by preference, 91 resulting in a passive targeting delivery of exosome cargo. Similar to other lipid‐bilayer nanocarriers, exosomes can be tissue‐specifically targeted by surface modifications to enhance and improve their targeting activity. 85 Also, exosomes administered through the inhalation route has shown a promising approach to enhance the efficacy of local therapy. 92 The combination therapy of exosomes and traditional immune‐modulating drugs may provide a synergic effect for the treatment of Covid‐19. 93 Two approaches have been used to load therapeutic cargos (drugs, nucleic acids, proteins, and peptides) into the exosomes. 94 (1) Passive methods; which are biological‐based cargo loading techniques. The passive methods can be achieved by incubating the cargo with the exosomes to introduce the cargo molecule to them through diffusion and hydrophobic interaction. Another strategy is to employee genetically engineered donor cells for overexpression of the cargo, which is finally is packaged into exosomes. 95 (2) Active methods; these approaches are rely on the temporary disruption of the exosome membrane by mechanical/chemical methods (i.e. sonication, extrusion, repetitive freeze‐thawing, electroporation, or using chemical reagents), providing the desired compounds to diffuse into the vesicles. 96 Although during these processes the native structure of the exosome can be damaged and may lost its therapeutic properties, active methods have shown higher loading efficacy compared to passive methods. 95 , 97
5. CONCLUSION
Covid‐19 has reached epidemic proportions globally, affecting all continents. Thus far, no specific antiviral drug is available for controlling patients with SARS‐CoV‐2 infection. Due to the inefficiency of most countries in rapid vaccination and a large number of cases, the development of new therapeutic strategies has been the focus of considerable research efforts to address this pressing clinical need. MSCs and their derived exosomes have attracted much attention in investigations of Covid‐19 treatment, owing to their promising properties, including regenerative, immunomodulatory, and anti‐inflammatory. Previous investigations have shown promise in preclinical studies and is now being studied in various clinical trials. MSC‐Es have shown significant influences in treating Covid‐19 and other inflammatory and tissue injury diseases. MSC‐Es have been suggested as a hopeful candidate for drug delivery and combination therapy, owing to their ability to encapsulate various therapeutic agents, their nano‐sized, and low immunogenicity. However, more investigation is needed on the biodistribution and the in vivo metabolic fate of both MSCs versus MSCs‐Es. The current challenges from the bench to clinical practice, include the qualification control, the biodistribution, and stability of each manufactory scale preparation of MSCs‐Es, which should be investigated in future.
CONFLICTS OF INTEREST
All authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Maryam Yousefi Dehbidi, Nima Goodarzi, and Mohammad H. Azhdari; Writing–original draft and Editing. Mohammad Doroudian; conceptualization, preparation, Writing, Reviewing, and Editing the Final manuscript.
ACKNOWLEDGEMENT
None.
Yousefi Dehbidi M, Goodarzi N, Azhdari MH, Doroudian M. Mesenchymal stem cells and their derived exosomes to combat Covid‐19. Rev Med Virol. 2022;32(2):e2281. 10.1002/rmv.2281
References
- 1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Organization WH. WHO Coronavirus (COVID‐19) Dashboard; 2021. https://covid19.who.int/?gclid=Cj0KCQjwlN32BRCCARIsADZJ4u4V1zhMUVPe0_HicDuVkxeL‐afUPq1sg1FXFw6Z9D_XflpDQInjuAaAu7oEALw_ [Google Scholar]
- 3. Vlachakis D, Papakonstantinou E, Mitsis T, et al. Molecular mechanisms of the novel coronavirus SARS‐CoV‐2 and potential anti‐COVID19 pharmacological targets since the outbreak of the pandemic. Food Chem Toxicol. 2020;146:111805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS‐CoV‐2. Nat Rev Microbiol. 2021;19(3):155‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vilar S, Isom DG. One year of SARS‐CoV‐2: How much has the virus changed? Biology. 2021;10(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giovanetti M, Benedetti F, Campisi G, et al. Evolution patterns of SARS‐CoV‐2: Snapshot on its genome variants. Biochem Biophysical Res Commun. 2021;538:88‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forni G, Mantovani A. COVID‐19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28 (2):626–639. 10.1038/s41418-020-00720-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem cell Res Ther. 2019;10(1):1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moghadasi S, Elveny M, Rahman HS, et al. A paradigm shift in cell‐free approach: the emerging role of MSCs‐derived exosomes in regenerative medicine. J Transl Med. 2021;19(1):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Bamba C, Singh SP, Choudhury S. Can mesenchymal stem cell therapy be the interim management of COVID‐19? Drug Discov Ther. 2020;14(3):139‐142. [DOI] [PubMed] [Google Scholar]
- 11. Hu S, Park J, Liu A, et al. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med. 2018;7(8):615‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahsavari A, Weeratunga P, Ovchinnikov DA, Whitworth DJ. Pluripotency and immunomodulatory signatures of canine induced pluripotent stem cell‐derived mesenchymal stromal cells are similar to harvested mesenchymal stromal cells. Sci Rep. 2021;11(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907‐1920. [DOI] [PubMed] [Google Scholar]
- 14. Phinney DG, Pittenger MF. Concise review: MSC‐derived exosomes for cell‐free therapy. Stem Cells. 2017;35(4):851‐858. [DOI] [PubMed] [Google Scholar]
- 15. Lindsay MA. microRNAs and the immune response. Trends Immunol. 2008;29(7):343‐351. [DOI] [PubMed] [Google Scholar]
- 16. Nahand JS, Vandchali NR, Darabi H, et al. Exosomal microRNAs: novel players in cervical cancer. Epigenomics. 2020;12(18):1651‐1660. [DOI] [PubMed] [Google Scholar]
- 17. Khalaj K, Figueira RL, Antounians L, Lauriti G, Zani A. Systematic review of extracellular vesicle‐based treatments for lung injury: are EVs a potential therapy for COVID‐19? J Extracell Vesicles. 2020;9(1):1795365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID‐19. J Pharm Anal. 2020;10(2):102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yadav P, Vats R, Bano A, Bhardwaj R. Mesenchymal stem cell immunomodulation and regeneration therapeutics as an ameliorative approach for COVID‐19 pandemics. Life Sci. 2020;263:118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashemian SMR, Aliannejad R, Zarrabi M, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID‐19‐induced ARDS patients: a case series. Stem Cell Research & Therapy. 2021;12(1):91. 10.1186/s13287-021-02165-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campbell GR, To RK, Hanna J, Spector SA. SARS‐CoV‐2, SARS‐CoV‐1, and HIV‐1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non‐classical pathway. iScience. 2021;24(4):102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lei X, Dong X, Ma R, et al. Activation and evasion of type I interferon responses by SARS‐CoV‐2. Nat Commun. 2020;11(1):3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schultze JL, Aschenbrenner AC. COVID‐19 and the human innate immune system. Cell. 2021;184(7):1671–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tidu A, Janvier A, Schaeffer L, et al. The viral protein NSP1 acts as a ribosome gatekeeper for shutting down host translation and fostering SARS‐CoV‐2 translation. RNA. 2020;27(3):253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zumla A, Wang FS, Ippolito G, et al. Reducing mortality and morbidity in patients with severe COVID‐19 disease by advancing ongoing trials of Mesenchymal Stromal (stem) Cell (MSC) therapy ‐ achieving global consensus and visibility for cellular host‐directed therapies. Int J Infect Dis. 2020;96:431‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X, Han P, Wang H, et al. Engineering mesenchymal stromal cells with neutralizing and anti‐inflammatory capability against SARS‐CoV‐2 infection. Molecular Therapy‐Methods & Clinical Development. 2021;21:754‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan HW, Xu YM, Lau AT. Angiotensin‐converting enzyme 2: the old door for new severe acute respiratory syndrome coronavirus 2 infection. Rev Med virology. 2020;30(5):e2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akbari A, Rezaie J. Potential therapeutic application of mesenchymal stem cell‐derived exosomes in SARS‐CoV‐2 pneumonia. Stem Cell Res Ther. 2020;11(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gallelli L, Zhang L, Wang T, Fu F. Severe acute lung injury related to COVID‐19 infection: a review and the possible role for escin. J Clin Pharmacol. 2020;60(7):815‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hardenberg JB, Stockmann H, Aigner A, et al. Critical illness and systemic inflammation are key risk factors of severe acute kidney injury in patients with COVID‐19. Kidney Int Rep. 2021;6(4):905‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS‐CoV‐2: from neurological manifestations of COVID‐19 to potential neurotropic mechanisms. J Neurol. 2020;267(8):2179‐2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Achar A, Ghosh C. COVID‐19‐Associated neurological disorders: the potential route of CNS invasion and blood‐brain relevance. Cells. 2020;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barros I, Silva A, de Almeida LP, Miranda CO. Mesenchymal stromal cells to fight SARS‐CoV‐2: Taking advantage of a pleiotropic therapy. Cytokine Growth Factor Rev. 2021;58:114‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID‐19 and the liver. J Hepatol. 2020;73(5):1231‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iwamura APD, Tavares da Silva MR, Hümmelgen AL, et al. Immunity and inflammatory biomarkers in COVID‐19: a systematic review. Rev Med Virology. 2021;31(4):e2199. [DOI] [PubMed] [Google Scholar]
- 37. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine. 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vieira C, Nery L, Martins L, Jabour L, Dias R, Simões ESAC. Downregulation of membrane‐bound angiotensin converting enzyme 2 (ACE2) receptor has a pivotal role in COVID‐19 immunopathology. Curr Drug Targets. 2021;22(3):254‐281. [DOI] [PubMed] [Google Scholar]
- 40. Taghavi‐Farahabadi M, Mahmoudi M, Soudi S, Hashemi SM. Hypothesis for the management and treatment of the COVID‐19‐induced acute respiratory distress syndrome and lung injury using mesenchymal stem cell‐derived exosomes. Med Hypotheses. 2020;144:109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dos Santos WG. Natural history of COVID‐19 and current knowledge on treatment therapeutic options. Biomed Pharmacother. 2020;129:110493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buonaguro FM, Ascierto PA, Morse GD, et al. Covid‐19: time for a paradigm change. Rev Med Virology. 2020;30(5):e2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Havervall S, Rosell A, Phillipson M, et al. Symptoms and functional impairment assessed 8 Months after mild COVID‐19 among health care workers. J Am Med Assoc. 2021;325(19):2015‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bernard I, Limonta D, Mahal LK, Hobman TC. Endothelium infection and dysregulation by SARS‐CoV‐2: evidence and caveats in COVID‐19. Viruses. 2020;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choudhery MS, Harris DT. Stem cell therapy for COVID‐19: possibilities and challenges. Cell Biol Int. 2020;44(11):2182‐2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells ‐ current trends and future prospective. Biosci Rep. 2015;35(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID‐19: present or future. Stem Cell Rev Rep. 2020;16(3):427‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bari E, Ferrarotti I, Saracino L, Perteghella S, Torre ML, Corsico AG. Mesenchymal stromal cell secretome for severe COVID‐19 infections: premises for the therapeutic use. Cells. 2020;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rajarshi K, Chatterjee A, Ray S. Combating COVID‐19 with mesenchymal stem cell therapy. Biotechnol Rep (Amst). 2020;26:e00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome‐guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Advanced Science. 2019;6(20):1900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xiao K, Hou F, Huang X, Li B, Qian ZR, Xie L. Mesenchymal stem cells: current clinical progress in ARDS and COVID‐19. Stem Cell Res Ther. 2020;11(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patel S. Danger‐associated molecular patterns (DAMPs): the derivatives and triggers of inflammation. Curr Allergy Asthma Rep. 2018;18(11):63. [DOI] [PubMed] [Google Scholar]
- 55. Lindner HA, Velasquez SY, Thiel M, Kirschning T. Lung protection vs. Infection resolution: interleukin 10 Suspected of double‐dealing in COVID‐19. Front Immunol. 2021;12:602130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chia YC, Anjum CE, Yee HR, et al. Stem cell therapy for neurodegenerative diseases: How do stem cells bypass the blood‐brain barrier and Home to the brain? Stem Cell Int. 2020;2020:8889061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2(‐) mesenchymal stem cells improves the outcome of patients with COVID‐19 pneumonia. Aging Dis 2020;11(2):216‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun L, Wang D, Liang J, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum 2010;62(8):2467‐2475. [DOI] [PubMed] [Google Scholar]
- 59. Hashmi S, Ahmed M, Murad MH, et al. Survival after mesenchymal stromal cell therapy in steroid‐refractory acute graft‐versus‐host disease: systematic review and meta‐analysis. Lancet Haematol 2016;3(1):e45‐e52. [DOI] [PubMed] [Google Scholar]
- 60. Chan MC, Kuok DI, Leung CY, et al. Human mesenchymal stromal cells reduce influenza A H5N1‐associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci U. S. A. 2016;113(13):3621‐3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen J, Hu C, Chen L, et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a Hint for COVID‐19 treatment. Engineering (Beijing). 2020;6(10):1153‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126(4):1139‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hwang I. Cell‐cell communication via extracellular membrane vesicles and its role in the immune response. Mol Cells. 2013;36(2):105‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus‐like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mohan A, Agarwal S, Clauss M, Britt NS, Dhillon NK. Extracellular vesicles: novel communicators in lung diseases. Respir Res. 2020;21(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Deng H, Sun C, Sun Y, et al. Lipid, protein, and MicroRNA composition within mesenchymal stem cell‐derived exosomes. Cell Reprogr. 2018;20(3):178‐186. [DOI] [PubMed] [Google Scholar]
- 69. Qiu G, Zheng G, Ge M, et al. Functional proteins of mesenchymal stem cell‐derived extracellular vesicles. Stem Cell Res Ther. 2019;10(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Dev Reprod Biol. 2015;13(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jelonek K, Widlak P, Pietrowska M. The influence of ionizing radiation on exosome composition, secretion and intercellular communication. Protein Pept Lett. 2016;23(7):656‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hu C, Zhao L, Zhang L, Bao Q, Li L. Mesenchymal stem cell‐based cell‐free strategies: safe and effective treatments for liver injury. Stem Cell Res Ther. 2020;11(1):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two‐edged sword in cancer therapy. Int J Nanomedicine. 2019;14:2847‐2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pocsfalvi G, Mammadova R, Ramos Juarez AP, Bokka R, Trepiccione F, Capasso G. COVID‐19 and extracellular vesicles: an intriguing interplay. Kidney and Blood Pressure Research. 2020;45(5):661‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Noronha NdC, Mizukami A, Caliári‐Oliveira C, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell‐based therapies. Stem Cell Res Ther. 2019;10(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ji L, Bao L, Gu Z, et al. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol Res. 2019;67(4‐5):432‐442. [DOI] [PubMed] [Google Scholar]
- 77. Chen W, Huang Y, Han J, et al. Immunomodulatory effects of mesenchymal stromal cells‐derived exosome. Immunol Res. 2016;64(4):831‐840. [DOI] [PubMed] [Google Scholar]
- 78. Chen X, Wu S, Tang L, et al. Mesenchymal stem cells overexpressing heme oxygenase‐1 ameliorate lipopolysaccharide‐induced acute lung injury in rats. J Cell physiology. 2019;234(5):7301‐7319. [DOI] [PubMed] [Google Scholar]
- 79. Huh JW, Kim WY, Park YY, et al. Anti‐inflammatory role of mesenchymal stem cells in an acute lung injury mouse model. Acute Crit Care. 2018;33(3):154‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Qin H, Zhao A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein & Cell. 2020;11(10):707‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li J, Deng X, Ji X, et al. Mesenchymal stem cell exosomes reverse acute lung injury through Nrf‐2/ARE and NF‐kappaB signaling pathways. PeerJ. 2020;8:e9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xiao K, He W, Guan W, et al. Mesenchymal stem cells reverse EMT process through blocking the activation of NF‐κB and Hedgehog pathways in LPS‐induced acute lung injury. Cell Death Dis. 2020;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kaspi H, Semo J, Abramov N, et al. MSC‐NTF (NurOwn(R)) exosomes: a novel therapeutic modality in the mouse LPS‐induced ARDS model. Stem Cell Res Ther. 2021;12(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Qiu G, Zheng G, Ge M, et al. Mesenchymal stem cell‐derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther. 2018;9(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baek G, Choi H, Kim Y, Lee HC, Choi C. Mesenchymal stem cell‐derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl Med. 2019;8(9):880‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schultz IC, Bertoni APS, Wink MR. Mesenchymal stem cell‐derived extracellular vesicles carrying miRNA as a potential multi Target therapy to COVID‐19: an in silico analysis. Stem Cell Rev Rep. 2021;17(2):341‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID‐19. Stem Cells Dev. 2020;29(12):747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ortega A, Martinez‐Arroyo O, Forner MJ, Cortes R. Exosomes as drug delivery systems: endogenous nanovehicles for treatment of systemic lupus erythematosus. Pharmaceutics. 2020;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Doroudian M, MacLoughlin R, Poynton F, Prina‐Mello A, Donnelly SC. Nanotechnology based therapeutics for lung disease. Thorax. 2019;74(10):965‐976. [DOI] [PubMed] [Google Scholar]
- 90. Doroudian M, O’ Neill A, Mac Loughlin R, Prina‐Mello A, Volkov Y, Donnelly SC. Nanotechnology in pulmonary medicine. Curr Opin Pharmacol. 2021;56:85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kostyushev D, Kostyusheva A, Brezgin S, et al. Gene editing by extracellular vesicles. Int J Mol Sci. 2020;21(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Popowski KD, Dinh PUC, George A, Lutz H, Cheng K. Exosome therapeutics for COVID‐19 and respiratory viruses. View. 2021;2:20200186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pinky S, Gupta V, Sharma Y, Dinda AK, Mohanty S. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID‐19. Stem Cell Rev Rep. 2021;17(1):33‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fu S, Wang Y, Xia X, Zheng JC. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. [Google Scholar]
- 95. Akuma P, Okagu OD, Udenigwe CC. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Frontiers in Sustainable Food Systems. 2019;3(23). [Google Scholar]
- 96. Li S‐p, Lin Z‐x, Jiang X‐y, Yu X‐y. Exosomal cargo‐loading and synthetic exosome‐mimics as potential therapeutic tools. Acta Pharmacol Sin. 2018;39(4):542‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9(4):1015‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]


