Summary
BNT162b2 and mRNA‐1273 are two types of mRNA‐based vaccine platforms that have received emergency use authorization. The emergence of novel severe acute respiratory syndrome (SARS‐CoV‐2) variants has raised concerns of reduced sensitivity to neutralization by their elicited antibodies. We aimed to systematically review the most recent in vitro studies evaluating the effectiveness of BNT162b2 and mRNA‐1273 induced neutralizing antibodies against SARS‐CoV‐2 variants of concern. We searched PubMed, Scopus, and Web of Science in addition to bioRxiv and medRxiv with terms including ‘SARS‐CoV‐2’, ‘BNT162b2’, ‘mRNA‐1273’, and ‘neutralizing antibody’ up to June 29, 2021. A modified version of the Consolidated Standards of Reporting Trials (CONSORT) checklist was used for assessing included study quality. A total 36 in vitro studies meeting the eligibility criteria were included in this systematic review. B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and B.1.617.2 (Delta) are four SARS‐CoV‐2 variants that have recently been identified as variants of concern. Included studies implemented different methods regarding pseudovirus or live virus neutralization assays for measuring neutralization titres against utilized viruses. After two dose vaccination by BNT162b2 or mRNA‐1273, the B.1.351 variant had the least sensitivity to neutralizing antibodies, while B.1.1.7 variant had the most sensitivity; that is, it was better neutralized relative to the comparator strain. P.1 and B.1.617.2 variants had an intermediate level of impaired naturalization activity of antibodies elicited by prior vaccination. Our review suggests that immune sera derived from vaccinated individuals might show reduced protection of individuals immunized with mRNA vaccines against more recent SARS‐CoV‐2 variants of concern.
Keywords: BNT162b2, mRNA‐1273, neutralizing antibody, SARS‐CoV‐2, variants of concern
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- CONSORT
Consolidated Standards of Reporting Trials
- COVID‐19
coronavirus disease 2019
- GISAID
Global Influenza Surveillance and Response System
- NTD
N‐terminal domain
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta‐Analyses
- PROSPERO
Prospective Register of Systematic Reviews
- RBD
receptor binding domain
- S1
spike 1
- S2
spike 2
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), caused the global pandemic of coronavirus disease 2019 (COVID‐19), which infected more than 181 million and killed 3.9 million people across the world as of June 29, 2021. 1 Since the emergence of COVID‐19, several drastic public health measures such as draconian lockdowns have been imposed to contain the virus spread and end the pandemic. Understandably, substantial focus has been given to the rapid manufacture and distribution of vaccines that would enable herd immunity for some countries as early as the second half of 2021. This is exemplified by early authorization for emergency use of Pfizer‐BioNtech (BNT162b2) 2 and Moderna (mRNA‐1273) 3 vaccines, knowing to be the first mRNA based platform vaccines rolled out on a global scale.
SARS‐CoV‐2 is a positive‐stranded RNA virus. The spike protein of the virus consists of two fragments: The spike 1 (S1) subunit, consisting of N‐terminal domain (NTD) and receptor binding domain (RBD), is responsible for viral attachment to the host cell through angiotensin‐converting enzyme 2 (ACE2), whereas the spike 2 (S2) subunit completes membrane fusion. 4 Because the spike protein is an important mechanism of viral cell entry, it has been a potential target for vaccine development. 5
Due to the great numbers of viral genome replications that occur in infected individuals and the error‐prone nature of RNA dependent RNA polymerase, 6 progressive accrual mutations do and will continue to occur. Despite ineffectiveness of most mutations to viral fitness, a few may provide beneficial features that could give the virus an opportunity to transmit more efficiently and evade host immune response. 7 As a result, efficient mutations could be the subject of natural selection and lead to emergence of novel SARS‐CoV‐2 variants that are able to expand rapidly across countries and overcome public health efforts to restrict the infection. However, as vaccines currently in circulation have been designed based on the spike sequence of the ancestral SARS‐CoV‐2 strain, outbreaks of novel variants could be a potential threat for compromising immunogenicity of these vaccines. 8 , 9 , 10
In recent months, several mutations have appeared in the spike protein, leading to identification of novel variants with several substitutions or deletions in the spike protein. The variants, which have potential to increase transmissibility, virulence, or evade available diagnostics, vaccines, and therapeutics, have been denoted as variants of concern. According to the World Health Organization (WHO), these variants which named Alpha (Lineage B.1.1.7), Beta (Lineage B.1.351), Gamma (Lineage P.1), and Delta (Lineage B.1.617.2) were first emerged in the United Kingdom, South Africa, Brazil, and India, respectively, where they have rapidly become dominant and are currently spreading across the globe (Figure S1 and S2). 11
Serum neutralization activity is a common predictor of protection against SARS‐CoV‐2 following natural infection or vaccination. 12 , 13 Due to variations observed in the spike genome, effective protection from SARS‐CoV‐2 infection requires a sufficient breadth of neutralizing antibodies rather than potency alone. 14 Preliminary studies have shown that mutant viruses increase the affinity of binding to host cell receptors and diminish the susceptibility of neutralization by pre‐existing antibodies raised through either prior infection or vaccination. 15 , 16 , 17 , 18
In the present study, effectiveness of neutralizing antibodies elicited by two doses of mRNA based vaccine platforms, including BNT162b2 and mRNA‐1273, was systematically evaluated according to variants of concern using data from in vitro studies.
2. METHODS
The guideline of Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) statement was followed for review reporting. 19 As the evaluation was on in vitro studies, the pre‐specified protocol could not be published on the International Prospective Register of Systematic Reviews (PROSPERO). However, the protocol was pre‐specified and no alterations to the proposed evaluation methods (including design and outcomes) occurred literature retrieval.
2.1. Search strategy
Three online databases (PubMed, Scopus and Web of Science) and two preprint servers (bioRxiv and medRxiv) were screened for relevant records up to June 29, 2021. Search terms included ‘SARS‐CoV‐2’, ‘COVID‐19’, ‘B.1.1.7’, ‘B.1.351’, ‘P.1’, ‘B.1.617.2’, ‘BNT162b2’, ‘mRNA‐1273’, and ‘neutralizing antibody’. No search filters were applied to any fields, including study type, publication date, or language. Details on the search strategy for each database are represented in Table S1. Several journals were screened directly from the associated portal, including the New England Journal of Medicine, Science, Nature, and Cell journals, while grey literature included manual screening of results from the first 100 pages of the Google Scholar search engine as well as forward and backward citation searching of reference lists from included studies to find further eligible publications.
2.2. Study selection
Search results were exported to EndNote X8.0 (Clarivate Analytics, Philadelphia, PA, USA) reference manager software. Following removal of duplicates, titles and abstracts from remaining articles were screened against inclusion and exclusion criteria by two independent review authors. Studies requiring full‐text review were again screened by two independent review authors, with discrepancies resolved through consensus, and where required a third author as arbiter. We contacted the corresponding authors for retrieving full‐text of articles that we could not access to their full‐texts.
Studies meeting the following criteria were included: (1) In vitro studies comparing 50% neutralization titre against SARS‐CoV‐2 variants of concern and a reference strain for samples obtained from vaccine recipients, (2) Studies reporting fold change of neutralization titre or displayed it in high resolution images, (3) Studies recruiting samples from individuals who received two doses of 30 μg BNT162b2 vaccine or 100 μg mRNA‐1273 vaccine, (4) Studies utilizing viruses with at least one set of mutations as reported by WHO for each variant of concern, 20 and (5) Studies reporting the exact mutation in the spike protein of utilized viruses or reporting the strain name and Global Influenza Surveillance and Response System (GISAID; https://www.gisaid.org/hcov19‐mutation‐dashboard/)‐ID. Exclusion criteria were as follows: (1) Samples were collected from convalescent individuals who were not vaccinated, (2) The vaccine type was not clearly determined, (3) Recruited individuals received only one dose of vaccine, (4) Studies were conducted on samples from vaccinated mice or non‐human primates, (5) Neutralization titre was measured for viruses with only a specific subset of mutations, and (6) Study types other than in vitro (e.g., clinical trials, animal studies, and systematic reviews).
2.3. Data extraction
Two independent reviewers extracted the following characteristics from identified studies using standardized templates: first author's name, title, publication date, country of origin, sample size, gender and age of vaccine recipients, type of vaccine, history of previous SARS‐CoV‐2 infection, and days passed from the second vaccine dose that samples were obtained. Variant of concern type and its spike protein mutation profiles, type of reference virus, stain name or GISAID‐ID for variants and reference viruses were also extracted. Where the mutation profile of spike protein was not reported, an additional source, that is the GISAID webpage were was searched. 21 Laboratory methods, including type of neutralization assay and source of the utilized viruses, fold changes in 50% neutralization titre, and relevant p‐values were also recorded. If fold changes were not reported, neutralization titres were digitized from figures in papers with a digital extraction tool. 22 Disagreements between review authors were resolved by discussion and consensus, or where required consultation with a third reviewer.
2.4. Risk of bias assessment
Given that no established guidelines currently exist for quality assessment of in vitro studies, two independent review authors used a modified version of the Consolidated Standards of Reporting Trials (CONSORT) tool, which was developed to appraise the quality of studies in dentistry. 23 Discrepancies were resolved through discussion and consultation with a third reviewer. The checklist contains 15 items enabling assessment of methodological quality for included studies, taking into account evidence presented in the abstract, introduction, methods, results, discussion, and other information sections. Each item is answered as a Yes (1 point) or a No (0 point) based on reporting the relevant information. Therefore, a maximum of 15 points and a minimum of 0 point could be assigned to each study for evaluating quality.
2.5. Data synthesis
Data analysis was performed using qualitative methodology with narrative synthesis. Included studies were categorized based on the variant of interest and type of vaccine. Tables summarizing outcome measures and related findings from each type of vaccine were made. Meta‐analysis was not possible due to substantial differences across investigation methods, which was a pre‐specified decision, therefore, no test for assessment of publication bias was performed.
3. RESULTS
The systematic search identified 782 records of which 120 were duplicates and excluded. Following title and abstract screening of the remaining 662 records, 69 full text publications were screened for eligibility. Eighteen studies evaluated the effects of single mutations, 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 six studies evaluated the effects of other variants which were not variants of concern, 42 , 43 , 44 , 45 , 46 , 47 three were review articles, 48 , 49 , 50 two studies were re‐analysis of previously published articles, 51 , 52 two studies did not differentiate type of vaccines, 53 , 54 two studies were conducted on animal subjects, 55 , 56 and one did not mention the exact mutations of viruses. 57 Finally, 36 publications met the inclusion criteria and were included in this systematic review (Figure 1).
FIGURE 1.
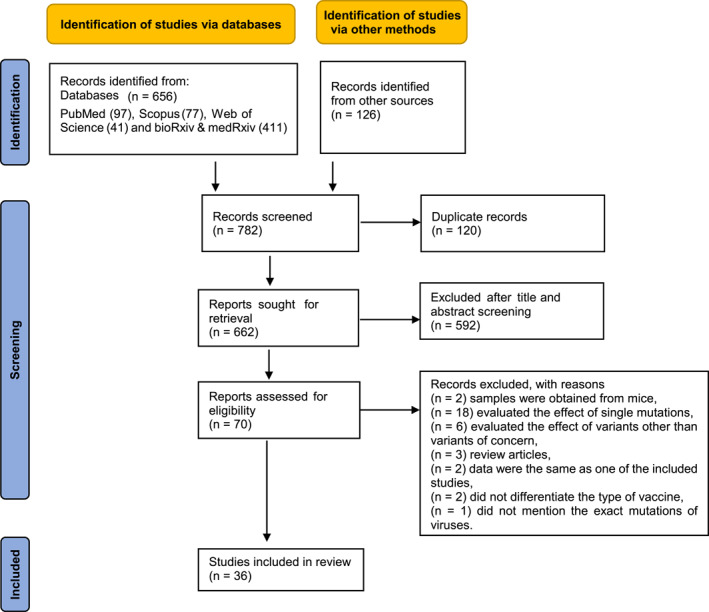
Study selection process
3.1. Study characteristics
3.1.1. B.1.1.7 variant
BNT162b2 vaccine
Twenty‐two studies 15 , 16 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 with a total of 968 samples evaluated the effect of B.1.1.7 variant on antibody neutralization activity elicited by BNT162b2 vaccine. Samples were obtained at least seven days and up to 91 days after the second dose of vaccine. The reference strain was a virus with D614G mutation in 10 studies, 60 , 61 , 62 , 66 , 67 , 68 , 70 , 72 , 73 , 77 a virus with no functional mutation in 11 studies, 15 , 16 , 58 , 59 , 63 , 64 , 65 , 69 , 71 , 74 , 75 and both types of viruses were used as comparator strains in one study. 76 Fourteen studies utilized live virus neutralization assays, 15 , 16 , 58 , 59 , 61 , 63 , 64 , 66 , 68 , 70 , 72 , 73 , 75 , 77 seven used pseudovirus neutralization assays, 60 , 62 , 65 , 67 , 69 , 71 , 74 and one used both types of assays. 76 Fifty percent neutralization titre was decreased as little a s2.6 fold or increased up to 3.8 fold in studies utilizing live virus neutralization assays, and decreased as little as 6.7 fold or increased up to 1.69 fold in studies utilizing pseudovirus neutralization assays, as compared with reference strain (Table 1).
TABLE 1.
Summary of included studies evaluating the neutralization activity of samples against B.1.1.7 variant obtained from vaccine recipients
| Vaccine type | Serological assay | Sample size | Days post second vaccine dose | Reference strain | Fold change in 50% neutralization titre | p‐value of change | History of previous SARS‐CoV‐2 infection | Age | Sex (number of female) | Country | Study ID |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BNT162b2 | Live virus neutralization test (FRNT) | 51 | 14–15 days | WT | −2.6 | <0.0001 | NA | 50 (21–82) | 28 | USA | Bates et al. 2021 b 59 |
| Live virus neutralization test (FRNT) | 24 | 7 days | D614G | −2.03 | <0.0001 | NA | 45 (26–64) | 15 | USA | Chen et al. 2021 61 | |
| Live virus neutralization test (FRNT) | 25 | 7–17 days | WT | −3.3 | <0.0001 | No | 43 (25–63) | 14 | UK | Dejnirattisai et al. 2021 63 | |
| Live virus neutralization test (PRNT) | 20 | 14–28 days | WT | 1.25 | 0.02 | No | 51 (23–69) | 14 | USA | Liu et al. 2021 15 | |
| Live virus neutralization test (PRNT) | 20 | 14–28 days | WT | 1.15 | 0.04 | No | 51 (23–69) | 14 | USA | Liu et al. 2021 a 16 | |
| Live virus neutralization test (PRNT) | 13 | 21–28 days | D614G | 2.17 | <0.0001 | Yes | 42 (34–56) | 10 | Netherlands | Geers et al. 2021 66 | |
| Live virus neutralization test (PRNT) | 12 | 21–28 days | D614G | 1.99 | <0.01 | No | 47 (34–55) | 11 | Netherlands | Geers et al. 2021 66 | |
| Live virus neutralization test (MN) | 159 | 21–37 days | WT | −2.6 | NA | No | 43 | 99 | UK | Wall et al. 2021 75 | |
| Live virus neutralization test (MN) | 25 | 7–17 days | WT | −2.4 | <0.0001 | No | NA | NA | UK | Skelly et al. 2021 b 64 | |
| Live virus neutralization test (MN) | 180 | 13–33 days | D614G | 1 | NS | NA | 43 (20–65) | 149 | Finland | Jalkanen et al. 2021 b 68 | |
| Live virus neutralization test (MN) | 60 | 30 days | WT | −1.1 | NS | No | 45 (25–65) | 38 | Italy | Anichini et al. 2021 58 | |
| Live virus neutralization test (microplate neutralization assay) | 10 | 7 days | WT | 1.3 | NS | NA | 42 (29–64) | 5 | USA | Wang et al. 2021 76 | |
| Live virus neutralization test (S‐Fuse neutralization assay) | 10 | 7 days | D614G | 1.45 | NS | NA | NA | NA | France | Planas et al. 2021 72 | |
| Live virus neutralization test (S‐Fuse neutralization assay) | 15 | 21 days | D614G | 3.8 | <0.05 | NA | NA | NA | France | Planas et al. 2021 72 | |
| Live virus neutralization test (S‐Fuse neutralization assay) | 16 | 35 days | D614G | 1.87 | NS | No | 60 (37–75) | 5 | France | Planas et al. 2021 b 73 | |
| Live virus neutralization test (S‐Fuse neutralization assay) | 13 | 91 days | D614G | 1.31 | NS | No | NA | NA | France | Planas et al. 2021 b 73 | |
| Live virus neutralization test (whole virus replication assay) | 29 | 7 days | D614G | −2.5 | <0.0001 | No | 55 (38–65) | 20 | France | Marot et al. 2021 b 70 | |
| Live virus neutralization test (cytopathic effect [CPE]‐based assay) | 37 | 10–20 days | D614G | 2.27 | <0.0001 | No | 46 (23–67) | 21 | Italy | Zani et al. 2021 77 | |
| Lentivirus‐vector pseudovirus neutralization test | 50 | 28 days | D614G | −1.6 | 0.0035 | No | >60–<35 | 31 | Netherlands | Caniels et al. 2021 b 60 | |
| Lentivirus‐vector pseudovirus neutralization test | 21 | 21 days | D614G | −1.9 | <0.001 | NA | NA | NA | UK | Collier et al. 2021 62 | |
| Lentivirus‐vector pseudovirus neutralization test | 21 | 21 days | D614G | −6.7 | <0.0001 | NA | NA | NA | UK | Collier et al. 2021 a 62 | |
| Lentivirus‐vector pseudovirus neutralization test | 30 | 7–32 days | WT | −2.1 | NS | No (except one suspected case) | 35 (26–66) | 18 | USA | Garcia‐Beltran et al. 2021 65 | |
| Lentivirus‐vector pseudovirus neutralization test | 16 | 14 days | WT | 1.69 | NS | Yes | 39 (29–44) | 11 | Spain | Trinité et al. 2021 b 74 | |
| Lentivirus‐vector pseudovirus neutralization test | 16 | 14 days | WT | −2.38 | NS | No | 45 (30–61) | 12 | Spain | Trinité et al. 2021 b 74 | |
| VSV‐vector pseudovirus neutralization test | 40 | 7–21 days | WT | −1.25 | <0.01 | No | 23–73 | NA | USA | Muik et al. 2021 71 | |
| VSV‐vector pseudovirus neutralization test | 10 | 7 days | D614G | −2 | 0.004 | NA | 42 (29–64) | 5 | USA | Wang et al. 2021 76 | |
| VSV‐vector pseudovirus neutralization test | 15 | 13–15 days | D614G | −1.77 | <0.01 | NA | 42 (25–65) | 4 | Germany | Hoffmann et al. 2021 67 | |
| VSV‐vector pseudovirus neutralization test | 30 | 22–68 days | WT | −2.2 | <0.0001 | NA | 36 (21–73) | 19 | USA | Liu et al. 2021 b 69 | |
| mRNA‐1273 | Live virus neutralization test (FRNT) | 24 | 14 days | D614G | −1.2 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 b 78 |
| Live virus neutralization test (FRNT) | 24 | 90 days | D614G | −1.3 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 b 78 | |
| Live virus neutralization test (FRNT) | 24 | 180 days | D614G | −1.3 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 b 78 | |
| Live virus neutralization test (FRNT) | 14 | 14 days | D614G | 1.2 | 0.04 | No | 18–55 | 8 | USA | Edara et al. 2021 b 79 | |
| Live virus neutralization test (FRNT) | 14 | 14 days | WT | −1.77 | 0.02 | No | 18–55 | 8 | USA | Edara et al. 2021 80 | |
| Live virus neutralization test (microplate neutralization assay) | 12 | 15 days | WT | 1.6 | NS | No | 18–>70 | NA | USA | Wang et al. 2021 76 | |
| Lentivirus‐vector pseudovirus neutralization test | 35 | 7–177 days | WT | −2.3 | NS | No (except one suspected case) | 47.3 (25–67) | 25 | USA | Garcia‐Beltran et al. 2021 65 | |
| Lentivirus‐vector pseudovirus neutralization test | 24 | 14 days | D614G | −1.5 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 b 78 | |
| Lentivirus‐vector pseudovirus neutralization test | 24 | 90 days | D614G | −1.6 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 b 78 | |
| Lentivirus‐vector pseudovirus neutralization test | 24 | 180 days | D614G | −1.6 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 b 78 | |
| Lentivirus‐vector pseudovirus neutralization test | 29 | 28 days | D614G | −2 | <0.0001 | No | 18–>71 | 18 | USA | Shen et al. 2021 81 | |
| VSV‐vector pseudovirus neutralization test | 19 | 24–49 days | WT | −1.48 | NS | NA | 39 (20–65) | 12 | USA | Liu et al. 2021 b 69 | |
| VSV‐vector pseudovirus neutralization test | 12 | 15 days | D614G | −1.8 | 0.003 | No | 18–>70 | NA | USA | Wang et al. 2021 76 | |
| VSV‐vector pseudovirus neutralization test | 8 | 7 days | D614G | −1.2 | NS | No | NA | NA | USA | Wu et al. 2021 18 | |
| VSV‐vector pseudovirus neutralization test | 8 | 7 days | D614G | −3.1 | 0.008 | No | NA | NA | USA | Wu et al. 2021 a 18 |
Abbreviations: FRNT, focus reduction neutralization test; MN, microneutralization assay; NA, Not available; NS, Not significant; PRNT, plaque reduction neutralization test; VSV, vesicular stomatitis virus; WT, wild type (Wuhan strain).
The utilized B.1.1.7 mutant viruses had an additional E484K substitution.
Preprint article.
mRNA‐1273 vaccine
Eight studies 18 , 65 , 69 , 76 , 78 , 79 , 80 , 81 with a total of 295 samples evaluated the effect of B.1.1.7 variant on antibody neutralization activity elicited by mRNA‐1273 vaccine. Samples were obtained at least seven days and up to 180 days after second dose of vaccine. The reference strain was a virus with D614G mutation in four studies, 18 , 78 , 79 , 81 a virus with no functional mutation in three studies, 65 , 69 , 80 and both types of viruses were used as a comparator strain in one study. 76 Two studies utilized live virus neutralization assays, 79 , 80 while four used pseudovirus neutralization assays, 18 , 65 , 69 , 81 and two used both type of assays. 76 , 78 Fifty percent neutralization titre was decreased as little as 1.77 fold or increased up to 1.6 fold in studies utilizing live virus neutralization assays and it was decreased as little as 3.1 fold in studies utilizing pseudovirus neutralization assays, as compared with reference strain (Table 1).
3.1.2. B.1.351 variant
BNT162b2 vaccine
Twenty‐one studies 15 , 58 , 59 , 60 , 61 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 72 , 73 , 75 , 76 , 77 , 82 , 83 , 84 with a total of 891 samples evaluated the effect of B.1.351 variant on antibody neutralization activity elicited by the BNT162b2 vaccine. Samples were obtained at least seven days and up to 91 days after second dose of vaccine. The reference strain was a virus with D614G mutation in 12 studies, 60 , 61 , 66 , 67 , 68 , 70 , 72 , 73 , 77 , 82 , 83 , 84 a virus with no functional mutation in eight studies, 15 , 58 , 59 , 63 , 64 , 65 , 69 , 75 and both types of viruses were used as a comparator strain in one study. 76 Fourteen studies utilized live virus neutralization assays, 15 , 58 , 59 , 61 , 63 , 64 , 66 , 68 , 70 , 72 , 73 , 75 , 77 , 82 while six used pseudovirus neutralization assays, 60 , 65 , 67 , 69 , 83 , 84 and one used both types of assays. 76 Fifty percent neutralization titre was decreased up to 22.83 fold in studies utilizing live virus neutralization assays and up to 41.2 fold in studies utilizing pseudovirus neutralization assays, as compared with reference strains (Table 2).
TABLE 2.
Summary of included studies evaluating the neutralization activity of samples against B.1.351 variant obtained from vaccine recipients
| Vaccine type | Serological assay | Sample size | Days post second vaccine dose | Reference strain | Fold change in 50% neutralization titre | p‐value of change | History of previous SARS‐CoV‐2 infection | Age | Sex (number of female) | Country | Study ID |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BNT162b2 | Live virus neutralization test (FRNT) | 24 | 7 days | D614G | −10.2 | <0.0001 | NA | 45 (26–64) | 15 | USA | Chen et al. 2021 61 |
| Live virus neutralization test (FRNT) | 51 | 14–15 days | WT | −8.8 | <0.0001 | NA | 50 (21–82) | 28 | USA | Bates et al. 2021 b 59 | |
| Live virus neutralization test (FRNT) | 25 | 7–17 days | WT | −7.6 | <0.0001 | No | 43 (25–63) | 14 | UK | Dejnirattisai et al. 2021 a 63 | |
| Live virus neutralization test (PRNT) | 20 | 14–28 days | WT | −2.74 | <0.001 | No | 51 (23–69) | 14 | USA | Liu et al. 2021 15 | |
| Live virus neutralization test (PRNT) | 13 | 21–28 days | D614G | −3.34 | <0.0001 | Yes | 42 (34–56) | 10 | Netherlands | Geers et al. 2021 a 66 | |
| Live virus neutralization test (PRNT) | 12 | 21–28 days | D614G | −3.07 | NA | No | 48 (34–55) | 11 | Netherlands | Geers et al. 2021 a 66 | |
| Live virus neutralization test (MN) | 159 | 21–37 days | WT | −4.9 | NA | No | 43 | 99 | UK | Wall et al. 2021 a 75 | |
| Live virus neutralization test (MN) | 25 | 7–17 days | WT | −4.49 | 0.000001 | No | NA | NA | UK | Skelly et al. 2021 a b 64 | |
| Live virus neutralization test (MN) | 180 | 13–33 days | D614G | −4.57 | <0.0001 | NA | 43 (20–65) | 149 | Finland | Jalkanen et al. 2021 a b 68 | |
| Live virus neutralization test (MN) | 60 | 30 days | WT | −4.2 | <0.001 | No | 45 (25–65) | 38 | Italy | Anichini et al. 2021 a 58 | |
| Live virus neutralization test (microplate neutralization assay) | 10 | 7 days | WT | −10.3 | 0.002 | NA | 42 (29–64) | 5 | USA | Wang et al. 2021 76 | |
| Live virus neutralization test (S‐Fuse neutralization assay) | 10 | 7 days | D614G | −4.66 | <0.01 | NA | NA | NA | France | Planas et al. 2021 a 72 | |
| Live virus neutralization test (S‐Fuse neutralization assay) | 15 | 21 days | D614G | −14.23 | <0.05 | NA | NA | NA | France | Planas et al. 2021 a 72 | |
| Live virus neutralization test (S‐Fuse neutralization assay) | 16 | 35 days | D614G | −8.83 | <0.001 | No | 60 (37–75) | 5 | France | Planas et al. 2021 b 73 | |
| Live virus neutralization test (S‐Fuse neutralization assay) | 13 | 91 days | D614G | −22.83 | <0.0001 | No | NA | NA | France | Planas et al. 2021 b 73 | |
| Live virus neutralization test (virus neutralization test) | 7 | 12 days | D614G | −5.1 | NA | NA | NA | NA | Germany | Becker et al. 2021 a 82 | |
| Virus neutralization test (whole virus replication assay) | 29 | 7 days | D614G | −4.8 | <0.0001 | No | 55 (38–65) | 20 | France | Marot et al. 2021 a b 70 | |
| Live virus neutralization test (cytopathic effect [CPE]‐based assay) | 37 | 20–10 days | D614G | −1.73 | <0.0001 | No | 46 (23–67) | 21 | Italy | Zani et al. 2021 77 | |
| Lentivirus‐vector pseudovirus neutralization test | 50 | 28 days | D614G | −5.1 | <0.0001 | No | >60–<35 | 31 | Netherlands | Caniels et al. 2021 a b 60 | |
| Lentivirus‐vector pseudovirus neutralization test | 30 | 7–32 days | WT | −34.5 | <0.0001 | No (except one suspected case) | 35 (26–66) | 18 | USA | Garcia‐Beltran et al. 2021 65 | |
| Lentivirus‐vector pseudovirus neutralization test | 30 | 7–32 days | WT | −41.2 | <0.0001 | No (except one suspected case) | 35 (26–66) | 18 | USA | Garcia‐Beltran et al. 2021 a 65 | |
| Lentivirus‐vector pseudovirus neutralization test | 5 | 7 days | D614G | −3.1 | ≤0.01 | No | NA | NA | USA | Tada et al. 2021 a 83 | |
| VSV‐vector pseudovirus neutralization test | 10 | 7 days | D614G | −6.5 | 0.002 | NA | 42 (29–64) | 5 | USA | Wang et al. 2021 a 76 | |
| VSV‐vector pseudovirus neutralization test | 15 | 13–15 days | D614G | −7.85 | <0.05 | NA | 42 (25–65) | 4 | Germany | Hoffmann et al. 2021 67 | |
| VSV‐vector pseudovirus neutralization test | 15 | 24–31 days | D614G | −11.13 | <0.001 | NA | 43 (26–58) | 12 | Germany | Hoffmann et al. 2021 84 | |
| VSV‐vector pseudovirus neutralization test | 30 | 22–68 days | WT | −10.46 | <0.0001 | NA | 36 (21–73) | 19 | USA | Liu et al. 2021 a b 69 | |
| mRNA‐1273 | Live virus neutralization test (FRNT) | 24 | 14 days | D614G | −5 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 a b 78 |
| Live virus neutralization test (FRNT) | 24 | 90 days | D614G | −6.1 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 a b 78 | |
| Live virus neutralization test (FRNT) | 24 | 180 days | D614G | −4.3 | NA | No | 18‐>71 | NA | USA | Pegu et al. 2021 a b 78 | |
| Live virus neutralization test (FRNT) | 19 | 14 days | D614G | −3.8 | <0.0001 | No | >56 | NA | USA | Edara et al. 2021 a 85 | |
| Live virus neutralization test (microplate neutralization assay) | 12 | 15 days | WT | −12.4 | 0.002 | No | 18–>70 | NA | USA | Wang et al. 2021 76 | |
| Lentivirus‐vector pseudovirus neutralization test | 35 | 7‐177 days | WT | −27.7 | <0.0001 | No (except one suspected case) | 47 (25–67) | 25 | USA | Garcia‐Beltran et al. 2021 65 | |
| Lentivirus‐vector pseudovirus neutralization test | 35 | 7‐177 days | WT | −20.8 | <0.0001 | No (except one suspected case) | 35 (26–66) | 25 | USA | Garcia‐Beltran et al. 2021 a 65 | |
| Lentivirus‐vector pseudovirus neutralization test | 26 | 28 days | D614G | −9.7 | <0.001 | No | 18–>71 | 17 | USA | Shen et al. 2021 a 17 | |
| Lentivirus‐vector pseudovirus neutralization test | 24 | 14 days | D614G | −9.1 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 a b 78 | |
| Lentivirus‐vector pseudovirus neutralization test | 24 | 90 days | D614G | −9.7 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 a b 78 | |
| Lentivirus‐vector pseudovirus neutralization test | 24 | 180 days | D614G | −7 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 a b 78 | |
| VSV‐vector pseudovirus neutralization test | 12 | 15 days | D614G | −8.6 | 0.0005 | No | 18–>70 | NA | USA | Wang et al. 2021 a 76 | |
| VSV‐vector pseudovirus neutralization test | 8 | 7 days | D614G | −6.4 | 0.008 | No | NA | NA | USA | Wu et al. 2021 a 18 | |
| VSV‐vector pseudovirus neutralization test | 19 | 24–49 days | WT | −7.56 | <0.0001 | NA | 39 (20–65) | 12 | USA | Liu et al. 2021 a b 69 |
Abbreviations: FRNT, focus reduction neutralization test; MN, microneutralization assay; NA, not available; NS, not significant; PRNT, plaque reduction neutralization test; VSV, vesicular stomatitis virus; WT, wild type (Wuhan strain).
The utilized B.1.351 mutant viruses had an additional L18f substitution.
Preprint article.
mRNA‐1273 vaccine
Seven studies 17 , 18 , 65 , 69 , 76 , 78 , 85 with a total of 310 samples evaluated the effect of the B.1.351 variant on antibody neutralization activity elicited by the mRNA‐1273 vaccine. Samples were obtained at least seven days and up to 180 days after second dose of vaccine. The reference strain was a virus with D614G mutation in four studies, 17 , 18 , 78 , 85 a virus with no functional mutation in two studies, 65 , 69 and both types of viruses were used as comparator strains in one study. 76 One study utilized live virus neutralization assays, 85 while four used pseudovirus neutralization assays, 17 , 18 , 65 , 69 and two used both types of assays. 76 , 78 Fifty percent neutralization titre was decreased up to 12.4 fold in studies utilizing live virus neutralization assays and up to 27.7 fold in studies utilizing pseudovirus neutralization assays, as compared with reference strains (Table 2).
3.1.3. P.1 variant
BNT162b2 vaccine
Nine studies 15 , 58 , 60 , 61 , 63 , 65 , 67 , 77 , 86 with 272 samples evaluated the effect of the P.1 variant on antibody neutralization activity elicited by the BNT162b2 vaccine. Samples were obtained at least seven days and up to 32 days after the second dose of vaccine. The reference strain was a virus with D614G mutation in four studies, 60 , 61 , 67 , 77 a virus with no functional mutation in four studies, 15 , 58 , 63 , 65 and both types of viruses were used as comparator strains in one study. 86 Five studies utilized live virus neutralization assays, 15 , 58 , 61 , 63 , 77 while three used pseudovirus neutralization assays, 60 , 65 , 67 and one used both types of assays. 86 Fifty percent neutralization titre was decreased up to 2.99 fold in studies utilizing live virus neutralization assays and it up to 6.7 fold in studies utilizing pseudovirus neutralization assays, as compared with reference strain (Table 3).
TABLE 3.
Summary of included studies evaluating the neutralization activity of samples against P.1 variant obtained from vaccine recipients
| Vaccine type | Serological assay | Sample size | Days post second vaccine dose | Reference strain | Fold change in 50% neutralization titre | p‐value of change | History of previous SARS‐CoV‐2 infection | Age | Sex (number of female) | Country | Study ID |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BNT162b2 | Live virus neutralization test (FRNT) | 15 | 7 day | D614G | −2.23 | 0.002 | NA | NA | NA | USA | Chen et al. 2021 61 |
| Live virus neutralization test (FRNT) | 25 | 7–17 days | WT | −2.6 | <0.0001 | No | 43 (25–63) | 14 | UK | Dejnirattis et al. 2021 63 | |
| Live virus neutralization test (PRNT) | 20 | 14–28 days | WT | −2.99 | NS | No | 51 (23–69) | 14 | USA | Liu et al. 2021 15 | |
| Live virus neutralization test (MN) | 60 | 30 days | WT | −1.2 | 0.03 | No | 45 (25–65) | 38 | Italy | Anichini et al. 2021 58 | |
| Live virus neutralization test (microplate neutralization assay) | 10 | 7 days | WT | −3.8 | 0.002 | NA | 42 (29–64) | 5 | USA | Wang et al. 2021 86 | |
| Live virus neutralization test (cytopathic effect [CPE]‐based assay) | 37 | 20–10 days | D614G | −1.38 | 0.0002 | No | 46 (23–67) | 21 | Italy | Zani et al. 2021 77 | |
| Lentivirus‐vector pseudovirus neutralization test | 50 | 28 days | D614G | −2 | <0.0001 | No | >60–<35 | 31 | Netherlands | Caniels et al. 2021 a 60 | |
| Lentivirus‐vector pseudovirus neutralization test | 30 | 7–32 days | WT | −6.7 | <0.0001 | No (except one suspected case) | 35 (26–66) | 18 | USA | Garcia‐Beltran et al. 2021 65 | |
| VSV‐vector pseudovirus neutralization test | 15 | 13–15 days | D614G | −5.12 | <0.01 | NA | 42 (25–65) | 4 | Germany | Hoffmann et al. 2021 67 | |
| VSV‐vector pseudovirus neutralization test | 10 | 7 days | D614G | −2.2 | 0.002 | NA | 42 (29–64) | 5 | USA | Wang et al. 2021 86 | |
| mRNA‐1273 | Live virus neutralization test (microplate neutralization assay) | 12 | 15 days | WT | −4.8 | 0.0005 | No | 18–>70 | NA | USA | Wang et al. 2021 86 |
| VSV‐vector pseudovirus neutralization test | 8 | 7 days | D614G | −3.5 | 0.008 | No | NA | NA | USA | Wu et al. 2021 18 | |
| VSV‐vector pseudovirus neutralization test | 12 | 15 days | D614G | −2.8 | 0.0005 | No | 18–>70 | NA | USA | Wang et al. 2021 86 | |
| Lentivirus‐vector pseudovirus neutralization test | 24 | 14 days | D614G | −2.8 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 a 78 | |
| Lentivirus‐vector pseudovirus neutralization test | 35 | 7–117 days | WT | −4.5 | <0.001 | No (except one suspected case) | 47 (25–67) | 25 | USA | Garcia‐Beltran et al. 2021 65 | |
| Lentivirus‐vector pseudovirus neutralization test | 24 | 180 days | D614G | −3.8 | NA | No | 18–>71 | NA | USA | Pegu et al. 2021 a 78 |
Abbreviations: FRNT, focus reduction neutralization test; MN, microneutralization assay; NA, not available; NS, not significant; PRNT, plaque reduction neutralization test; VSV, vesicular stomatitis virus; WT, wild type (Wuhan strain).
Preprint article.
mRNA‐1273 vaccine
Four studies 18 , 65 , 78 , 86 with 115 samples evaluated the effect of P.1 variant on antibody neutralization activity elicited by mRNA‐1273 vaccine. Samples were obtained at least seven days and up to 180 days after second dose of vaccine. The reference strain was a virus with D614G mutation in one study, 18 a virus with no functional mutation in one study, 65 and both types of viruses were used as comparator strains in two studies. 78 , 86 Three studies utilized pseudovirus neutralization assays, 18 , 65 , 78 while one used both types of assays. 86 Fifty percent neutralization titre was decreased to 4.8 fold in a study utilizing live virus neutralization assays and up to 4.5 fold in studies utilizing pseudovirus neutralization assays, as compared with reference strain (Table 3).
3.1.4. B.1.617.2 variant
Five studies 73 , 75 , 87 , 88 , 89 with 275 samples evaluated the effect of the B.1.617.2 variant on antibody neutralization activity elicited by BNT162b2 vaccine. Samples were obtained at least seven days and up to 91 days after the second dose of vaccine. The reference strain was a virus with D614G mutation in two studies 73 , 89 and a virus with no functional mutation in three studies. 75 , 87 , 88 All of the included studies utilized live virus neutralization assays except one study that used both pseudovirus and live virus assays. 89 Fifty percent neutralization titre was decreased up to 8.4 fold as compared with the reference strain (Table 4). No investigation assessed the effect of B.1.617.2 variant on neutralization activity of antibodies elicited by the mRNA‐1273 vaccine.
TABLE 4.
Summary of included studies evaluating the neutralization activity of samples against B.1.617.2 variant obtained from BNT162b2 vaccine recipients
| Serological assay | Sample size | Days post second vaccine dose | Reference strain | Fold change in 50% neutralization titre | p‐value of change | History of previous SARS‐CoV‐2 infection | Age | Sex (number of female) | Country | Study ID |
|---|---|---|---|---|---|---|---|---|---|---|
| Live virus neutralization test (MN) | 159 | 21–37 days | WT | −5.8 | NA | No | 43 | 99 | UK | Wall et al. 2021 a 75 |
| Live virus neutralization test (S‐Fuse neutralization assay) | 16 | 35 days | D614G | −1.54 | NS | No | 60 (37–75) | 5 | France | Planas et al. 2021 c 73 |
| Live virus neutralization test (S‐Fuse neutralization assay) | 13 | 91 days | D614G | −2.36 | <0.05 | No | NA | NA | France | Planas et al. 2021 c 73 |
| Live virus neutralization test (PRNT) | 20 | 14–28 days | WT | −1.46 | 0.004 | No | 51 (23–69) | 14 | USA | Liu et al. 2021 88 |
| Live virus neutralization test (FRNT) | 25 | 7–17 days | WT | −2.5 | <0.0001 | No | 43 (25–63) | 14 | UK | Liu et al. 2021 b 89 |
| Live virus neutralization test | 10 | 24–29 days | D614G | −8.4 | <0.01 | NA | NA | NA | UK | Mlcochova et al. 2021 c 90 |
| Lentivirus‐vector pseudovirus neutralization test | 32 | 24–29 days | D614G | −2.9 | <0.0001 | NA | 71 (46–83) | 13 | UK | Mlcochova et al. 2021 c 90 |
Abbreviations: FRNT, focus reduction neutralization test; MN, microneutralization assay; NA, not available; NS, not significant; PRNT, plaque reduction neutralization test; VSV, vesicular stomatitis virus; WT, wild type (Wuhan strain).
The utilized B.1.617.2 mutant virus had additional K77R and A222V substitution.
The utilized B.1.617.2 mutant virus had additional A222V substitution.
Preprint article.
3.2. Quality assessment
Quality of included studies ranged from 4 to 9, using the modified version of the CONSORT checklist (Table S2). The average score was 7.8 points, reflecting 52% of the possible total score of 15. Potential sources of bias were primarily attributed to issues concerning sample size estimation and randomization methods, including sequence generation, allocation concealment mechanism, implementation, and blinding. In addition, a registered pre‐specified protocol was not provided for any of the included studies. Furthermore, limitations were discussed in only half of the eligible studies. Figure 2 illustrates a summary of the modified CONSORT checklist per item.
FIGURE 2.
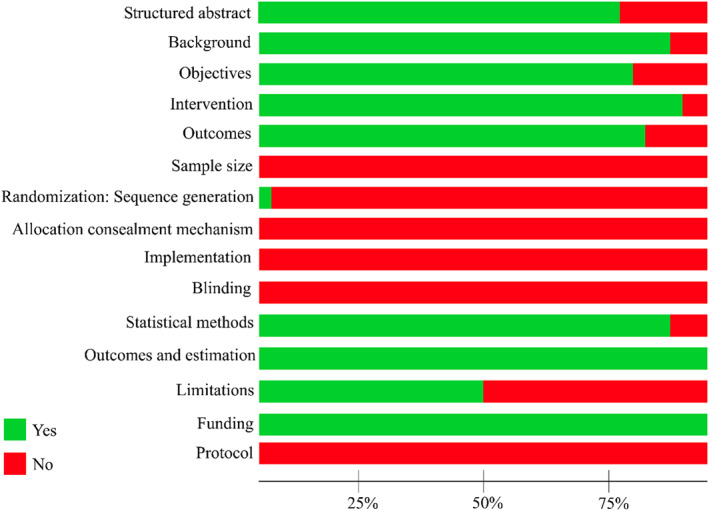
Percentage of modified Consolidated Standards of Reporting Trials (CONSORT) scores for included studies per item
4. DISCUSSION
This systematic review found that the B.1.351 variant had the most reduced sensitivity against antibody neutralization induced by vaccination with BNT162b2 or mRNA‐1273, while the B.1.1.7 variant had the least, and P.1 and B.1.617.2 variants had an intermediate phenotype, using either live virus or pseudovirus neutralization assays. In line with a recent review, the emergence of ongoing SARS‐CoV‐2 variants may potentially compromise current monoclonal antibodies and vaccine effectiveness. 90 These findings are of great importance since the immune escape of SARS‐CoV‐2 variants could confer an unpredictable threat to the whole world vaccination program, which could increase the risk of infection with mutant viruses, particularly later post decline of antibody titres.
4.1. RBD mutations as potential threat against vaccine efficacy
Vaccination is a key component of a long lasting strategy to bring the COVID‐19 pandemic under control. Pfizer‐BioNTech and Moderna vaccines are two lipid nanoparticled‐mRNA encoding perfusion stabilized forms of the full‐length SARS‐CoV‐2 spike protein, with more than 94% efficacy at preventing disease. 13 , 92 How previously vaccinated individuals with either BNT162b2 or mRNA‐1273 have responded to novel SARS‐CoV‐2 variants has been the subject of intense scrutiny over recent months. All of the variants of concern have mutations in the RBD region, which is the main target for neutralizing antibodies, 93 , 94 resulting in ineffectiveness of immune protection provided by natural infection or vaccination. Given that the RBD has functional plasticity, 94 , 95 ongoing mutations in this region would be feasible as the pandemic continues to evolve potentially compromising efficacy of current vaccines. The impact of changes in neutralization titres is challenging to predict, since it remains difficult to estimate precisely to what extent the reduction in neutralizing antibodies will affect vaccine efficacy leading to increase the risk of breakthrough infections or higher COVID‐19 severity in vaccinated populations.
4.2. Mutations in variants of concern that are responsible for escaping from neutralizing antibodies
Mutations that occur in the RBD region of spike protein are of the greatest concern due to their potential to promote escape from the vaccine induced neutralizing antibody response, which predominantly targets this region. N501Y substitution is shared among the RBD region of B.1.1.7, B.1.351, and P.1 spike genome. Although this mutation has been suggested to enhance the ACE2 binding affinity, 95 it has no pronounced effect on neutralizing activity of sera from vaccinated individuals. 31 , 39 , 76 , 79 , 97 , 98 While both B.1.351 and P.1 have mutations at 417 residue, studies have reported that this mutation has no potential impact on reduced sensitivity to neutralizing antibodies. 36 , 61 It appears that E484K substitution in B.1.351 and P.1 variants likely plays a crucial role in reducing the susceptibility of being neutralized by sera from vaccinated individuals. The E484 residue importantly is critical for binding of highly potent neutralizing antibodies. 98 This significant effect on serum neutralization can be elucidated by the dominance of RBD neutralizing antibodies, corroborated by studies indicating reduced neutralization titres mediated by the E484K mutation alone. 27 , 36 , 39 , 41 , 76 , 98 , 100 , 101 In addition, the original B.1.1.7 variant did not have E484K substitution in RBD, but there have been some reports that it recently poses E484 101 and it vitro studies have shown that while B.1.1.7 has minimal impact on serum neutralization, B.1.1.7 mutations alongside E484K could significantly reduce efficacy of neutralizing antibodies. 18 , 62 Therefore, E484K mutation located in the RBD would become a serious threat to the protection efficacy of mRNA‐based SARS‐CoV‐2 vaccines.
It is worth noting that the RBD of spike has the major impact on responding to neutralizing antibodies, however, despite the same encoded mutations in RBD of P.1 and B.1.351 (E484K, K417N/T and N501Y), neutralization of the P.1 variant is not compromised as severely as neutralization of B.1.351 when using vaccine sera immunized by earlier SARS‐CoV‐2 variants. This could be presumably justified by other distinct sets of mutations or deletions, particularly those introduced in NTD of viral spike. 103 , 104 , 105
Despite the great number of investigations on the efficacy of current vaccines for B.1.1.7, B.1.351, and P.1 variants, there is a lack of studies for the B.1.617.2 variant, which has recently been identified as variant of concern by WHO. B.1.617.2 harbours two mutations at 452 and 478 residues. In vitro experiments have demonstrated that L452R could compromise the neutralization titres, 106 , 107 while there were no studies evaluating the impact of the T478K mutation.
4.3. An urgent need for standardization of methodology of in vitro studies
In recent decades, several high throughput methods have been developed for quantification of neutralizing antibodies and the COVID‐19 pandemic has provided a beneficial opportunity for expediting research on upgrading neutralization assays. 107 It is noteworthy that included studies in this present review used different methods, including pseudovirus assays or live virus assays for testing neutralization activity of antibodies, that make comparability and therefore reliability of results challenging. Using authentic viruses or pseudovirus particles may have different impacts on neutralization due to the additional mutations outside of the spike region or differences in the density of spike protein per virion, which may alter sensitivity to neutralizing antibodies. However, recent studies have reported a high degree of concordance between pseudovirus and live virus neutralization assays, evaluating antibody response to SARS‐CoV‐2. 109 , 110 , 111 , 112 Furthermore, D614G mutation is one of the earliest substitutions that emerged and rapidly became globally dominant. Thereafter, in vitro studies aiming to make a comparison on neutralization activity of sera between an emerging variant and a reference strain utilized either virus bearing the D614G mutation or Wuhan strain with no functional mutations as comparator strain. It has been revealed that viruses with mutation in 614 residue may be neutralized better than the Wuhan strain by sera from vaccinated individuals. 18 , 37 , 113 Consequently, the reduction fold of neutralization titres against a certain variant may be potentially dependent on the comparator strain. Taken together, despite the questionable scientific relevance of distinct methods, there is an urgent need for standardization of neutralization assays and methods that in vitro studies utilize for comparing vaccine elicited immune effectors between different viruses to avoid diverse interpretations of final results.
Moreover, the status of individuals who received vaccines for previous SARS‐CoV‐2 infection was not clear in several included studies, which may impact the potency of antibodies to neutralize emerging variants. It has been demonstrated that sera from individuals who had recovered from SARS‐CoV‐2 infection prior to vaccination could not only neutralize B.1.351 more effectively than those who had been SARS‐CoV‐2 naïve, but there were also no significant reductions in neutralization titres against B.1.351 as compared to wild type strain after two dose vaccination. 53
4.4. Cellular immunity as another predictor of protection against novel variants
While the effect of humoural immunity on protection against mutant viruses for mRNA vaccine platforms has been extensively investigated, there is a need to study how the cellular immunity could contribute to protecting vaccinated individuals against novel variants. However, recent studies have suggested that T cell responses raised to early SARS‐CoV‐2 strains might be minimally impacted by emerging variants. 66 , 114 , 115
4.5. Real‐world effectiveness of mRNA vaccines over the expansion of variants of concern
During the second and third wave of SARS‐COV‐2 infection in Qatar, the B.1.1.7 and B.1.351 variants became dominant and nearly all of the infected cases were caused by these two variants. At the same time period, several individuals have been vaccinated with at least one dose of BNT162b2. Comparing the infection rate between vaccinated and unvaccinated individuals, two dose vaccination was effective at 89.5% and 75.0% in reducing the risk of infection by B.1.1.7 and B.1.351, respectively. Interestingly, the vaccine reaches 100% effectiveness in preventing severe, critical, or fatal disease for both variants. 115 Another study evaluating the effectiveness of BNT162b2 vaccine against B.1.617.2 variant reported that while two dose vaccination was effective at 93.4% in preventing B.1.1.7 infection, it reduces minimally to 87.9% against B.1.617.2 variant. 116 Therefore, confirming the results of in vitro investigation, the B.1.351 would be responsible for greatest rate of breakthrough infections.
4.6. Potent neutralization of variants of concern by single dose vaccination in convalescent individuals
For previously SARS‐CoV‐2 infected cases, a single dose of vaccines may act as booster dose following natural infection. Indeed, single dose vaccination post infection achieves similar levels of neutralizing antibodies to two doses in naïve vaccinated cases and second dose vaccination following the first dose in previously infected individuals offers no additional enhancement. 118 , 119 , 120 , 121 It has been reported that SARS‐CoV‐2 naïve immune cases were not capable of making detectable neutralizing antibody response against B.1.1.7 and B.1.351 variants after single dose vaccination. In contrast, vaccinated post infection individuals showed a strong neutralizing antibody response against B.1.1.7 and B.1.351 variants after single dose vaccination. 53 , 54 , 122 Consequently, the robust boosting of neutralizing antibodies in these subjects after one dose may have implications in settings where supply is limited.
4.7. Development of next generation vaccines with spike sequence of emerging variants
Given the ongoing emergence of SARS‐CoV‐2 variants, designing next generation vaccines as booster doses with diverse mutated spike sequences appears to be the only countermeasure strategy for combating the pandemic. Indeed, multiple vaccine companies have announced that they already initiated working on reformulating current vaccines with variants of concern. In this regard, Moderna has recently started the evaluation of booster dose containing the B.1.351 spike sequence in a phase 1 clinical trial. 122 However, efficacy of reformulated vaccines incorporating new viral strains may be challenging for individuals with pre‐existing immunity to ancestral strains. Whether the modified areas in spike protein will be capable of eliciting the unique antibody response, rather than boosting memory response against early strains is yet to be determined. 123 Therefore, intensive studies are needed to examine efficacy of booster doses for novel SARS‐CoV‐2 variants. Furthermore, based on our review, B.1.351 is the variant of greatest concern since it results in the largest reduction in neutralization titres, thus, despite the individuals who have already been vaccinated against SARS‐CoV‐2 globally, new variants such as B.1.351 may lead to a significant reinfection risk and it would be reasonable that developing booster vaccines constructs to B.1.351 should be prioritized.
4.8. Overview and future outlook
The emergence of variants of concern highlights the beginning of viral antigenic drift, which may be going in a direction that eventually leads to escape from our current prophylactic interventions against the spike protein. It is therefore imperative to closely monitor the emergence of novel SARS‐CoV‐2 variants and functional impacts that their mutations may have on vaccine efficacy. While viral sequence surveillance for detecting novel mutations in the SARS‐CoV‐2 genome has been established in several countries, global coverage is still insufficient. Consequently, suppression of viral replication by multiplying mitigation measures and expediting vaccine deployment is critical in reducing the risk of a new generation of SARS‐CoV‐2 variants. Finally, worldwide research on development of a general SARS‐CoV‐2 vaccine and challenges that mutations pose on vaccine program development, would offer a unique period in human history that can be studied and used to outline strategies for future infectious disease outbreaks.
5. LIMITATIONS
Our study relies on in vitro investigations, therefore, reduction in neutralization titres of antibodies against novel variants may not be generalizable to in vivo environments. In addition, the time period for collecting specimens following the second vaccination dose ranged from a few days to one month across studies, therefore, it is possible that antibody titres would have decreased over time to levels no longer able to provide adequate protection against mutant viruses. For instance, it was shown that almost half of vaccinated individuals with mRNA‐1273 were unable to neutralize the B.1.351 variant after three months. 78 Thus, long‐term evaluation of neutralizing antibody titres in vaccinated individuals is required to assess durability of protection against emerging SARS‐CoV‐2 variants. Although serum neutralizing antibody titre is a potent predictor of protection, mRNA vaccines could also elicit other immune effectors such as CD4+ and CD8+ T cells, complement deposition, and non‐neutralizing antibodies 124 that induce antibody‐dependent cytotoxicity. As a result, mechanisms other than neutralization by antibodies could confer further substantial vaccine‐mediated protection necessitating the need for further investigations. Considering the methodology of present systematic review, we cannot rule out the probability of missing some studies that our searching process might have failed to find. Additionally, there is no approved guideline for apprising the quality of in vitro studies, so we used the modified version of CONSORT checklist that have not been evaluated for its measurement properties. Finally, there is potential for publication and reporting bias, which has not been quantified as part of this review. Despite these limitations, to the best of our knowledge, this is the first article systematically reviewing the effectiveness of current mRNA vaccines against novel SARS‐CoV‐2 variants by eliciting neutralizing antibodies.
6. CONCLUSION
The recent emergence of multiple SARS‐CoV‐2 variants has disrupted confidence in the current generation of vaccines to provide adequate protection against COVID‐19. Our review suggests that immune sera derived from vaccinated individuals might fail to protect people immunized by mRNA vaccines against more recent SARS‐CoV‐2 variants of concern; mutations present in B.1.351 were found to have the most impact on impairing antibody naturalization activity, with B.1.1.7 showing minimal impact, and P.1 and B.1.617.2 showing an intermediate effect. With the emergence of ongoing mutations, it is likely that SARS‐CoV‐2 vaccines will require updating in the near future, with immunity monitored to compensate for viral evolution.
CONFLICT OF INTEREST
The authors declare no competing interests.
ETHICS STATEMENT
No ethical approval required for this article.
AUTHORS CONTRIBUTION
Maryam Noori and Seyed Aria Nejadghaderi designed and wrote the first draft of the manuscript; Shahnam Arshi, Kristin Carson‐Chahhoud, Khalil Ansarin, Ali‐Asghar Kolahi and Saeid Safiri critically revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the support of the National Institutes for Medical Research Development (NIMAD), Tabriz University of Medical Sciences and Shahid Beheshti University of Medical Sciences. The present study was funded by NIMAD and Tabriz University of Medical Sciences (grant number: 68009). In addition, the present study was partially supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant number: 28935). Registration and protocol: Since in vitro studies were the focus of this review, the pre‐specified protocol could not be published on the International Prospective Register of Systematic Reviews (PROSPERO).
Noori M, Nejadghaderi SA, Arshi S, et al. Potency of BNT162b2 and mRNA‐1273 vaccine‐induced neutralizing antibodies against severe acute respiratory syndrome‐CoV‐2 variants of concern: a systematic review of in vitro studies. Rev Med Virol. 2022;32(2):e2277. 10.1002/rmv.2277
Contributor Information
Shahnam Arshi, Email: s.arshi@sbmu.ac.ir.
Saeid Safiri, Email: safiris@tbzmed.ac.ir.
DATA AVAILABILITY STATEMENT
Data supporting findings from this study are available from the corresponding author upon reasonable request.
References
- 1. WHO Coronavirus (COVID‐19) Dashboard. https://covid19.who.int/ [Google Scholar]
- 2. EUA AT, Agnihothram S. Emergency Use Authorization (EUA) for an unapproved product review memorandum identifying information. [Google Scholar]
- 3. WHO lists Moderna vaccine for emergency use. https://www.who.int/news/item/30‐04‐2021‐who‐lists‐moderna‐vaccine‐for‐emergency‐use [Google Scholar]
- 4. Huang Y, Yang C, Xu X‐f, Xu W, Liu S‐w. Structural and functional properties of SARS‐CoV‐2 spike protein: potential antivirus drug development for COVID‐19. Acta Pharmacol Sin. 2020;41:1141‐1149. 10.1038/s41401-020-0485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Du L, He Y, Zhou Y, Liu S, Zheng B‐J, Jiang S. The spike protein of SARS‐CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denison MR, Graham RL, Donaldson EF, Eckerle LD, Baric RS. Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol. 2011;8:270‐279. 10.4161/rna.8.2.15013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volz E, Hill V, McCrone JT, et al. Evaluating the effects of SARS‐CoV‐2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2021;184:64‐75. e11. 10.1016/j.cell.2020.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore JP. Approaches for optimal use of different COVID‐19 vaccines: issues of viral variants and vaccine efficacy. J Am Med Assoc. 2021;325:1251‐1252. 10.1001/jama.2021.3465 [DOI] [PubMed] [Google Scholar]
- 9. Williams TC, Burgers WA. SARS‐CoV‐2 evolution and vaccines: cause for concern? Lancet Respir Med. 2021;9:333‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karim SSA. Vaccines and SARS‐CoV‐2 variants: the urgent need for a correlate of protection. Lancet. 2021;397:1263‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tracking SARS‐CoV‐2 variants. 2021. [Google Scholar]
- 12. McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS‐CoV‐2 in rhesus macaques. Nature. 2021;590:630‐634. 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N. Engl J Med. 2020;383:2603‐2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183‐186. 10.1126/science.1225416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2‐elicited serum. N. Engl J Med. 2021;384:1466‐1468. 10.1056/NEJMc2102017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Y, Liu J, Xia H, et al. BNT162b2‐Elicited neutralization against new SARS‐CoV‐2 spike variants [Online ahead of print] 2021. N. Engl J Med. 10.1056/NEJMc2106083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen X, Tang H, Pajon R, et al. Neutralization of SARS‐CoV‐2 variants B.1.429 and B.1.351. N. Engl J Med. 2021;384:2352‐2354. 10.1056/NEJMc2103740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu K, Werner AP, Koch M, et al. Serum neutralizing activity elicited by mRNA‐1273 vaccine. N. Engl J Med. 2021;384:1468‐1470. 10.1056/NEJMc2102179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . COVID‐19 weekly epidemiological update. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19‐‐‐11‐may‐2021 [Google Scholar]
- 21. Global influenza surveillance and response system (GISAID). https://www.gisaid.org/ [Google Scholar]
- 22. Get data graph digitizer version 2.26.0.20. http://getdata‐graph‐digitizer.com/ [Google Scholar]
- 23. Faggion CM, Jr . Guidelines for reporting pre‐clinical in vitro studies on dental materials. J Evid Base Dent Pract. 2012;12:182‐189. 10.1016/j.jebdp.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 24. Amanat F, Thapa M, Lei T, et al. The plasmablast response to SARS‐CoV‐2 mRNA vaccination is dominated by non‐neutralizing antibodies and targets both the NTD and the RBD. medRxiv. 2021. [Google Scholar]
- 25. Cherian S, Potdar V, Jadhav S, et al. Convergent evolution of SARS‐CoV‐2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID‐19 in Maharashtra, India. BioRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng X, Garcia‐Knight MA, Khalid MM, et al. Transmission, infectivity, and neutralization of a spike L452R SARS‐CoV‐2 variant. Cell. 2021;184(13):3426–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jangra S, Ye C, Rathnasinghe R, et al. SARS‐CoV‐2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe. 2021;2:e283‐e284. 10.1016/S2666-5247(21)00068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jangra S, Ye C, Rathnasinghe R, et al. The E484K mutation in the SARS‐CoV‐2 spike protein reduces but does not abolish neutralizing activity of human convalescent and post‐vaccination sera. MedRxiv. 2021. [Google Scholar]
- 29. Kuzmina A, Khalaila Y, Voloshin O, et al. SARS‐CoV‐2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post‐vaccination sera. Cell Host microbe. 2021;29:522‐528. e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuzmina A, Khalaila Y, Voloshin O, et al. SARS CoV‐2 escape variants exhibit differential infectivity and neutralization sensitivity to convalescent or post‐vaccination sera. Available at SSRN 3789258 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rathnasinghe R, Jangra S, Cupic A, et al. The N501Y mutation in SARS‐CoV‐2 spike leads to morbidity in obese and aged mice and is neutralized by convalescent and post‐vaccination human sera. medRxiv. 2021. 10.1101/2021.01.19.21249592 [DOI] [Google Scholar]
- 32. Rees‐Spear C, Muir L, Griffith SA, et al. The effect of spike mutations on SARS‐CoV‐2 neutralization. Cell Rep. 2021;34:108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sahin U, Muik A, Derhovanessian E, et al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH 1 T cell responses. Nature. 2020;586:594‐599. [DOI] [PubMed] [Google Scholar]
- 34. Shi P‐Y, Xie X, Zou J, et al. Neutralization of N501Y mutant SARS‐CoV‐2 by BNT162b2 vaccine‐elicited sera. bioRxiv. 2021. [Google Scholar]
- 35. Strengert M, Becker M, Ramos GM, et al. Cellular and humoral immunogenicity of a SARS‐CoV‐2 mRNA vaccine in patients on hemodialysis. medRxiv. 2021. 10.1101/2021.05.26.21257860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine‐elicited antibodies to SARS‐CoV‐2 and circulating variants. Nature. 2021;592:616‐622. 10.1038/s41586-021-03324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weissman D, Alameh MG, deSilva T, et al. D614G spike mutation increases SARS CoV‐2 susceptibility to neutralization. Cell Host Microbe. 2021;29:23‐31. e24. 10.1016/j.chom.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Widera M, Wilhelm A, Hoehl S, et al. Bamlanivimab does not neutralize two SARS‐CoV‐2 variants carrying E484K in vitro. medRxiv. 2021. 10.1101/2021.02.24.21252372 [DOI] [Google Scholar]
- 39. Xie X, Liu Y, Liu J, et al. Neutralization of SARS‐CoV‐2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine‐elicited sera. Nat Med. 2021;27:620‐621. 10.1038/s41591-021-01270-4 [DOI] [PubMed] [Google Scholar]
- 40. Zou J, Xie X, Fontes‐Garfias CR, et al. The effect of SARS‐CoV‐2 D614G mutation on BNT162b2 vaccine‐elicited neutralization. NPJ Vaccines. 2021;6:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chang X, Augusto GS, Liu X, et al. BNT162b2 mRNA COVID‐19 vaccine induces antibodies of broader cross‐reactivity than natural infection, but recognition of mutant viruses is up to 10‐fold reduced [Online ahead of print] 2021. Allergy. 10.1111/all.14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCallum M, Bassi J, DeMarco A, et al. SARS‐CoV‐2 immune evasion by the B.1.427/B.1.429 variant of concern [Online ahead of print]. Science. 10.1126/science.abi7994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tada T, Zhou H, Dcosta BM, Samanovic MI, Mulligan MJ, Landau NR. The spike proteins of SARS‐CoV‐2 B. 1.617 and B. 1.618 variants identified in India provide partial resistance to vaccine‐elicited and therapeutic monoclonal antibodies. BioRxiv. 2021. [Google Scholar]
- 44. West AP, Jr , Wertheim JO, Wang JC, et al. Detection and characterization of the SARS‐CoV‐2 lineage B. 1.526. bioRxiv; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou H, Dcosta BM, Samanovic MI, Mulligan MJ, Landau NR, Tada TB1. B.1.526 SARS‐CoV‐2 variants identified in New York City are neutralized by vaccine‐elicited and therapeutic monoclonal antibodies. bioRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bayarri‐Olmos R, Rosbjerg A, Johnsen LB, et al. The SARS‐CoV‐2 Y453F mink variant displays a pronounced increase in ACE‐2 affinity but does not challenge antibody neutralization. J Biol Chem. 2021;296:100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pei‐Yong S, Jianying L, Yang L, et al. Neutralization of SARS‐CoV‐2 variants B.1.617.1 and B.1.525 by BNT162b2‐elicited sera. Nature Portfolio. 2021. 10.21203/rs.3.rs-540721/v1 [DOI] [Google Scholar]
- 48. Bian L, Gao F, Zhang J, et al. Effects of SARS‐CoV‐2 variants on vaccine efficacy and response strategies. Expet Rev Vaccine. 2021;20(4):365‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Focosi D, Maggi F. Neutralising antibody escape of SARS‐CoV‐2 spike protein: risk assessment for antibody‐based Covid‐19 therapeutics and vaccines [Online ahead of print]. Rev Med Virol. 10.1002/rmv.2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abdool Karim SS, deOliveira T. New SARS‐CoV‐2 variants—clinical, public health, and vaccine implications. N. Engl J Med. 2021;384:1866‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS‐CoV‐2 variant B.1.351 from natural and vaccine‐induced sera. Cell. 2021;184:2348‐2361. e2346. 10.1016/j.cell.2021.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Supasa P, Zhou D, Dejnirattisai W, et al. Reduced neutralization of SARS‐CoV‐2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201‐2211. e2207. 10.1016/j.cell.2021.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS‐CoV‐2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6:6. 10.1126/sciimmunol.abi6950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stamatatos L, Czartoski J, Wan Y‐H, et al. mRNA vaccination boosts cross‐variant neutralizing antibodies elicited by SARS‐CoV‐2 infection. Science. 2021;372:eabg9175. 10.1126/science.abg9175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang Y, Zang J, Xu S, et al. Efficacy of ancestral receptor‐binding domain, S1 and trimeric spike protein vaccines against SARS‐CoV‐2 variants B. 1.1. 7, B. 1.351, and B.1.617. 1. bioRxiv. 2021. [Google Scholar]
- 56. Corbett KS, Edwards DK, Leist SR, et al. SARS‐CoV‐2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stankov MV, Cossmann A, Bonifacius A, et al. Humoral and cellular immune responses against SARS‐CoV‐2 variants and human coronaviruses after single BNT162b2 vaccination. medRxiv. 2021. 10.1101/2021.04.16.21255412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anichini G, Terrosi C, Gori Savellini G, Gandolfo C, Franchi F, Cusi MG. Neutralizing antibody response of vaccinees to SARS‐CoV‐2 variants. Vaccines. 2021;9:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bates TA, Leier HC, Lyski ZL, et al. Neutralization of SARS‐CoV‐2 variants by convalescent and vaccinated serum. medRxiv. 2021. 10.1101/2021.04.04.21254881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Caniels TG, Bontjer I, vander Straten K, et al. Emerging SARS‐CoV‐2 variants of concern evade humoral immune responses from infection and vaccination. medRxiv. 2021. 10.1101/2021.05.26.21257441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen RE, Zhang X, Case JB, et al. Resistance of SARS‐CoV‐2 variants to neutralization by monoclonal and serum‐derived polyclonal antibodies. Nat Med. 2021;27:717‐726. 10.1038/s41591-021-01294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Collier DA, De Marco A, Ferreira IATM, et al. Sensitivity of SARS‐CoV‐2 B.1.1.7 to mRNA vaccine‐elicited antibodies. Nature. 2021;593:136‐141. 10.1038/s41586-021-03412-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dejnirattisai W, Zhou D, Supasa P, et al. Antibody evasion by the P.1 strain of SARS‐CoV‐2. Cell. 2021;184:2939‐2954. e2939. 10.1016/j.cell.2021.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Donal TS, Adam CH, Javier G‐J, et al. Two doses of SARS‐CoV‐2 vaccination induce more robust immune responses to emerging SARS‐CoV‐2 variants of concern than does natural infection. Research Square. 2021. 10.21203/rs.3.rs-226857/v2 [DOI] [Google Scholar]
- 65. Garcia‐Beltran WF, Lam EC, Denis St.K, et al. Multiple SARS‐CoV‐2 variants escape neutralization by vaccine‐induced humoral immunity. Cell. 2021;184:2372‐2383. e2379. 10.1016/j.cell.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Geers D, Shamier MC, Bogers S, et al. SARS‐CoV‐2 variants of concern partially escape humoral but not T‐cell responses in COVID‐19 convalescent donors and vaccinees. Science Immunology. 2021;6:eabj1750. 10.1126/sciimmunol.abj1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hoffmann M, Arora P, Groß R, et al. SARS‐CoV‐2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184:2384‐2393. e2312. 10.1016/j.cell.2021.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jalkanen P, Kolehmainen P, Häkkinen H, et al. COVID‐19 mRNA vaccine induced antibody responses and neutralizing antibodies against three SARS‐CoV‐2 variants; 2021. [DOI] [PMC free article] [PubMed]
- 69. Liu J, Bodnar BH, Wang X, et al. Correlation of vaccine‐elicited antibody levels and neutralizing activities against SARS‐CoV‐2 and its variants. bioRxiv. 2021. 10.1101/2021.05.31.445871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marot S, Malet I, Leducq V, et al. Neutralization heterogeneity of United Kingdom and South‐African SARS‐CoV‐2 variants in BNT162b2‐vaccinated or convalescent COVID‐19 healthcare workers. Clin Infect Dis [Online ahead of print]. 10.1093/cid/ciab492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Muik A, Wallisch A‐K, Sänger B, et al. Neutralization of SARS‐CoV‐2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371:1152‐1153. 10.1126/science.abg6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Planas D, Bruel T, Grzelak L, et al. Sensitivity of infectious SARS‐CoV‐2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27:917‐924. 10.1038/s41591-021-01318-5 [DOI] [PubMed] [Google Scholar]
- 73. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of infectious SARS‐CoV‐2 variant B.1.617.2 to monoclonal antibodies and sera from convalescent and vaccinated individuals. bioRxiv. 2021. 10.1101/2021.05.26.445838 [DOI] [Google Scholar]
- 74. Trinité B, Pradenas E, Marfil S, et al. Previous SARS‐CoV‐2 Infection Increases B.1.1.7 Cross‐Neutralization by Vaccinated Individuals. Viruses. 2021;13(6):1–12. 10.1101/2021.03.05.433800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS‐CoV‐2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397(10292):2331–2333. 10.1016/S0140-6736(21)01290-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS‐CoV‐2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130‐135. 10.1038/s41586-021-03398-2 [DOI] [PubMed] [Google Scholar]
- 77. Zani A, Caccuri F, Messali S, Bonfanti C, Caruso A. Serosurvey in BNT162b2 vaccine‐elicited neutralizing antibodies against authentic B.1, B.1.1.7, B.1.351, B.1.525 and P.1 SARS‐CoV‐2 variants. Emerg Microb Infect. 2021;10:1‐1243. 10.1080/22221751.2021.1940305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pegu A, O'Connell S, Schmidt SD, et al. Durability of mRNA‐1273‐induced antibodies against SARS‐CoV‐2 variants. bioRxiv. 2021. 10.1101/2021.05.13.444010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Edara VV, Floyd K, Lai L, et al. Infection and mRNA‐1273 vaccine antibodies neutralize SARS‐CoV‐2 UK variant. medRxiv. 2021. 10.1101/2021.02.02.21250799 [DOI] [Google Scholar]
- 80. Edara VV, Hudson WH, Xie X, Ahmed R, Suthar MS. Neutralizing antibodies against SARS‐CoV‐2 variants after infection and vaccination. J Am Med Assoc. 2021;325:1896‐1898. 10.1001/jama.2021.4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shen X, Tang H, McDanal C, et al. SARS‐CoV‐2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29:529‐539. e523. 10.1016/j.chom.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Becker M, Dulovic A, Junker D, et al. Immune response to SARS‐CoV‐2 variants of concern in vaccinated individuals. Nat Commun. 2021;12:3109. 10.1038/s41467-021-23473-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tada T, Dcosta BM, Samanovic MI, et al. Convalescent‐phase sera and vaccine‐elicited antibodies largely maintain neutralizing titer against global SARS‐CoV‐2 variant spikes. Mbio. 2021;12:e00696‐00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hoffmann M, Hofmann‐Winkler H, Krüger N, et al. SARS‐CoV‐2 variant B.1.617 is resistant to Bamlanivimab and evades antibodies induced by infection and vaccination [Online ahead of print] 2021. Cell Rep. 109415. 10.1016/j.celrep.2021.109415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Edara VV, Norwood C, Floyd K, et al. Infection‐ and vaccine‐induced antibody binding and neutralization of the B.1.351 SARS‐CoV‐2 variant. Cell Host Microbe. 2021;29:516‐521. e513. 10.1016/j.chom.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang P, Casner RG, Nair MS, et al. Increased resistance of SARS‐CoV‐2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747‐751. e744. 10.1016/j.chom.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu J, Liu Y, Xia H, et al. BNT162b2‐elicited neutralization of B.1.617 and other SARS‐CoV‐2 variants [Online ahead of print] 2021. Nature. 10.1038/s41586-021-03693-y [DOI] [PubMed] [Google Scholar]
- 88. Liu C, Ginn HM, Dejnirattisai W, et al. Reduced neutralization of SARS‐CoV‐2 B.1.617 by vaccine and convalescent serum [Online ahead of print] 2021. Cell. 10.1016/j.cell.2021.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mlcochova P, Kemp SA, Shanker Dhar M, et al. SARS‐CoV‐2 B.1.617.2 Delta variant emergence and vaccine breakthrough. bioRxiv. 2021. 10.1101/2021.05.08.443253 [DOI] [Google Scholar]
- 90. Focosi D, Tuccori M, Baj A, Maggi F. SARS‐CoV‐2 variants: a synopsis of in vitro efficacy data of convalescent plasma, currently marketed vaccines, and monoclonal antibodies. Viruses. 2021;13(7):1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baden LR, ElSahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2020;384:403‐416. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cerutti G, Guo Y, Zhou T, et al. Potent SARS‐CoV‐2 neutralizing antibodies directed against spike N‐terminal domain target a single supersite. Cell Host Microbe. 2021;29:819‐833. e817. 10.1016/j.chom.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Piccoli L, Park Y‐J, Tortorici MA, et al. Mapping neutralizing and immunodominant sites on the SARS‐CoV‐2 spike receptor‐binding domain by structure‐guided high‐resolution serology. Cell. 2020;183:1024‐1042. e1021. 10.1016/j.cell.2020.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Greaney AJ, Starr TN, Gilchuk P, et al. Complete mapping of mutations to the SARS‐CoV‐2 spike receptor‐binding domain that escape antibody recognition. Cell Host Microbe. 2021;29:44‐57. e49. 10.1016/j.chom.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ali F, Kasry A, Amin M. The new SARS‐CoV‐2 strain shows a stronger binding affinity to ACE2 due to N501Y mutant. Medicine in Drug Discovery. 2021;10:100086. 10.1016/j.medidd.2021.100086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wu K, Werner AP, Moliva JI, et al. mRNA‐1273 vaccine induces neutralizing antibodies against spike mutants from global SARS‐CoV‐2 variants. bioRxiv. 2021. 10.1101/2021.01.25.427948 [DOI] [Google Scholar]
- 97. Kuzmina A, Khalaila Y, Voloshin O, et al. SARS‐CoV‐2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post‐vaccination sera. Cell Host Microbe. 2021;29(4):522‐528. 10.1016/j.chom.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barnes CO, Jette CA, Abernathy ME, et al. SARS‐CoV‐2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682‐687. 10.1038/s41586-020-2852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tada T, Dcosta BM, Samanovic‐Golden M, et al. Neutralization of viruses with European, South African, and United States SARS‐CoV‐2 variant spike proteins by convalescent sera and BNT162b2 mRNA vaccine‐elicited antibodies. bioRxiv. 2021. 10.1101/2021.02.05.430003 [DOI] [Google Scholar]
- 100. Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS‐CoV‐2 spike protein variants. Elife. 2020;9. e61312. 10.7554/eLife.61312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Public Health England . Public Health England. Investigation of novel SARS‐CoV‐2 variant: technical briefing 4. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/957504/Variant_of_Concern_VOC_202012_01_Technical_Briefing_5_England.pdf [Google Scholar]
- 102. Chi X, Yan R, Zhang J, et al. A neutralizing human antibody binds to the N‐terminal domain of the Spike protein of SARS‐CoV‐2. Science. 2020;369:650‐655. 10.1126/science.abc6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liu L, Wang P, Nair MS, et al. Potent neutralizing antibodies against multiple epitopes on SARS‐CoV‐2 spike. Nature. 2020;584:450‐456. 10.1038/s41586-020-2571-7 [DOI] [PubMed] [Google Scholar]
- 104. Brouwer PJM, Caniels TG, vander Straten K, et al. Potent neutralizing antibodies from COVID‐19 patients define multiple targets of vulnerability. Science. 2020;369:643‐650. 10.1126/science.abc5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ferreira I, Datir R, Kemp S, et al. SARS‐CoV‐2 B.1.617 emergence and sensitivity to vaccine‐elicited antibodies. bioRxiv. 2021. 10.1101/2021.05.08.443253 [DOI] [Google Scholar]
- 106. Tada T, Zhou H, Dcosta BM, Samanovic MI, Mulligan MJ, Landau NR. The spike proteins of SARS‐CoV‐2 B.1.617 and B.1.618 variants identified in India provide partial resistance to vaccine‐elicited and therapeutic monoclonal antibodies. bioRxiv. 2021. 10.1101/2021.05.14.444076 [DOI] [Google Scholar]
- 107. Focosi D, Maggi F, Mazzetti P, Pistello M. Viral infection neutralization tests: a focus on severe acute respiratory syndrome‐coronavirus‐2 with implications for convalescent plasma therapy. Rev Med Virol. 2021;31(2):e2170. 10.1002/rmv.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Schmidt F, Weisblum Y, Muecksch F, et al. Measuring SARS‐CoV‐2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020;217:217. 10.1084/jem.20201181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Noval MG, Kaczmarek ME, Koide A, et al. High titers of multiple antibody isotypes against the SARS‐CoV‐2 spike receptor‐binding domain and nucleoprotein associate with better neutralization. bioRxiv. 2020. 10.1101/2020.08.15.252353 [DOI] [Google Scholar]
- 110. Case JB, Rothlauf PW, Chen RE, et al. Neutralizing antibody and soluble ACE2 inhibition of a replication‐competent VSV‐SARS‐CoV‐2 and a clinical isolate of SARS‐CoV‐2. Cell Host Microbe. 2020;28:475‐485. e475. 10.1016/j.chom.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yang R, Huang B, A R, et al. Development and effectiveness of pseudotyped SARS‐CoV‐2 system as determined by neutralizing efficiency and entry inhibition test in vitro. Biosafety and Health. 2020;2:226‐231. 10.1016/j.bsheal.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lilleri D, Cassaniti I, Bergami F, et al. SARS‐CoV‐2 mRNA vaccine BNT162b2 elicited a robust humoral and cellular response against SARS‐CoV‐2 variants; 2021. [Google Scholar]
- 113. Woldemeskel BA, Garliss CC, Blankson JN. SARS‐CoV‐2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS‐CoV‐2 variants and HCoV‐NL63. J Clin Investig. 2021;131(10):e149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gallagher KME, Leick MB, Larson RC, et al. SARS ‐CoV‐2 T‐cell immunity to variants of concern following vaccination. bioRxiv. 2021. 10.1101/2021.05.03.442455 [DOI] [Google Scholar]
- 115. Abu‐Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 covid‐19 vaccine against the B.1.1.7 and B.1.351 variants. N. Engl J Med. 2021;385:187‐189. 10.1056/NEJMc2104974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bernal JL, Andrews N, Gower C, et al. Effectiveness of COVID‐19 vaccines against the B.1.617.2 variant. medRxiv. 2021. 10.1101/2021.05.22.21257658 [DOI] [PubMed] [Google Scholar]
- 117. Bradley T, Grundberg E, Selvarangan R, et al. Antibody responses after a single dose of SARS‐CoV‐2 mRNA vaccine. N. Engl J Med. 2021;384:1959‐1961. 10.1056/NEJMc2102051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med. 2021;384:1372‐1374. 10.1056/NEJMc2101667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mazzoni A, Di Lauria N, Maggi L, et al. First‐dose mRNA vaccination is sufficient to reactivate immunological memory to SARS‐CoV‐2 in subjects who have recovered from COVID‐19. J Clin Invest. 2021;131:131. 10.1172/jci149150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Focosi D, Baj A, Maggi F. Is a single COVID‐19 vaccine dose enough in convalescents? Hum Vaccin Immunother. 2021:1‐3. 10.1080/21645515.2021.1917238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Reynolds CJ, Pade C, Gibbons JM, et al. Prior SARS‐CoV‐2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372:1418‐1423. 10.1126/science.abh1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Safety and immunogenicity study of a SARS‐CoV‐2 (COVID‐19) variant vaccine (mRNA‐1273.351) in naïve and previously vaccinated adults. https://clinicaltrials.gov/ct2/show/NCT04785144 [Google Scholar]
- 123. Noori M, Nejadghaderi SA, Rezaei N. “Original antigenic sin”: a potential threat beyond the development of booster vaccination against novel SARS‐CoV‐2 variants [Online ahead of print] 2021. Infect Control Hosp Epidemiol. 1‐2. 10.1017/ice.2021.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Huang AT, Garcia‐Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. 10.1038/s41467-020-18450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data supporting findings from this study are available from the corresponding author upon reasonable request.


