Abstract
Background
Evidence recommends that vitamin D might be a crucial supportive agent for the immune system, mainly in cytokine response regulation against COVID‐19. Hence, we carried out a systematic review and meta‐analysis in order to maximise the use of everything that exists about the role of vitamin D in the COVID‐19.
Methods
A systematic search was performed in PubMed, Scopus, Embase and Web of Science up to December 18, 2020. Studies focused on the role of vitamin D in confirmed COVID‐19 patients were entered into the systematic review.
Results
Twenty‐three studies containing 11 901 participants entered into the meta‐analysis. The meta‐analysis indicated that 41% of COVID‐19 patients were suffering from vitamin D deficiency (95% CI, 29%‐55%), and in 42% of patients, levels of vitamin D were insufficient (95% CI, 24%‐63%). The serum 25‐hydroxyvitamin D concentration was 20.3 ng/mL among all COVID‐19 patients (95% CI, 12.1‐19.8). The odds of getting infected with SARS‐CoV‐2 are 3.3 times higher among individuals with vitamin D deficiency (95% CI, 2.5‐4.3). The chance of developing severe COVID‐19 is about five times higher in patients with vitamin D deficiency (OR: 5.1, 95% CI, 2.6‐10.3). There is no significant association between vitamin D status and higher mortality rates (OR: 1.6, 95% CI, 0.5‐4.4).
Conclusion
This study found that most of the COVID‐19 patients were suffering from vitamin D deficiency/insufficiency. Also, there is about three times higher chance of getting infected with SARS‐CoV‐2 among vitamin‐D‐deficient individuals and about five times higher probability of developing the severe disease in vitamin‐D‐deficient patients. Vitamin D deficiency showed no significant association with mortality rates in this population.
Review Criteria
Following database search, paper screening, data extraction and quality assessment were done based on inclusion and exclusion criteria by independent researchers.
Message for the Clinic
Our study demonstrated a significant association between vitamin D deficiency/insufficiency and SARS‐CoV‐2 infection, which can be helpful to consider in the clinical setting.
1. INTRODUCTION
Following the emergence of a novel coronavirus from Wuhan, China, in December 2019, the respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has affected the whole world and is declared a pandemic by World Health Organisation (WHO) on March 26, 2020. 1 According to Worldometer metrics, this novel virus has been responsible for approximately 83,848,186 infections, of which 59,355,654 cases are recovered, and 1,826,530 patients have died worldwide up to January 01, 2021.
After months of medical communities’ efforts, one of the hottest topics is still the role of Vitamin D in the prevention or treatment of COVID‐19. Several functions, such as modulating the adaptive immune system and cell‐mediated immunity, as well as an increase of antioxidative‐related genes expression, have been proven for Vitamin D as an adjuvant in the prevention and treatment of acute respiratory infections. 2 , 3 , 4 According to available investigations, it seems that such functions lead to cytokine storm suppression and avoid Acute Respiratory Distress Syndrome (ARDS), which has been studied on other pandemics and infectious diseases in recent years. 4 , 5 , 6 , 7
To the best of our knowledge, unfortunately, after several months, there is no adequate high‐quality data on different treatment regimens, which raise questions about gaps in scientific works. On this occasion, when there is an essential need for controlled randomised trials, it is surprising to see only observational studies without a control group or non‐randomised controlled studies with retrospective nature covering a small number of patients. The same issue is debatable for 25‐hydroxyvitamin D (25(OH)D); hence, concerning all of the limitations and analyse difficulties, we carried out a systematic review and meta‐analysis to try for maximising the use of everything that exists about the role of this vitamin in the COVID‐19.
2. METHODS
2.1. Search Strategy
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guideline was considered for the study plan. A systematic search through databases of PubMed, Scopus, Embase and Web of Science was done up to December 18, 2020. Moreover, to obtain more data, we considered grey literature and references of eligible papers. The search strategy included all MeSH terms and free keywords found for COVID‐19, SARS‐CoV‐2 and Vitamin D (Table S1). There was no time/location/language limitation in this search.
2.2. Criteria study selection
Four researchers have screened and selected the papers independently, and the supervisor solved the disagreements. Studies met the following criteria included in the meta‐analysis: 1) comparative or non‐comparative studies with retrospective or prospective nature; and 2) studies reported the role of vitamin D in confirmed COVID‐19 patients. Studies were excluded if they were: 1) in vitro studies, experimental studies, reviews, 2) duplicate publications.
2.3. Data extraction and quality assessment
Two researchers (H.J and M.M) have evaluated the papers’ quality assessment and extracted data from selected papers. The supervisor (D.Sh) resolved any disagreements in this step. The data extraction checklist included the name of the first author, publication year, region of study, number of patients, comorbidity, vitamin D Status, serum 25‐hydroxyvitamin D levels, ethnicity, mean age, medication dosage, treatment duration, adverse effects, radiological results and mortality. The Newcastle‐Ottawa Scale (NOS) checklist 8 and its modified version for cross‐sectional studies 9 and Jadad scale 10 for randomised clinical trials were used to value the studies concerning various aspects of the methodology and study process.
2.4. Vitamin D cut‐off 11
In this case, according to most of the studies, vitamin D cut‐off points were considered as follows:
Vitamin D sufficiency: 25(OH)D concentration greater than 30 ng/mL.
Vitamin D insufficiency: 25(OH)D concentration of 20‐30 ng/mL.
Vitamin D deficiency: 25(OH)D level less than 20 ng/mL.
2.5. Targeted outcomes
(a) Frequency of Vitamin D status in COVID‐19 patients; (b) Mean 25(OH)D concentration; (c) Association between Vitamin D Deficiency and SARS‐CoV‐2 infection; (d) Association between Vitamin D Deficiency and COVID‐19 severity; (e) Association between Vitamin D Deficiency and COVID‐19 mortality; (f) Comorbidity frequency; (g) Ethnicity frequency.
2.6. Heterogeneity assessment
I‐square (I 2) statistic was used for heterogeneity evaluation. Following Cochrane Handbook for Systematic Reviews of Interventions, 12 the I 2 was interpreted as follows: “0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity. The importance of the observed value of I2 depends on (i) magnitude and direction of effects and (ii) strength of evidence for heterogeneity (eg, P‐value from the chi‐squared test, or a confidence interval for I2).” Thus, the random‐effects model was used for pooling the outcomes in case of heterogeneity; otherwise, the inverse variance fixed‐effect model was used. Forest plots were presented to visualise the degree of variation between studies.
2.7. Data analysis
Meta‐analysis was performed using Comprehensive Meta‐Analysis (CMA) v. 2.2.064 software. The pooling of effect sizes was done with 95% Confident Interval (CI). The fixed/random‐effects model was used according to heterogeneities. In the case of zero frequency, the correction value of 0.1 was used.
2.8. Publication bias
Begg's and Egger's tests were used for publication bias evaluation. A P‐value of less than .05 was considered as statistically significant.
3. RESULTS
3.1. Study selection process
The first search through databases resulted in 1382 papers. After removing duplicated papers and first‐step screening based on title and abstract, 121 papers were assessed for eligibility. Finally, 23 articles were entered into the meta‐analysis. PRISMA flow diagram for the study selection process is presented in Figure 1.
FIGURE 1.
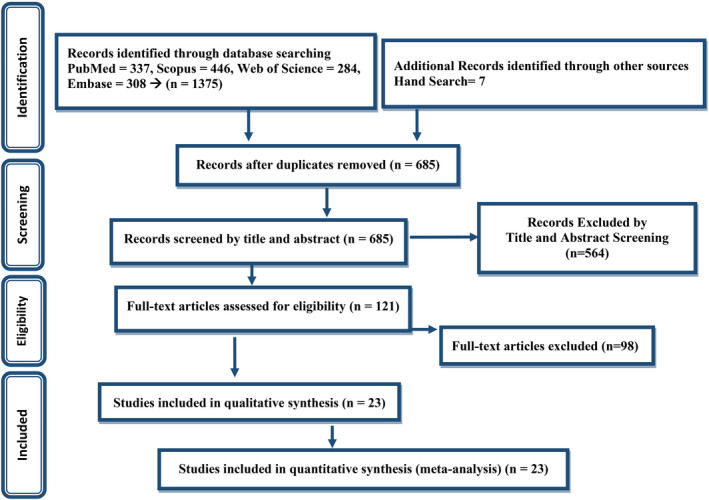
PRISMA flow diagram for the study selection process
3.2. Study characteristics
Among the 23 studies included in the meta‐analysis, all were designed in retrospective nature, except for five studies in prospective nature. The studies’ sample size ranged from 19 to 7807, including 11 901 participants. Characteristics of studies entered into the systematic review are presented in Table 1.
TABLE 1.
Characteristics of studies entered into the systematic review
| Study | Country | Study design | No. of patients (cases) (male/female) | Controls (male/female) |
Mean (±SD) Median (IQR) age of patients (cases) |
Comorbidity of patients (cases) | Vitamin D status of patients (cases) | Ethnicity of patients (cases) | Quality score b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | I | D | CS | AC | O | ||||||||
| Im et al 81 | South Korea | Case‐control study | 50 | 150 | 57.5 (34.5‐68.0) | — | 13 | — | 37 | — | — | — | 7/9 |
| Maghbooli et al 82 | Iran | Retrospective cross sectional | 235 | — | 58.72 (±15.2) *mean |
Diabetes: 86 Hypertension: 104 Respiratory disease: 72 Cancer: 2 |
77 | — | — | — | — | — | 7/10 |
| Baktash et al 83 | UK | Prospective cohort study | 70 (42/28) | — | ≥65 |
Hypertension: 34 Diabetes mellitus: 26 Ischaemic heart disease: 15 Chronic respiratory disease: 13 Heart failure: 12 Stroke: 9 Dementia: 6 CKD: 16 Atrial fibrillation: 14 Cancer: 3 Endocrinological disease: 3 |
31 | — | 39 | 50 | — | 20 | 9/10 |
| Meltzer et al 84 | US | Retrospective cohort study | 71 | — | — |
Hypertension:261 Diabetes:137 COPD:117 Pulmonary circulation disorders: 20 Depression: 119 CKD:116 Liver disease: 56 Comorbidities with immunosuppression: 105 |
39 | — | 32 | — | — | — | 9/10 |
| Faul et al 85 | Ireland | Retrospective cross sectional | 33 (33/0) | — | ≥40 | — | 21 | — | 12 | 33 | — | — | 5/10 |
| Merzon et al 86 | Israel | Case‐control study | 782 (385/397) | 7025 (2849, 4176) | 35.58 |
Depression/Anxiety: 73 Schizophrenia: 15 Dementia: 27 Diabetes mellitus: 154 Hypertension: 174 Cardiovascular disease: 78 Chronic lung disorders: 66 Obesity: 235 |
79 | 598 | 105 | — | — | — | 6/9 |
| Panagiotou et al 87 | UK | Retrospective cross sectional | 134 (73/61) | — | — |
Hypertension: 56 Diabetes: 38 Obesity: 14 Malignancy: 15 Respiratory: 42 Cardiovascular disease: 20 Kidney and Liver diseases: 19 |
— | — | 44 | 132 | 1 | 1 | 6/10 |
| Carpagnano et al 88 | Italy | Retrospective cohort study | 42 (30/12) | — | 65 (±13) *mean |
Hypertension: 26 Cardiovascular disease: 16 CKD: 16 Diabetes type II: 11 Cerebrovascular disease: 5 Psychosis, depression, anxiety: 10 Malignancy: 5 COPD: 5 Asthma: 2 |
8 | 11 | 23 | — | — | — | 8/9 |
| Nicola et al 89 | Italy | Retrospective cohort study | 112 (52/60) | — | 47.2 (±16.4) | — | — | — | — | — | — | — | 6/9 |
| Macaya et al 90 | Spain | Retrospective cohort study | 80 (35/45) | — | 67.65 (50‐84) |
Hypertension: 50 Diabetes mellitus: 32 Cardiac disease: 19 |
— | — | 45 | — | — | — | 7/9 |
| Karahan et al 91 | Turkey | Retrospective cohort study | 149 (81/68) | — | 63.5 (±15.3) |
Coronary artery disease: 32 Hypertension: 85 Dyslipidaemia: 39 Diabetes mellitus: 61 Cerebrovascular accident: 9 COPD: 15 Malignancy: 23 CKD: 29 Chronic atrial fibrillation: 15 Congestive heart failure: 18 Acute kidney injury: 16 |
12 | 34 | 103 | — | — | — | 8/9 |
| Abdollahi et al 92 | Iran | Case‐control study | 201 (66/135) | 201 (66/135) | 48 (±16.95) |
Hypothyroidism: 15 Diabetes mellitus: 42 Splenectomy: 1 Heart failure and hypertension: 20 Respiratory infections: 14 Autoimmune diseases: 11 AIDS: 4 |
39 | 161 | 1 | — | — | — | 7/9 |
| Arvinte et al 93 | US | Prospective cohort study (pilot study) | 21 (15/6) | — |
60.2 (±17.4) 61 (20‐94) |
— | — | — | — | 4 | — | 17 | 6/9 |
| Cereda et al 94 | Italy | Prospective cohort study | 129 (70/59) | — | 77 (65.0‐85.0) |
COPD: 16 Diabetes: 39 Hypertension: 89 Ischaemic heart disease: 52 Cancer: 27 CKD: 24 |
— | 30 a | 99 | — | — | — | 7/9 |
| Hamza et al 95 | Pakistan | Randomised controlled trial study | 168 (94/74) | — | 42.26 (±13.69) | — | 22 | 47 | 98 | — | — | — | 3/5 |
| Hernandez et al 96 | Spain | Case‐control study | 19 (7/12) | 197 (123/74) | 60.0 (59.0‐75.0) |
Hypertension: 12 Diabetes: 0 Cardiovascular disease: 3 COPD: 2 Active cancer: 0 Immunosuppression: 6 |
— | — | — | — | — | — | 7/9 |
| Jain et al 97 | India | Prospective cohort study | 154 (95/69) | — | 46.05 (±8.8) | — | — | — | 90 | — | — | — | 8/9 |
| Ling et al 98 | UK | Retrospective cohort study | 444 (245/199) | — | 74 (63‐83) |
Diabetes mellitus: 129 COPD: 100 Asthma: 52 IHD: 73 ACS: 48 Heart failure: 54 Hypertension: 197 TIA: 40 Dementia: 59 Obesity: 20 Malignancy of solid organ: 71 Malignancy of skin: 8 Haematological malignancy: 8 Solid organ transplant: 4 Inflammatory arthritis: 16 Inflammatory bowel disease: 5 |
63 | 80 | 87 | 386 | 5 | 53 | 8/9 |
| Luo et al 99 | China | Retrospective cross‐sectional study | 335 (148/187) | 560 (257/303) | 56.0 (43.0‐64.0) | Comorbidity status: 147 | — | — | 218 | — | — | — | 7/10 |
| Radujkovic et al 100 | Germany | Retrospective cohort study | 185 (95/90) | 93 (59/34) | 60 (49‐70) |
Cardiovascular disease: 58 Diabetes: 19 Chronic kidney disease: 8 Chronic lung disease: 15 Active or history of malignancy: 17 |
— | — | 41 | — | — | — | 7/9 |
| Vassiliou et al 101 | Greece | Retrospective cohort study | 39 (31/8) | — | 61.17 (±13) |
Hypertension: 18 COPD: 1 Hyperlipidaemia: 9 Diabetes: 6 CAD: 4 Asthma: 1 |
— | 7 | 32 | — | — | — | 6/9 |
| Ye et al 102 | China | Case‐control study | 62 (23/39) | 80 (32/48) | 43 (32‐59) |
Diabetes: 5 Hypertension; 6 Liver injury: 1 COPD: 1 Asthma: 0 Renal failure: 16 |
— | — | 26 | — | — | — | 6/9 |
| Karonova et al 103 | Russia | Retrospective cohort study | 80 (43/37) | — | 53.2 (±15.7) |
Obesity: 18 Ischaemic heart disease: 21 Diabetes: 12 |
7 | 16 | 57 | — | — | — | 6/9 |
Abbreviations: SD, standard deviation; IQR, interquartile range; US, United States; UK, United Kingdom; N, normal; I, insufficient; D, deficient; CS, Caucasian; AC, Afro‐Caribbean; O, other; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; AIDS, acquired immunodeficiency syndrome; ACS, acute coronary syndrome (Current or previous); TIA, transient ischaemic attack.
In the study defined as patients with 25(OH)Vitamin D > 20 ng/mL.
Quality assessment tools were mentioned and cited in the method section.
3.3. Quality assessment
Results of quality assessment for studies entered into meta‐analysis were fair.
3.4. Publication bias
The findings of Begg's and Egger's tests were as follows for publication bias in main analysis: frequency of vitamin D status (P B = .38; P E = .02); mean 25(OH)D concentration (P B = .80; P E = .76); vitamin D deficiency and SARS‐CoV‐2 infection (P B = 1.00; P E = .55); Vitamin D deficiency and COVID‐19 severity (P B = .12; P E = .14) and vitamin D deficiency and COVID‐19 mortality (P B = .54; P E = .62).
3.5. Meta‐analysis findings
3.5.1. Frequency of Vitamin D status in COVID‐19 patients
The meta‐analysis of event rates in peer‐reviewed papers showed that 41% of COVID‐19 patients were suffering from vitamin D deficiency (95% CI, 29%‐55%), in 42% of patients, levels of vitamin D were lower than the normal range (95% CI, 24%‐63%) and only 19% of patients had normal vitamin D levels (95% CI, 11%‐32%) (Figure 2).
FIGURE 2.
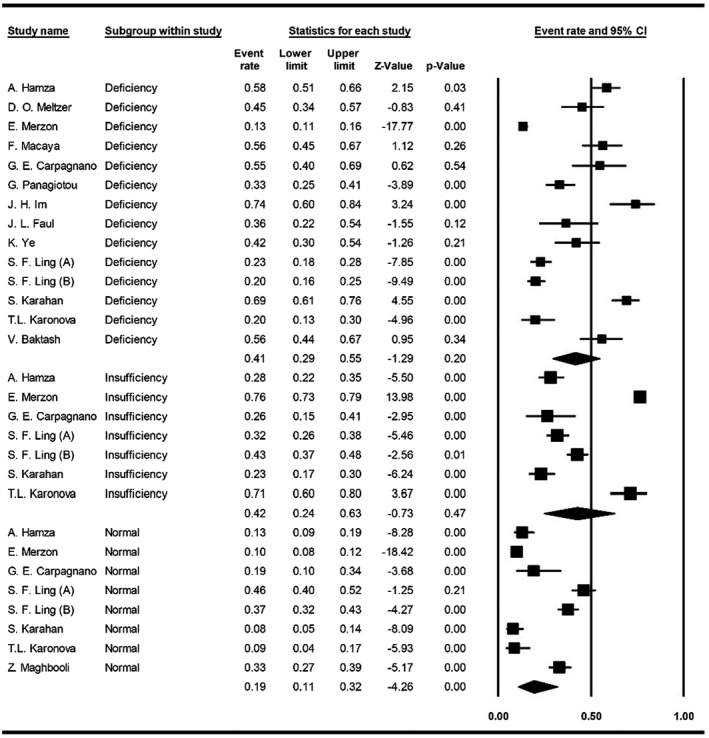
Forest plot for pooling events of vitamin D status
3.5.2. Mean serum 25‐hydroxyvitamin D concentration
The meta‐analysis of mean 25(OH)D concentration was 20.3 ng/mL among all COVID‐19 patients (95% CI, 11.5‐28.1), 16.0 ng/mL in severe cases (95% CI, 12.1‐19.8) and 24.5 ng/mL in non‐severe cases (95% CI, 20.0‐29.0) (Figure 3).
FIGURE 3.
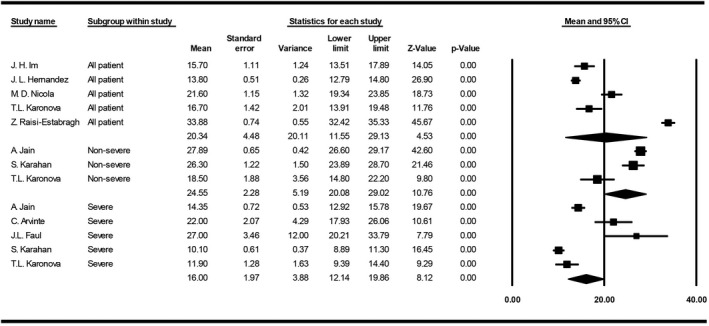
Forest plot for pooling mean 25(OH)D concentrations
3.5.3. Vitamin D Deficiency and SARS‐CoV‐2 infection
The meta‐analysis indicated that odds of getting infected with SARS‐CoV‐2 increase by 3.3 times in individuals with vitamin D deficiency (95% CI, 2.5‐4.3) (Figure 4).
FIGURE 4.
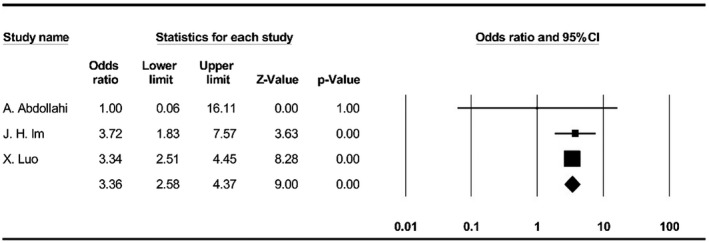
Forest plot for pooling odds ratios of vitamin D deficiency and SARS‐CoV‐2 infection
3.5.4. Vitamin D Deficiency and COVID‐19 severity
The meta‐analysis showed that the probability of developing severe stages of COVID‐19 is 5.1 times higher in patients with vitamin D deficiency (95% CI, 2.6‐10.3) (Figure 5).
FIGURE 5.
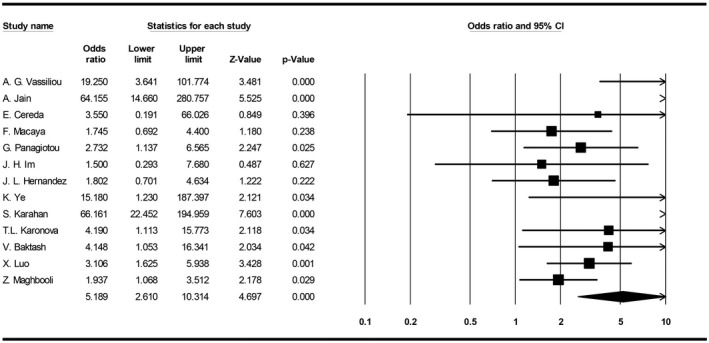
Forest plot for pooling odds ratios of vitamin D deficiency and COVID‐19 severity
3.5.5. Vitamin D Deficiency and COVID‐19 mortality
The meta‐analysis indicated no significant higher COVID‐19 mortality related to vitamin‐D‐deficient patients (OR: 1.6, 95% CI, 0.5‐4.4) (Figure 6).
FIGURE 6.
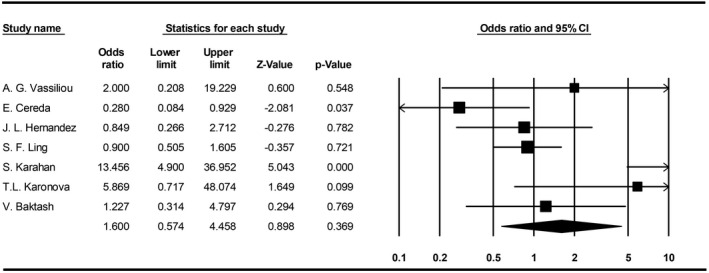
Forest plot for pooling odds ratios of vitamin D deficiency and COVID‐19 mortality
3.6. Comorbidities
Meta‐analysis of available data on comorbidities frequency in COVID‐19 patients was as follows: in non‐severe cases, 13% cancer, 12% chronic kidney disease (CKD), 18% cardiovascular diseases (CVD), 21% diabetes, 29% hypertension (HTN), 12% obesity and 13% respiratory diseases (Figure S1); in severe cases, 13% cancer, 34% CKD, 31% CVD, 35% diabetes, 64% HTN, 33% obesity and 17% respiratory diseases (Figure S2); in overall, 8% cancer, 20% CKD, 26% CVD, 5% dementia, 15% depression/anxiety, 22% obesity, 26% diabetes, 49% HTN and 15% respiratory diseases (Figure S3).
3.7. Ethnicity frequency
Pooling available data regarding ethnicity distribution among COVID‐19 patients resulted in 2% Afro‐Caribbean, 13% Asian and 87% Caucasian (Figure S4). The results for severe cases were as follows: 2% Asian, 68% Caucasian and 81% Hispanic (Figure S5).
4. DISCUSSION
4.1. Epidemiological and clinical aspects
Although comparing global statistics of COVID‐19 outcomes is difficult, it is clear that the mortality rate is higher in several countries. It seems that among various factors such as age, healthcare system quality, general health status, socioeconomic status, etc, one of the underestimated factors that might be associated with COVID‐19 outcome is the vitamin D status in every population. In recent years, vitamin D deficiency/insufficiency has become a global health issue, and its impact has been studied on respiratory viral infections. Most of the epidemiological studies have been reported a higher risk of developing the infection to the severe stages and death in patients with low levels of vitamin D. 13 , 14 , 15 , 16 Besides, vitamin D clinical interventions have demonstrated a significantly reduced risk of respiratory tract infection (RTI), further proposed as a prophylactic or treatment approach against RTIs by WHO in 2017. 17 , 18 , 19
Concerning all of the limitations and lack of high‐quality data about the relation of vitamin D status and COVID‐19 after several months, we have conducted this systematic review and meta‐analysis to maximise the use of every available data, which would give us an overview towards further studies like what we have done recently on the effectiveness of hydroxychloroquine in COVID‐19 patients, 20 which have underestimated first, but the value was revealed after a while.
According to available data entered into our meta‐analysis, we could find that approximately 43% of the patients infected with SARS‐CoV‐2 were suffering from vitamin D deficiency, and this vitamin was insufficient in about 42% of them. We have also found that mean 25(OH)D levels were low (~20 ng/mL) in all COVID‐19 patients. More importantly, our analysis showed that the chance of infecting with SARS‐CoV‐2 is about three times higher in individuals with vitamin D deficiency and the probability of developing the severe disease in such patients is about five times higher than others. However, vitamin D deficiency did not substantially affect mortality rates in such patients.
These findings are in the same line with studies that have debated the association of vitamin D and COVID‐19. 21 , 22 , 23 , 24 , 25 Recently, Kaufman et al 26 studied the relation of SARS‐CoV‐2 positivity rates with circulation 25(OH)D among 191,779 patients retrospectively. They found the highest SARS‐CoV‐2 positivity rate among patients with vitamin D deficiency (12.5%, 95% CI, 12.2%‐12.8%). Overall, the study indicated a significant inverse relation between SARS‐CoV‐2 positivity and circulating 25(OH)D levels in COVID‐19 patients.
Along with all observational studies, a pilot randomised clinical trial performed by Castillo et al 27 on 76 hospitalised COVID‐19 patients indicated a promising result for calcifediol therapy in these individuals. In this study, high‐dose oral calcifediol significantly reduced the need for intensive care unit (ICU) treatment. However, because of the small sample size, more extensive, well‐organised clinical trials are needed to robust and confirm this study's findings.
Additionally, in the case of vitamin D supplements’ benefits against acute respiratory tract infections, Martineau et al conducted a meta‐analysis of randomised controlled on 10.933 participants and resulted in an inverse association between vitamin D levels and risk of acute respiratory tract infections. Thus, it can be concluded that patients with lower vitamin D levels or patients with vitamin D deficiency are at higher risk of developing the disease to the severe form. 17
4.2. Comorbidities
After months of investigation on COVID‐19, several factors, such as male sex, older age, CVD, HTN, chronic lung disease, obesity and CKD, are proposed to be risk factors towards deteriorating COVID‐19 patients’ outcomes. 28 , 29 , 30 , 31 Interestingly, one of the conditions that lead to most of the considered risk factors is vitamin D deficiency. Studies indicated that malignancies, diabetes, HTN and CVDs are significantly related to vitamin D deficiency. Also, studies reported the important role of vitamin D deficiency in older males. 32 , 33 , 34 Evidence shows that ageing, physical activity, obesity, seasonal variation, less vitamin D absorption, pregnancy, thyroid disorders, prolonged use of corticosteroids and ethnicity/race can substantially affect the circulating 25(OH)D levels. 35 , 36 , 37 , 38 , 39 , 40 , 41
Hence, although studies reported vitamin D deficiency as one of the critical risk factors in clinical outcomes of COVID‐19 patients, it seems that it can also be in a strong relationship with basic underlying risk factors and diseases in such patients.
In this case, our analyses indicated that HTN, CVDs, CKDs, diabetes, obesity and respiratory diseases were the most frequent comorbidities in COVID‐19 patients. According to the facts mentioned above and our findings, it is plausible that both vitamin D deficiency and underlying diseases, which affect each other, may worsen the condition of these patients more than others.
4.3. Ethnicity
From the beginning of the COVID‐19 pandemic, different studies have been reported probable associations between COVID‐19 and the ethnicity of these patients. Most studies found that the mortality rate among black people is higher than the other ethnic groups. 42 , 43 , 44 , 45 , 46 However, other challenges, such as human resources, healthcare systems budgetary, poor management, etc, have to be considered among such people and low‐income countries, 47 , 48 , 49 which unavoidably affects the subject significantly. In recent years, many studies have focused on vitamin D mechanisms and status among various ethnic groups to find the roles of vitamin D and its relationships with any factors or disorders in various ethnicities. 50 , 51 , 52 , 53
Herein, our findings demonstrated that the most frequent ethnic group has belonged to Caucasians, followed by Hispanic, Asian and Afro‐Caribbean. Although there is some evidence on the role of genetic variants in COVID‐19 patients, the subject is still not clear enough. 54 , 55
In contrast to many studies about vitamin D status in different ethnicities, Aloia et al have reported that serum 25(OH)D concentration is the same in cross‐racial comparison. They found an inconsistency between monoclonal and polyclonal assays for detecting vitamin D‐binding protein. 56 Hence, the approach for considering serum 25(OH)D concentration is much important.
4.4. Vitamin D mechanisms and COVID‐19
Vitamin D metabolism has been well studied throughout history. Numerous investigations indicate vitamin D’s roles in reducing microbial infections through a physical barrier, natural immunity and adaptive immunity. 2 , 57 , 58 , 59 , 60 , 61 , 62 For example, investigations on respiratory infections indicated that 25(OH)D could effectively induce the host defence peptides against bacterial or viral agents. Vitamin D insufficiency/deficiency can lead to non‐communicable and infectious diseases. 2 , 63 , 64 The other potential role of vitamin D is reducing inflammatory induced following SARS‐CoV‐2 infection by suppressing inflammatory cytokines, reducing leukocytes’ infiltration, interaction with polymorphonuclear leukocytes and inhibiting complement component C3. 13 , 65 , 66 , 67 , 68 , 69 Also, according to the available evidence for infections and malignancies, 70 , 71 vitamin D may enhance the serological response and CD8+ T lymphocytes performance against COVID‐19 when the T cells’ exhaustion is related to the critical stages of the disease. 72 , 73 , 74
Besides, according to the revealed association of SARS‐CoV‐2 and angiotensin‐converting enzyme 2 (ACE2), this virus can substantially down‐regulate the ACE2 expression, which seems to lead the COVID‐19 patients to deterioration. 75 , 76 , 77 In contrast, vitamin D affects the renin‐angiotensin system pathway and promotes the expression of ACE2. 78 , 79 However, since the high expression of ACE2 can be a risk factor for the severity of the disease, 80 it is not yet clear enough to conclude how much vitamin D helps the condition. Hence, more evidence and trials are needed to design a treatment plan for three groups of mild, moderate and severe patients.
It is worth noticing that the current meta‐analysis includes the following limitations: (a) most of studies entered into the meta‐analysis were retrospective in nature; (b) There are inevitable challenges with the reliability of data due to different strategies in a testing (eg, vitamin D measurement, COVID‐19 test, etc), various subpopulations, etc; (c) other immunomodulatory factors (eg, vitamin C, zinc, selenium, etc), which might be influential in the outcome of COVID‐19 patients, have not considered in included studies and (d) type II statistical errors following studies with small sample size. Eventually, to overcome the limitations and bias, the study's results should be confirmed by robustly large multicentre randomised clinical trials.
5. CONCLUSION
The conditional evidence recommends that vitamin D might be a critical supportive agent for the immune system, mainly in cytokine response regulation against pathogens. In this systematic review and meta‐analysis, we found that mean serum 25(OH)D level was low (~20 ng/mL) in all COVID‐19 patients and most of them were suffering from vitamin D deficiency/insufficiency. Also, there is about three times higher chance of getting infected with SARS‐CoV‐2 among vitamin‐D‐deficient individuals and five times higher probability of developing the severe disease in such patients. Vitamin D deficiency showed no significant association with mortality rates in these population. The Caucasian was the dominant ethnic group, and the most frequent comorbidities in COVID‐19 patients were HTN, CVDs, CKDs, diabetes, obesity and respiratory diseases, which might be affected by vitamin D deficiency directly or indirectly. However, further large clinical trials following comprehensive meta‐analysis should be taken into account to achieve more reliable findings.
DISCLOSURES
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Fig S1
Table S1
ACKNOWLEDGEMENTS
We would like to express our appreciation to the Student Research Committee of Mazandaran University of Medical Sciences for approving this student research proposal with the code 7904. It is also remarkable that the manuscript was published on a pre‐print server (available at https://doi.org/10.1101/2020.06.05.20123554).
Ghasemian R, Shamshirian A, Heydari K, et al. The role of vitamin D in the age of COVID‐19: A systematic review and meta‐analysis. Int J Clin Pract. 2021;75:e14675. 10.1111/ijcp.14675
Contributor Information
Amir Shamshirian, Email: shamshirian.amir@gmail.com.
Danial Shamshirian, Email: shamshirian@sbmu.ac.ir.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Organization WH . Coronavirus Disease (COVID‐2019) Situation Reports. WHO; 2020. [Google Scholar]
- 2. Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240‐4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27:e1909. [DOI] [PubMed] [Google Scholar]
- 4. Mercola J, Grant WB, Wagner CL. Evidence regarding vitamin D and risk of COVID‐19 and its severity. Nutrients. 2020;12:3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aranow C. Vitamin D and the immune system. J Invest Med. 2011;59:881‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goncalves‐Mendes N, Talvas J, Dualé C, et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo‐controlled trial. Front Immunol. 2019;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grant WB, Giovannucci E. The possible roles of solar ultraviolet‐B radiation and vitamin D in reducing case‐fatality rates from the 1918–1919 influenza pandemic in the United States. Dermato‐endocrinology. 2009;1:215‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wells GA, Shea B, O’Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. In: Oxford; 2000.
- 9. Modesti PA, Reboldi G, Cappuccio FP, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta‐analysis. PLoS One. 2016;11:e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halpern SH, Douglas MJ, eds. Appendix: Jadad scale for reporting randomized controlled trials. Evidence‐based Obstetric Anesthesia. Blackwell Publishing Ltd.; 2005:237‐238. [Google Scholar]
- 11. Giustina A, Adler RA, Binkley N, et al. Controversies in vitamin D: summary statement from an international conference. J Clin Endocrinol Metab. 2019;104:234‐240. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. 2nd ed. John Wiley & Sons; 2019. [Google Scholar]
- 13. Teymoori‐Rad M, Shokri F, Salimi V, Marashi SM. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29:e2032. [DOI] [PubMed] [Google Scholar]
- 14. McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44:981‐988. [DOI] [PubMed] [Google Scholar]
- 15. Belderbos ME, Houben ML, Wilbrink B, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513‐e1520. [DOI] [PubMed] [Google Scholar]
- 16. Inamo Y, Hasegawa M, Saito K, et al. Serum vitamin D concentrations and associated severity of acute lower respiratory tract infections in Japanese hospitalized children. Pediatrics Int. 2011;53:199‐201. [DOI] [PubMed] [Google Scholar]
- 17. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ (Clinical Research Ed). 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergman P, Lindh AU, Björkhem‐Bergman L, Lindh JD. Vitamin D and respiratory tract infections: a systematic review and meta‐analysis of randomized controlled trials. PLoS One. 2013;8:e65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aponte R, MSHN, Palacios C. Vitamin D for prevention of respiratory tract infections. WHO; e‐Library of Evidence for Nutrition Actions (eLENA); 2017. https://www.who.int/elena/titles/commentary/vitamind_pneumonia_children/en/. [Google Scholar]
- 20. Shamshirian A, Hessami A, Heydari K, et al. the role of hydroxychloroquine in COVID‐19: a systematic review and meta‐analysis. Ann Acad Med Singapore. 2020;49:789‐800. [PubMed] [Google Scholar]
- 21. Carter SJ, Baranauskas MN, Fly AD. Considerations for obesity, vitamin D, and physical activity amid the COVID‐19 pandemic. Obesity. 2020;28:1176‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jakovac H. COVID‐19 and vitamin D—is there a link and an opportunity for intervention? Am J Physiol‐Endocrinol Metab. 2020;318:E589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molloy E, Murphy N. Vitamin D, covid‐19 and children. Ir Med J. 2020;113:64. [PubMed] [Google Scholar]
- 25. Zemb P, Bergman P, Camargo CA Jr, et al. Vitamin D deficiency and the COVID‐19 pandemic. J Global Antimicrobial Resist. 2020;22:133‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS‐CoV‐2 positivity rates associated with circulating 25‐hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID‐19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol. 2020;8:823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease C, Prevention . People with certain medical conditions. 2020.
- 30. Chow N, Fleming‐Dutra K, Gierke R, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 ‐ United States, February 12‐March 28, 2020. MMWR Morbidity Mortality Weekly Rep. 2020;69:382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hessami A, Shamshirian A, Heydari K, et al. Cardiovascular diseases burden in COVID‐19: systematic review and meta‐analysis. Am J Emergency Med. 2020;46:382‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orwoll E, Nielson CM, Marshall LM, et al. Vitamin D deficiency in older men. J Clin Endocrinol Metab. 2009;94:1214‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mosekilde L. Vitamin D and the elderly. Clin Endocrinol (Oxf). 2005;62:265‐281. [DOI] [PubMed] [Google Scholar]
- 34. La Vignera S, Cannarella R, Condorelli RA, Torre F, Aversa A, Calogero AE. Sex‐Specific SARS‐CoV‐2 mortality: among hormone‐modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis D . Int J Mol Sci. 2020;21:2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang C‐Y, Leung PSC, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:217‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vranić L, Mikolašević I, Milić S. Vitamin D deficiency: consequence or cause of obesity? Medicina (Kaunas). 2019;55:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim D. The role of vitamin D in thyroid diseases. Int J Mol Sci. 2017;18:1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kdekian A, Alssema M, Van Der Beek EM, et al. Impact of isocaloric exchanges of carbohydrate for fat on postprandial glucose, insulin, triglycerides, and free fatty acid responses‐a systematic review and meta‐analysis. Eur J Clin Nutr. 2020;74:1‐8. [DOI] [PubMed] [Google Scholar]
- 39. Cashman KD, Dowling KG, Škrabáková Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weishaar T, Rajan S, Keller B. Probability of vitamin D deficiency by body weight and race/ethnicity. J Am Board Fam Med. 2016;29:226‐232. [DOI] [PubMed] [Google Scholar]
- 41. Correia A, Azevedo Mdo S, Gondim F, Bandeira F. Ethnic aspects of vitamin D deficiency. Arquivos brasileiros de endocrinologia e metabologia. 2014;58:540‐544. [DOI] [PubMed] [Google Scholar]
- 42. Yancy CW. COVID‐19 and African Americans. JAMA. 2020;323:1891. [DOI] [PubMed] [Google Scholar]
- 43. Milam AJ, Furr‐Holden D, Edwards‐Johnson J, et al. Are clinicians contributing to excess African American COVID‐19 deaths? Unbeknownst to them, They may be . Health equity. 2020;4:139‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laurencin CT, McClinton A. The COVID‐19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethnic Health Disparities. 2020;7:398‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khunti K, Singh AK, Pareek M, Hanif W. Is ethnicity linked to incidence or outcomes of covid‐19? BMJ (Clinical Research Ed). 2020;369:m1548. [DOI] [PubMed] [Google Scholar]
- 46. Hastie CE, Mackay DF, Ho F, et al. Vitamin D concentrations and COVID‐19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14:561‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moszynski P. WHO report highlights Africa's health challenges. BMJ (Clinical Research Ed). 2006;333:1088. [Google Scholar]
- 48. Oleribe OO, Momoh J, Uzochukwu BS, et al. Identifying key challenges facing healthcare systems in Africa and potential solutions. Int J Gen Med. 2019;12:395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Organization WH . The African regional health report 2014: The health of the people. 2014.
- 50. Yuen AW, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39‐44. [DOI] [PubMed] [Google Scholar]
- 51. Word AP, Perese F, Tseng LC, Adams‐Huet B, Olsen NJ, Chong BF. 25‐Hydroxyvitamin D levels in African‐American and Caucasian/Hispanic subjects with cutaneous lupus erythematosus. Br J Dermatol. 2012;166:372‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Powe CE, Evans MK, Wenger J, et al. Vitamin D‐binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991‐2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bikle D, Christakos S. New aspects of vitamin D metabolism and action ‐ addressing the skin as source and target. Nat Rev Endocrinol. 2020;16:234‐252. [DOI] [PubMed] [Google Scholar]
- 54. Darbeheshti F, Rezaei N. Genetic predisposition models to COVID‐19 infection. Med Hypotheses. 2020;142:109818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. COVID‐19 Host Genetics Initiative . The COVID‐19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS‐CoV‐2 virus pandemic. Eur J Hum Genetics: EJHG. 2020;28:715‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aloia J, Mikhail M, Dhaliwal R, et al. Free 25(OH)D and the Vitamin D paradox in African Americans. J Clin Endocrinol Metabol. 2015;100:3356‐3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266‐281. [DOI] [PubMed] [Google Scholar]
- 58. Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012;76:315‐325. [DOI] [PubMed] [Google Scholar]
- 59. Beard JA, Bearden A, Striker R. Vitamin D and the anti‐viral state. J Clin Virol. 2011;50:194‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abhimanyu A, Coussens AK. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem Photobiol Sci. 2017;16:314‐338. [DOI] [PubMed] [Google Scholar]
- 61. Rondanelli M, Miccono A, Lamburghini S, et al. Self‐care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds‐practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid Based Complement Alternat Med. 2018;2018:5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grant WB, Boucher BJ. Yes, vitamin D can be a magic bullet. Clin Nutr. 2020;39:1627. [DOI] [PubMed] [Google Scholar]
- 63. Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol (Baltimore, Md: 1950). 2008;181:7090‐7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Olliver M, Spelmink L, Hiew J, Meyer‐Hoffert U, Henriques‐Normark B, Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J Infect Dis. 2013;208:1474‐1481. [DOI] [PubMed] [Google Scholar]
- 65. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID‐19 infections and deaths. Nutrients. 2020;12:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu H, Soruri A, Gieseler RKH, Peters JH. 1, 25‐Dihydroxyvitamin D3 exerts opposing effects to IL‐4 on MHC class‐II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38:535‐540. [DOI] [PubMed] [Google Scholar]
- 67. Hirsch D, Archer FE, Joshi‐Kale M, Vetrano AM, Weinberger B. Decreased anti‐inflammatory responses to vitamin D in neonatal neutrophils. Mediators Inflamm. 2011;2011:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Risitano AM, Mastellos DC, Huber‐Lang M, et al. Complement as a target in COVID‐19? Nat Rev Immunol. 2020;20:343‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gralinski LE, Sheahan TP, Morrison TE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018;9:e01753‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Karkeni E, Morin SO, Bou Tayeh B, et al. Vitamin D controls tumor growth and CD8+ T cell infiltration in breast cancer. Front Immunol. 2019;10:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chadha MK, Fakih M, Muindi J, et al. Effect of 25‐hydroxyvitamin D status on serological response to influenza vaccine in prostate cancer patients. Prostate. 2011;71:368‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17:533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moon C. Fighting COVID‐19 exhausts T cells. Nat Rev Immunol. 2020;20:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Minton K. DAMP‐driven metabolic adaptation. Nat Rev Immunol. 2020;20:1. [DOI] [PubMed] [Google Scholar]
- 75. Xiao L, Sakagami H, Miwa N. ACE2: the key molecule for understanding the pathophysiology of severe and critical conditions of COVID‐19: demon or angel? Viruses. 2020;12:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van de Veerdonk F, Netea MG, van Deuren M, et al. Kinins and cytokines in COVID‐19: a comprehensive pathophysiological approach. 2020.
- 77. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mitchell F. Vitamin‐D and COVID‐19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Machado CDS, Ferro Aissa A, Ribeiro DL, Antunes LMG. Vitamin D supplementation alters the expression of genes associated with hypertension and did not induce DNA damage in rats. J Toxicol Environ Health Part A. 2019;82:299‐313. [DOI] [PubMed] [Google Scholar]
- 80. Gracia‐Ramos AE. Is the ACE2 overexpression a risk factor for COVID‐19 infection? Arch Med Res. 2020;51:345‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID‐19. Int J Infect Dis. 2020;100:390‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Maghbooli Z, Sahraian MA, Ebrahimi M, et al. Vitamin D sufficiency, a serum 25‐hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID‐19 infection. PLoS One. 2020;15:e0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83. Baktash V, Hosack T, Patel N, et al. Vitamin D status and outcomes for hospitalised older patients with COVID‐19. Postgrad Med J. 2020;97:442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID‐19 test results. JAMA network open. 2020;3:e2019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Faul JL, Kerley CP, Love B, et al. Vitamin D deficiency and ARDS after SARS‐CoV‐2 infection. Ir Med J. 2020;113:84. [PubMed] [Google Scholar]
- 86. Merzon E, Tworowski D, Gorohovski A, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: an Israeli population‐based study. FEBS J. 2020;287:3693‐3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Panagiotou G, Tee SA, Ihsan Y, et al. Low serum 25‐hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID‐19 are associated with greater disease severity. Clin Endocrinol. 2020;93:508‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Carpagnano GE, Di Lecce V, Quaranta VN, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID‐19. J Endocrinol Invest. 2021;44:765‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Di Nicola M, Dattoli L, Moccia L, et al. Serum 25‐hydroxyvitamin D levels and psychological distress symptoms in patients with affective disorders during the COVID‐19 pandemic. Psychoneuroendocrinology. 2020;122:104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Macaya F, Espejo Paeres C, Valls A, et al. Interaction between age and vitamin D deficiency in severe COVID‐19 infection. Nutr Hosp. 2020;37:1039‐1042. [DOI] [PubMed] [Google Scholar]
- 91. Karahan S, Katkat F. Impact of serum 25(OH) vitamin D level on mortality in patients with COVID‐19 in Turkey. J Nutr Health Aging. 2020;25:189‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Abdollahi A, Sarvestani HK, Rafat Z, et al. The association between the level of serum 25(OH) vitamin D, obesity, and underlying diseases with the risk of developing COVID‐19 infection: a case‐control study of hospitalized patients in Tehran, Iran. J Med Virol. 2020;93:2359‐2364. [DOI] [PubMed] [Google Scholar]
- 93. Arvinte C, Singh M, Marik PE. Serum levels of vitamin C and vitamin D in a cohort of critically Ill COVID‐19 patients of a North American community hospital intensive care unit in May 2020: a pilot study. Med Drug Discovery. 2020;8:100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cereda E, Bogliolo L, Klersy C, et al. Vitamin D 25OH deficiency in COVID‐19 patients admitted to a tertiary referral hospital. Clin Nutr (Edinburgh, Scotland). 2020;40:2469‐2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hamza A, Ahmed M, Ahmed K, Durrani AB. Role of vitamin D in pathogenesis and severity of coronavirus disease 2019 (COVID‐19) infection. Pak J Med Health Sci. 2020;14:462‐465. [Google Scholar]
- 96. Hernández JL, Nan D, Fernandez‐Ayala M, et al. Vitamin D Status in Hospitalized Patients with SARS‐CoV‐2 Infection. J Clin Endocrinol metab. 2020;106:e1343‐e1353. [DOI] [PubMed] [Google Scholar]
- 97. Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID‐19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10:20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ling SF, Broad E, Murphy R, et al. High‐dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID‐19: a cross‐sectional multi‐centre observational study. Nutrients. 2020;12:3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Luo X, Liao Q, Shen Y, Li H, Cheng L. Vitamin D deficiency is inversely associated with COVID‐19 incidence and disease severity in chinese people. J Nutr. 2020;151:98‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Radujkovic A, Hippchen T, Tiwari‐Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D deficiency and outcome of COVID‐19 patients. Nutrients. 2020;12:2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vassiliou AG, Jahaj E, Pratikaki M, et al. Vitamin D deficiency correlates with a reduced number of natural killer cells in intensive care unit (ICU) and non‐ICU patients with COVID‐19 pneumonia. Hellenic J Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ye K, Tang F, Liao X, et al. Does serum vitamin D level affect COVID‐19 infection and its severity? A case‐control study. J Am Coll Nutr. 2020;1‐ 8. [DOI] [PubMed] [Google Scholar]
- 103. Karonova T, Andreeva A, Vashukova M. Serum 25 (OH) D level in patients with COVID‐19. J Infectol. 2020;12:21‐27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Data Availability Statement
Not applicable.


