Abstract
Introduction
The COVID‐19 vaccination programme is under way worldwide. Anecdotal evidence is increasing that some people with type 1 diabetes mellitus (T1DM) experience temporary instability of blood glucose (BG) levels post‐vaccination which normally settles within 2‐3 days. We report an analysis of BG profiles of 20 individuals before/after vaccination.
Methods
We examined the BG profile of 20 consecutive adults (18 years of age or more) with T1DM using the FreeStyle Libre flash glucose monitor in the period immediately before and after COVID‐19 vaccination. The primary outcome measure was percentage (%) BG readings in the designated target range 3.9‐10 mmmol/L as reported on the LibreView portal for 7 days prior to the vaccination (week −1) and the 7 days after the vaccination (week +1).
Results
There was a significant decrease in the %BG on target following the COVID‐vaccination for the 7 days following vaccination (mean 45.2% ± SE 4.2%) vs pre‐COVID‐19 vaccination (mean 52.6% ± SE 4.5%). This was mirrored by an increase in the proportion of readings in other BG categories 10.1%‐13.9%/≥14%. There was no significant change in BG variability in the 7days post‐COVID‐19 vaccination. This change in BG proportion on target in the week following vaccination was most pronounced for people taking Metformin/Dapagliflozin+basal‐bolus insulin (−23%) vs no oral hypoglycaemic agents (−4%), and median age <53 vs ≥53 years (greater reduction in %BG in target for older individuals (−18% vs −9%)).
Conclusion
In T1DM, we have shown that COVID‐19 vaccination can cause temporary perturbation of BG, with this effect more pronounced in patients talking oral hypoglycaemic medication plus insulin, and in older individuals. This may also have consequences for patients with T2DM who are currently not supported by flash glucose monitoring.
What's known
The COVID‐19 vaccination programme is under way in the United Kingdom as elsewhere. Flash glucose monitoring has given a new insight into blood glucose variability in T1DM.
What's new
We here describe that COVID‐19 vaccination can cause temporary perturbation of BG, with this effect more pronounced in patients talking oral hypoglycaemic medication, plus insulin and in older individuals. There was no relation between the type of vaccine given and the likelihood of BG perturbation.
1. INTRODUCTION
Since its arrival in 2019, the COVID‐19 pandemic has challenged all healthcare systems across the world. 1 , 2 The focus on mitigating the effects of the virus has led to many routine healthcare services being disrupted and to millions of people with diabetes across the world being fearful regarding the potential for infection with COVID‐19 to make them very seriously unwell. 3
Continuous glucose monitoring (CGM) devices which display an estimate of blood glucose levels, along with trends in direction, are increasingly being adopted for routine care in people with type 1 diabetes (T1DM). 4 Flash glucose monitoring, allows users retrospectively to review the preceding 8 hours of continuous glucose data, 5 along with a contemporary estimated blood glucose value and trend line. In the eyes of many, they are proving to be a step‐change in diabetes management. The use of CGM is associated with a reduction in HbA1c. 4 , 6
The COVID‐19 vaccination programme is now well under way in the UK utilising the Pfizer/Biontech or the Oxford/AstraZeneca vaccine. 7 At the time of writing, more than 25 million people in the UK have been vaccinated with their first dose. We have found in routine clinical practice that some people with T1DM experience temporary instability of blood glucose (BG) levels after vaccination.
We collected data from 20 consecutive individuals with T1DM who routinely use flash glucose monitoring, and who have recently received their first dose of vaccine. We here report an analysis of the blood glucose profiles of these 20 patients before and after vaccination.
2. METHODS
We examined the blood glucose profile of 20 consecutive adults (18 years of age or more) with T1DM using the FreeStyle Libre flash glucose monitor in the period immediately before and after COVID‐19 vaccination. All were under the care of the National Health Service (NHS) specialist diabetes service in Eastern Cheshire UK.
The Libre View reporting system 8 provides a number of metrics over the selected time period for each patient that is all dependant on underlying patient BG control, these include average BG, BG variability, % of BG results falling within given ranges, 3.9‐10, 10.1‐13.9, ≥14 mmol/L, from these on can also calculated the % <3.9 mmol/L. In order to select a primary metric all the above metrics were evaluated across the 20 patients for the 7 days before vaccination and the 7 days directly after vaccination. The primary outcome metric was chosen on the basis of the highest difference and significant P‐value.
Data for that metric was also extracted for the weeks −2 and +2 to evaluate the BG stability in the period before and the speed of return after the main measurement period.
Other data that might have an impact on the results was also taken from the patient records. These included gender, type of vaccine given, medication, age, duration with T1DM and body mass index (BMI). For continuous indicators, the patients were split into 2 groups across the median value of each variable.
This was a quality improvement project. Ethics approval was not obtained for this study, as this mode of monitoring of BG is part of standard care for T1DM individuals, according to National Institute for Health and Clinical Excellence (NICE) guidance. 9 All individual patient data were anonymised prior to statistical analysis.
2.1. Statistical analysis
Excel 64‐bit with Analyse‐it add‐in was used to perform the analysis. Shapiro‐Wilkes testing confirmed that the patient glucose results fell in a normal distribution. Two tailed paired t‐test for the five outcome measures compared results in weeks −1 against +1 to establish difference and P values.
The mean and standard deviation of the selected indicators was then calculated for the total cohort and split into two classes for each potential factor. The trend and standard error over the 4 weeks for these variables were plotted graphically.
3. RESULTS
The median age was 53 years (overall range 26‐70 years); 11 (55%) of the patients were female. Baseline demographics are detailed in Table 1. COVID‐19 vaccination occurred between 14 January and 7 March 2021. 8/20 individuals received the Pfizer/Biotech and 12/20 individuals the Oxford/AstraZeneca vaccine. Pre‐vaccination HbA1c was in the range 46 mmol/mol (6.4%) to 92.0 mmol/mol (10.6%) (median 56.5 mmol/mol (7.3%)) with body mass index (BMI) in the range 21.5 to 41.6 kg/m2 (median 28.1 kg/m2).
TABLE 1.
Baseline characteristics for 20 T1DM individuals
| Men (n = 9) | Women (n = 11) | |
|---|---|---|
| Age (years) (SE) | 46.9 (5.0) | 51.5 (3.0) |
| BMI (kg/m2) (SE) | 28.1 (1.0) | 29.5 (2.1) |
| Duration of diagnosed T1DM (years) (SE) | 25.1 (4.5) | 23.5 (3.5) |
| Estimated HbA1c (mmol/mol) (SE) | 57.0 (2.0) | 61.2 (3.6) |
| % given Pfizer/Biontech vaccine | 33 | 45 |
| % given Oxford/AstraZeneca vaccine | 67 | 55 |
Abbreviations: BMI, body mass index; HbA1c, glycosylated haemoglobin; SE, standard error; T1DM, type 1 diabetes.
All 20 individuals were on a basal‐bolus regime of long acting analogue insulin (Insulin Degludec or Glargine) and prandial short acting analogue insulin (Insulin Aspart or Insulin Lispro). Nine individuals were additionally on Metformin (n = 7) or Dapagliflozin (n = 2) (licensed for adjunctive treatment in people with T1DM). Mean HbA1c for these patients was 57.0 mmol/mol (7.4%) ± standard error (SE) 1.7 mmol/mol (0.25%) vs 61.2 mmol/mol (7.7%) ± 3.7 mmol/mol (0.5%) for those on insulin alone. Thus there was no significant difference in HbA1c between these groups.
The %BG on target was parametrically distributed. The range of %BG on target (3.9‐10 mmol/L) pre‐COVID‐19 vaccination was 1% to 88% (mean 52.6% ± (SE) 4.5%). The results for the individual patients pre‐ and post‐COVID‐19 vaccination are shown in Figure 1. 5/20 patients showed an increase in the % BG in the target range with the rest showing a fall.
FIGURE 1.
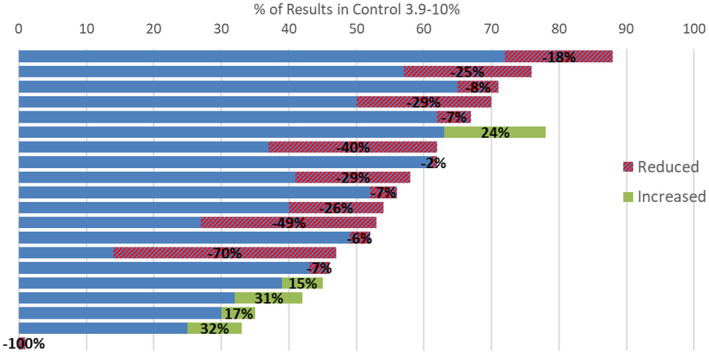
Individual patient results, % of results in control range (3.9‐10 mmol/L) over 7 days before vaccination and change to 7 days after vaccination (% change given in brackets)
Overall there was a significant decrease in the %BG on target following the COVID‐vaccination in the 7 days following vaccination (range 0%‐78%; mean 45.2% ± 4.2%) (Figure 2A and Table 2). This equated to 14% fall in the %BG in the target range 3.9‐10 mmol/L (P = .020). This was mirrored by an increase the proportion of readings in other BG categories 10.1‐13.9 and ≥14 mmol/L (Table 2). There was no significant change in BG variability in the 7 days post‐COVID‐19 vaccination compared with the previous week.
FIGURE 2.
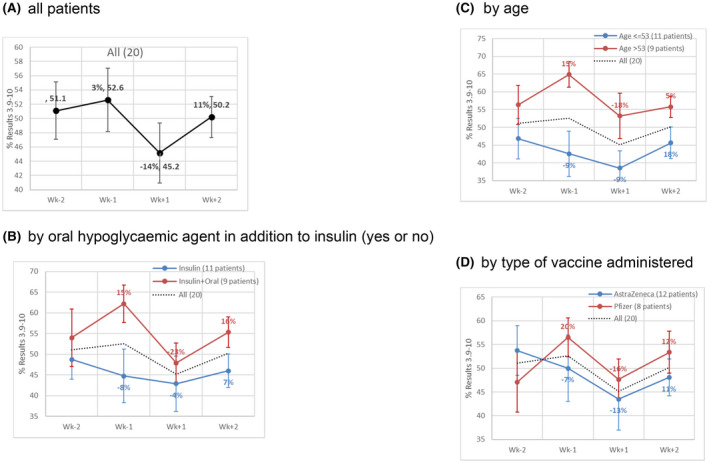
(A‐D) Development of indicator values over the 4 weeks. The vaccination takes place on the transition between Week −1 and Week +1. The % shown change reflect the change to the previous week. The bar reflects the standard error (SE). (A) All patients; (B) by oral hypoglycaemic agent in addition to insulin (yes or no); (C) by age and (D) by type of vaccine administered
TABLE 2.
Glycaemic profile pre‐ and post‐COVID‐19 vaccination
| All results | Pre‐ vax mean (%) | Pre‐ vax SE (%) | Post‐vax mean (%) | Post‐vax SE (%) | % Change | P value |
|---|---|---|---|---|---|---|
| % Results in control 3.9‐10 mmol/L | 52.6 | 4.5 | 45.2 | 4.2 | −14% | .020 |
| % Results 10.1‐13.9 mmol/L | 26.6 | 2.7 | 30.5 | 2.6 | +14% | .110 |
| % Results ≥14.0 mmol/L | 16.3 | 4.3 | 20.0 | 4.2 | +23% | .038 |
| % Results <3.9 mmol/L | 4.5 | 1.1 | 4.4 | 0.8 | −0.02% | .52 |
| Average G (mmol/L) | 9.8 | 0.6 | 10.4 | 0.5 | +6% | .034 |
| Variability | 36.0 | 1.7 | 35.7 | 1.6 | −1% | .772 |
Abbreviations: G, glucose; SE, standard error; Vax, vaccination.
This change BG proportion on target in the week following vaccination was most pronounced for people taking Metformin or Dapagliflozin in addition to the basal‐bolus insulin. The fall in the % on target categorised by additional Metformin/Dapagliflozin greater fall (−23%) vs no oral hypoglycaemic agents (−4%), and median age <53 vs ≥53 years greater fall for older patients (−18% vs −9%) is shown in Figure 2B,C (data shown for weeks −2 to +2 in relation to vaccine administration). The fall in % on target was also greater for those with median BMI of 28.1 kg/m2 or more (−22%) vs BMI < 28.1 kg/m2 (−4%). There was no significant difference in the change in proportion on target by type of vaccine (Figure 2D), pre‐vaccination HbA1c (−14% for ≤median HbA1c 56.5 mmol/mol vs −15% for >median HbA1c 56.5 mmol/mol) or duration of diagnosed T1DM.
A representative BG profile is shown in Figure 3 for one representative patient to demonstrate the change in BG profile in the week postvaccination. On review of the clinical records, in all the individuals there was no evidence of any other factor than the vaccination to account for the changes in BG profile—that is there is no evidence of intercurrent illness or other events that would significantly influence BG levels. 65% of patients did report systemic symptoms after vaccination including cough, headache, shakiness, feeling generally unwell, “jelly legs”, nausea and general malaise.
FIGURE 3.
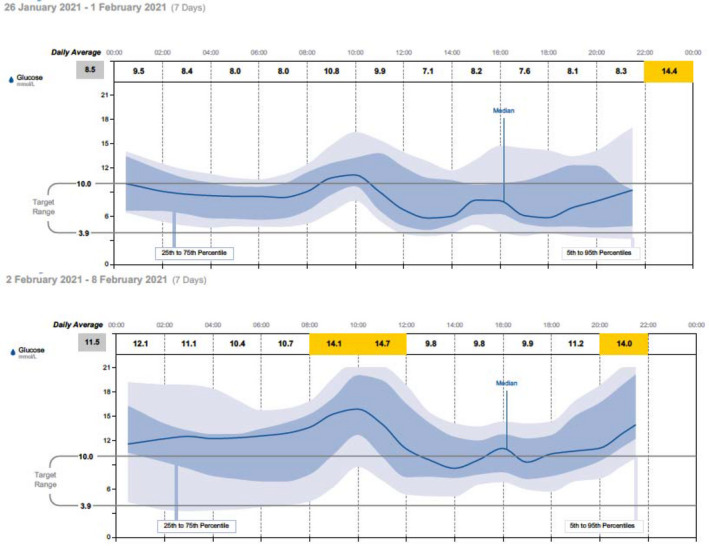
Representative patient trace for the week before and the week after COVID‐19 vaccination
There were no clinically reported inflammatory reactions at injection sites.
4. DISCUSSION
In this representative group of people with T1DM (in terms of age and BMI), we have here shown that COVID‐19 vaccination can cause temporary relative hyperglycaemia in people with T1DM (for at least one week) with this effect more pronounced in patients talking oral hypoglycaemic medication in addition to insulin. There was a non‐significant fall in %BG on target for patients on insulin alone—thus we report an unexpected influence of oral medication on the BG control of people with T1DM following COVID‐19 vaccination.
This in no way suggests that vaccination should be withheld. Clinical data supports a robust neutralizing antibody response in COVID‐19 patients with diabetes. 10 Our findings do indicate that patients with T1DM should be warned about the potential for transient hyperglycaemia following the vaccine. 11
We do not have sufficient data to differentiate at this stage the degree of difference between the Pfizer/Biontech Oxford/AstraZeneca vaccines in relation to their metabolic effect in the days after vaccination.
As to the mechanism underlying what we have reported, both the COVID‐19 vaccines available in the UK work by stimulating an antibody response to the spike protein on the virus. 12 , 13 Vaccination for influenza has been noted to cause blood glucose levels to become unstable for a time, perhaps related not only to a reaction to the virus but also to the excipients in the administered vaccine. 14 The UK government has recently published data of all UK spontaneous reports (received from 9 December 2020 to 7 March 2021) for mRNA Pfizer/BioNTech vaccine in which there were 27 cases of hyperglycaemia (not restricted to type 1 diabetes). 15 Similar reporting found 54 cases of hyperglycaemia (from 4 January to 7 March 2021) for COVID‐19 vaccine Oxford University/AstraZeneca. 16 Our use of flash glucose monitoring allows identification of subclinical trends in dysglycaemia that may escape other forms of monitoring. 4 , 5 , 6
Transient fluctuations in blood glucose have many causes. With our analysis of the cases revealing no other contributory factors such as infection or hypersensitivity to the excipients, it seems likely that the observed hyperglycaemia was associated with the COVID‐19 vaccination.
One possible mechanism for the hyperglycaemia is stimulation of the immune system resulting in a stress response, to a milder degree than would typically occur with a COVID‐19 infection. Physiologic stress has the potential to increase counter regulatory hormone levels. 17 Most notable among these are adrenaline, growth hormone and cortisol and/or glucagon in those with alpha cell reserve. People with T1DM may be less able to rapidly counteract such elevations in blood glucose. 18 This series comprises individuals having their first COVID vaccine. It has been reported that people with prior covid‐19 infection, reported side effects from the vaccine more frequently after the first dose. 19 We did not have serological data in our patient group for prior infection. However, in an important 2018 paper, Sestan et al 20 reported that viral‐induced inflammation leads to insulin resistance in skeletal muscle, followed by compensatory hyperinsulinemia, which promotes the anti‐viral effector response of CD8+ T cells. The potential mechanisms by which the COVID‐19 vaccination may cause relative hyperglycaemia remain to be determined.
Vaccinations by nature of their intended purpose elicit an immune response, often to varying degrees within and between individuals determined by a wide range of factors some of which reside within the vaccine, for example type of adjuvant or within the host eg immune response genes. It is not surprising that such immune responses have complex downstream effects on metabolism including regulation of blood glucose levels. A range of cytokines produced through immune‐driven inflammation is known to impact on blood glucose levels and insulin resistance within tissues. 21 Such actions are likely to have complex and further biological interplay with factors including adipokines, hormones and cortisol. In individuals with existing impaired glucose control, this is likely to be more pronounced.
Individual patient knowledge and involvement remain the cornerstones of diabetes management. Therefore, it is important to inform individuals with T1DM about the phenomenon reported here, while future research may shed more light on the underlying mechanisms. Finally, we speculate that fact that the deleterious effect on BG levels was more pronounced in older people and those with a higher BMI, and those with T1DM but also on oral medication raises the question as to whether what we report here may also be occurring in people with T2DM who currently do not have access to Libre glucose measurement.
4.1. Strengths/limitations
While we report these results in a small (n = 20) group of people with T1DM, this is based on day to day flash glucose monitoring over a period of 4 weeks, with more than 95% of glucose readings in that period being uploaded by the individuals studied.
A limitation is that we have not analysed what (if any) changes were made in the insulin doses during the week following the COVID‐19 vaccine. The change in %BG on target post‐COVID‐19 vaccination could have been larger than we have seen, with subsequent mitigation by measures that were taken by the patients studied.
5. CONCLUSION
In a representative group of individuals with T1DM, we have shown that COVID‐19 vaccination can cause temporary perturbation of BG in people with T1DM (for at least one week) with this effect more pronounced in patients talking oral hypoglycaemic medication in addition to insulin and in older individuals. This is of relevance to people with T1DM and to clinicians.
A larger patient multi‐site patient series is clearly necessary to investigate this further as the vaccination programme continues across the world.
DISCLOSURES
No author has any conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Jen Heath, Diabetes Specialist Nurse for her help in data collection and to Robert Moore for his observation that led to this paper.
Heald AH, Rea R, Horne L, et al. Analysis of continuous glucose tracking data in people with type 1 diabetes after COVID‐19 vaccination reveals unexpected link between immune and metabolic response, augmented by adjunctive oral medication. Int J Clin Pract. 2021;75:e14714. 10.1111/ijcp.14714
DATA AVAILABILITY STATEMENT
Any requests for data extracts will be considered by Dr Adrian Heald as the corresponding author.
REFERENCES
- 1. Rawaf S, Allen LN, Stigler FL, et al.; Global Forum on Universal Health Coverage and Primary Health Care . Lessons on the COVID‐19 pandemic, for and by primary care professionals worldwide. Eur J Gen Pract. 2020;26:129‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krist AH, DeVoe JE, Cheng A, Ehrlich T, Jones SM. Redesigning primary care to address the COVID‐19 pandemic in the midst of the pandemic. Ann Fam Med. 2020;18:349‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riddle MC, Buse JB, Franks PW, et al. COVID‐19 in people with diabetes: urgently needed lessons from early reports. Diabetes Care. 2020;43:1378‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta‐analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalra S, Gupta Y. Ambulatory glucose profile: flash glucose monitoring. J Pak Med Assoc. 2015;65:1360‐1362. [PubMed] [Google Scholar]
- 6. Yadegarfar G, Anderson SG, Khawaja Z, et al. The FreeStyle libre flash glucose monitoring system: how it has improved glycaemic control for people with type 1 diabetes in Eastern Cheshire, UK. Cardiovasc Endocrinol Metab. 2020;9(4):171‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. https://www.nhs.uk/conditions/coronavirus‐covid‐19/coronavirus‐vaccination/coronavirus‐vaccine/. Accessed March 19, 2021.
- 8. https://www.libreview.com/. Accessed March 19, 2021.
- 9. https://www.nice.org.uk/guidance/NG17. 2016. Accessed March 22, 2020.
- 10. Pal R, Bhadada SK, Misra A. COVID‐19 vaccination in patients with diabetes mellitus: current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15:505‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643‐2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polack FP, Thomas SJ, Kitchin N, et al.; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glaess SS, Benitez RM, Cross BM, Urteaga EM. Acute hyperglycemia after influenza vaccination in a patient with type 2 diabetes. Diabetes Spectr. 2018;31:206‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/970504/COVID‐19_mRNA_Pfizer‐_BioNTech_Vaccine_Analysis_Print.pdf. Accessed March 20, 2021
- 16. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/970505/COVID‐19_AstraZeneca_Vaccine_Analysis_Print.pdf. Accessed March 20. 2021
- 17. Mifsud S, Schembri EL, Gruppetta M. Stress‐induced hyperglycaemia. Br J Hosp Med. 2018;79:634‐663. [DOI] [PubMed] [Google Scholar]
- 18. Galassetti P, Tate D, Neill RA, Morrey S, Wasserman DH, Davis SN. Effect of antecedent hypoglycemia on counterregulatory responses to subsequent euglycemic exercise in type 1 diabetes. Diabetes. 2003;52:1761‐1769. [DOI] [PubMed] [Google Scholar]
- 19. Wise J. Covid‐19: people who have had infection might only need one dose of mRNA vaccine. BMJ. 2021;372:n308. 10.1136/bmj.n308 [DOI] [PubMed] [Google Scholar]
- 20. Šestan M, Marinović S, Kavazović I, et al. Virus‐induced interferon‐γ causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity. 2018;49:164‐177.e6. [DOI] [PubMed] [Google Scholar]
- 21. Shi J, Fan J, Su Q, Yang Z. Cytokines and abnormal glucose and lipid metabolism. Front Endocrinol. 2019;10:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any requests for data extracts will be considered by Dr Adrian Heald as the corresponding author.


