Abstract
Background
Healthcare workers (HCWs) are at increased risk since they are directly exposed to SARS‐CoV‐2‐infected patients, and nevertheless, some remain without the development of anti‐SARS‐CoV‐2 antibodies or related symptoms, suggesting less susceptibility to the infection.
Methods
This cross‐sectional, case‐control study aimed to compare SARS‐CoV‐2 T‐cell response by two different technologies, the analysis of IFN‐γ+ CD8+/CD4+ T cells by flow cytometry and the quantification of IFN‐γ release by ELISA‐related assay (without cell discrimination), both after SARS‐CoV‐2 stimulation among uninfected and convalescent HCWs.
Results
A high proportion of uninfected HCWs (53.8%) had pre‐existing IFN‐γ+ CD8 T‐cell response after stimulation with at least one of the structural viral proteins S, M or N, while 35.9% had pre‐existing IFN‐γ+ CD4 T‐cell response. This proportion was nearly or greater than 90% among convalescent HCWs. Interestingly, the magnitude of the response in uninfected was lower compared to that found in convalescent HCWs, using both methods. The concordance, quantifying the specific cellular response in convalescent HCWs, between both methods was 94.1% comparing CD8 T‐cell response and 89.7% comparing CD4 T‐cell response. This concordance was lower but still high in uninfected HCWs (76.5%) comparing CD8 T‐cell response and 71.8% comparing CD4 T‐cell response.
Conclusions
The good concordance between the proportion of individuals with IFN‐γ release after SARS‐COV‐2 stimulation with the proportion of individuals with specific IFN‐γ+ CD8/CD4 T cells found in this study drives IFN‐γ release assays to be a simple and easy way to determine the protective immunity to SARS‐CoV‐2 in a wide population.
Keywords: cellular immune response, COVID‐19, exposed healthcare workers, SARS‐CoV‐2
1. INTRODUCTION
The SARS‐CoV‐2 epidemic started in December 2019 in Wuhan (China) and has spread rapidly worldwide becoming a pandemic threatening public health. 1 , 2 , 3 Healthcare workers (HCW) have been at increased risk of SARS‐CoV‐2 infection since they have been continuous and directly exposed to infected individuals. 4 The seroprevalence of SARS‐CoV‐2 infection among healthcare workers is estimated to be up to 38.9% (23.7% in an intramural survey performed in April 2020 including 4968 health professionals of our hospital, data not published), 5 , 6 , 7 while it is up to 5.7% in the general population. 8 This higher prevalence among HCW is closely related to risk factors such as exposure to aerosol‐generating procedures, suboptimal handwashing after patient contact and suboptimal use of protective personal equipment during the first wave. 9 , 10
Although HCWs reported poor access to protective equipment while having very close contact with SARS‐CoV‐2 infected patients at the beginning of the pandemic, many have developed neither positive serology nor related symptoms, suggesting less susceptibility to the infection.
The immune response to SARS‐CoV‐2 infection involves not only the humoral response but also an important cellular response. 11 , 12 We hypothesized that these HCWs could have, at least in part, a cellular immune response that could limit the infection and/or the antibody development.
Here we analysed the SASR‐CoV‐2 immune response comparing two different technologies after SARS‐CoV‐2 stimulation, that is, analysing the proportion of IFN‐γ+ CD8+ and CD4+ T cells by flow cytometry, and the quantification of released soluble IFN‐γ that could be performed by standard ELISA or other multiparametric assays, in uninfected and convalescent HCWs.
2. DESIGN AND METHODS
This was a cross‐sectional, case‐control study (2:1 ratio), performed in a tertiary university Hospital in Madrid, Spain. Uninfected healthcare workers (HCW) with proven direct and continued care for COVID‐19 patients for more than two weeks during the first wave of the pandemic, and with no diagnosis of current or past SARS‐CoV‐2 infection, ascertained by the absence of clinical SARS‐CoV‐2‐compatible symptoms and negative serology for anti‐SARS‐CoV‐2 antibodies performed in an intramural survey (IgM/IgA and IgG antibodies, Novatec Immunodiagnostica, Germany), were included as cases. Convalescent HCWs, with similar exposure to COVID‐19 patients, who had been diagnosed by RT‐PCR (not performed in all of them because during the first wave this technology was only performed in individuals with compatible symptoms) and/or specific serology in the intramural survey (all individuals had anti‐SARS‐CoV‐2 IgG positive), were included as controls. The viral exposure level among HCWs included continuous care for COVID‐19 patients, incomplete personal protective equipment, aerosol‐generating procedures exposure 5 and additional close contact with infected households. Cases and controls were identified through informal interviews with hospital staff and by self‐identification and were included from 6 May to 1 June 2020. Exclusion criteria included having any kind of recent/current cancer have been under immunosuppress therapy and pregnancy. This study was approved by our Institutional Review Board (EC162/20) and performed according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all the participants. The global study was registered at clinicaltrials.gov (NCT04402827). Reporting of the study conforms to broad Equator guidelines. 13
Age, sex, COVID‐19 symptoms, exposure to SARS‐CoV‐2‐infected patients, handling of aerosol‐generating procedures and additional close contacts with an infected household were collected at the inclusion visit. At study inclusion, three serologic assays to detect the presence of SARS‐CoV‐2 antibodies were used: Anti‐SARS‐CoV‐2 IgG and IgM antibodies, (COVID‐19 IgG/IgM Rapid Test Kit; UNscience Biotechnology) and anti‐SARS‐CoV‐2 IgA antibodies (COVID‐19 ‐SARS‐CoV‐2 IgA ELISA; Demeditec). Quantitative measurement of ACE‐2 in plasma samples was performed with a sandwich enzyme immunoassay (Human Angiotensin Converting Enzyme 2—ACE2 ELISA kit; Reddot Biotech).
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA blood samples and stored in liquid nitrogen until use. After centrifugation at 200 g for 10 min, plasma fraction was collected and again centrifuged at 1200 g for 15 min, aliquoted and stored at −80℃. The cellular fraction was subjected to ficoll density gradient centrifugation at 500 g for 20 min. PBMCs were then washed and frozen in 90% foetal bovine serum (FBS; Sigma) and 10% dimethyl sulfoxide (DMSO; Sigma) in liquid nitrogen. PBMCs were thawed and cultured in RPMI‐1640 culture medium (Lonza) supplemented with 10% human serum (AB serum; Sigma), 100 IU of penicillin/streptomycin/mL (Lonza), 2 mM L‐glutamine (complete medium) during 24 hours before stimulation assay. SARS‐CoV‐2 peptide pools of proteins S, M and N were used for the stimulation of cultured PBMCs. Each peptide pool is composed of peptides consisting mainly of 15‐mer sequences with 11 amino acids overlap, covering the immunodominant sequence domains of the surface glycoprotein S, the complete sequence of the membrane glycoprotein M and the complete nucleocapsid phosphoprotein N of SARS‐CoV‐2 (PepTivator SARS‐CoV‐2 Prot S, M, and N; Miltenyi Biotec). In addition, unstimulated (only culture medium) and SEB (1.5 mg/well staphylococcal enterotoxin B; Sigma) stimulated PBMCs were also assayed as negative and positive controls, respectively. A sample with no response to SEB would be excluded from the analysis. PBMCs were plated in 96‐well flat‐bottom plates at 106 cells/well in RPMI‐1640 medium (Gibco) supplemented with 10% heat‐inactivated AB in duplicate for the abovementioned five conditions. IFN‐γ expression was finally analysed for either CD4 or CD8 T cells by multiparametric flow cytometry. For IFN‐γ +‐CD8 and IFN‐γ +‐CD4 cell analysis (Rapid Cytokine Inspector CD4/CD8 T‐cell kit; Miltenyi Biotec), cells were stimulated for 2 h and then treated with brefeldin A to stop cytokine release and kept in culture for other 14 h in an incubator at 37°C and 5% CO2. Then, cells were fixed and permeabilized according to the manufacturer´s instructions for intracellular staining using the following antibodies: CD3‐VioBlue, CD4‐APC, CD8‐FITC, CD14+CD20‐PerCP and IFN‐γ‐PE. Samples were analysed with a MACSQuant cytometer and the MACSQuantify software v2.13.1. To exclude dead cells, viability 405/520 fixable dye staining (Miltenyi, Cologne Germany) was added for the last 10 min of incubation. Single (FSC‐A/FSC‐H) dot plot and live cells were first selected. Cell debris and monocytes were excluded from the analysis with CD14‐ and CD20‐PerCP antibodies. Then, lymphocytes were selected with a FSC‐A/SSC‐A dot plot, and CD3 T cells were gated. IFN‐γ expression was finally analysed for either CD4 or CD8 T cells and considered positive when the percentage of cells expressing IFN‐γ increased twofold compared with the unstimulated control. A sample was considered reactive when the percentage of cells expressing IFN‐γ increased twofold compared with the unstimulated control. The flow cytometry strategy is shown in Figure 1A.
FIGURE 1.
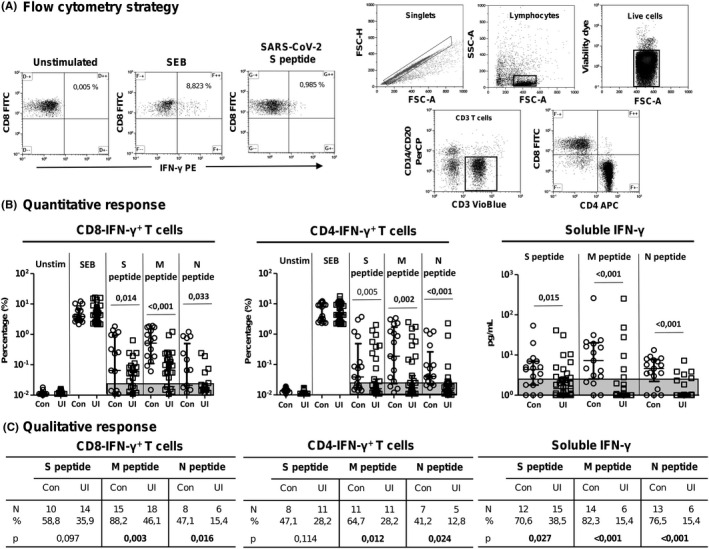
Response to SARS‐CoV‐2 peptides in healthcare workers (HCWs). (A) quantitative IFN‐γ‐producing CD8/CD4 T cells and IFN‐γ release after SARS‐CoV‐2 peptide in vitro stimulation (Mann‐Whitney U test). (B) comparison of qualitative response to different peptides between convalescent and uninfected HWCs (chi‐square test). Unstim, unstimulated; Con, convalescent HCWs; UI, uninfected HCWs. Significant when P < .005
For the quantification of soluble IFN‐γ production, cells were stimulated for 16 h. Then, 50 µl of supernatants was tested for cytokine quantification in duplicate using a human Th cytokine panel (Human MACSPlex Cytokine 12 kit; Miltenyi Biotec). Samples were analysed with a MACSQuant cytometer and the MACSQuantify v2.13.1 software. Samples were considered with significant positive results when the concentration in stimulated wells reached twofold the concentration in the unstimulated well. Response index was the ratio between the response to specific peptides divided by the unstimulated response.
2.1. Statistical analysis
The primary outcome of this study was the quantification of IFN‐γ release, including cell‐free supernatant and T‐cell‐associated release, after stimulation with specific antigens of the SARS‐CoV‐2 virus. Characteristics of both HCW groups were compared using two‐tailed statistical tests, chi‐square or Fisher's exact tests for categorical variables and Student's t test or Mann‐Whitney U tests for continuous variables. Categorical variables are shown as frequencies and proportions where continuous variables are shown as the mean and standard deviation or median and interquartile ranges (IQR). A P‐value below 0.05 was considered to be statistically significant. Statistics were performed with the IBM SPSS Statistics, version 23.0.
3. RESULTS
A total of 60 patients, 40 cases and 20 controls were included in the study. Four individuals were finally excluded, one case with positive serology and three controls with negative serology. Hence, a total of 39 cases (all positive for SARS‐CoV‐2‐specific IgG antibodies) and 17 controls were finally included for the analysis. The baseline characteristics of the participants are shown in Table 1. The mean age was 38 years, and 51% were female. The handling of aerosol‐generating procedures and/or additional risk contact with an infected household were similar in both groups (P = .224 and P = .440, respectively). Uninfected HCWs had a longer time of viral exposure compared to convalescents, with almost statistical significance (P = .084). Soluble plasma ACE‐2 was similar between both groups of HCWs. While ACE‐2 directly correlated with age (r = 0.337, P = .011), no correlation with sex was found (P = .924) (Figure S1).
TABLE 1.
Characteristics of the healthcare workers (HCWs) included in this study
|
Uninfected HCWs N = 39 |
Convalescent HCWs N = 17 |
p | |
|---|---|---|---|
| Age (years) | 39 [31‐51] | 38 [30‐50] | 0.894 |
| Gender (Female) | 20 (51.2%) | 9 (52.9%) | 0.519 |
| Health professionals | |||
| Physicians | 26 (66.6%) | 14 (83.3%) | 0.232 a |
| Nurses | 13 (33.3%) | 3 (17.6%) | 0.232 a |
| Time of exposure (weeks attending COVID‐19 patients) | 8 [6‐10] | 6 [5‐8] | 0.084 |
| Additional exposure | |||
| Aerosol generating procedures b | 33 (84.6%) | 12 (70.6%) | 0.224 a |
| SARS‐CoV‐2‐infected household | 5 (12.8%) | 1 (5.9%) | 0.440 a |
| Time from COVID‐19 diagnosis to serological survey (weeks) | — | 4 [2‐6] | |
| Time from serological survey to study inclusion (weeks) | 3 [2‐4] | 5 [3.5‐5] | <0.001 |
| Serology at serological survey c | |||
| IgM/IgA positive | 0 | 9 | |
| IgG positive | 0 | 17 | |
| Serology at study inclusion d | |||
| IgA positive | 0 | 3 | |
| IgM positive | 0 | 3 | |
| IgG positive | 0 | 17 | |
| SARS‐CoV‐2 RT‐PCR | |||
| Positive | — | 11 | |
| Negative | 6 | — | |
| Not performed | 33 | 6 | |
Data are expressed as median and interquartile range and percentage. Mann‐Whitney U test for statistical differences between continuous variables. HCW, healthcare workers.
Chi‐square test.
Aerosol‐generating procedures included airway suction, application of a high‐flow O2 instrument, bronchoscopy, endotracheal intubation, tracheostomy, nebulizer treatment, sputum induction, positive pressure ventilation, manual ventilation and cardiopulmonary resuscitation.
By ELISA.
IgA by ELISA and IgG and IgM by rapid chromatographic immunoassay. With statistical significance when P <.005.
The level of IFN‐γ+ CD8 T cells was higher in convalescents HCWs compared to uninfected HCWs for proteins S, M and N (P = .014, P < .001 and P = .033, respectively), as shown in Figure 1B. In parallel, the level of IFN‐γ+ CD4 T cells was higher in convalescents compared to uninfected for the three viral proteins (P = .005, P = .002 and P < .001, respectively). In parallel, the level of soluble IFN‐γ release was also higher in convalescents compared to uninfected HCWs to the three virus proteins (P = .015, P < .001 and P < .001, respectively).
In the same way, the number of individuals with positive IFN‐γ+ CD8 T‐cell response after peptide stimulation was higher in convalescents compared to uninfected HCWs with statistical significance for peptides M and N (P = .003 and P = .016), and almost with statistical significance for peptide S (P = .097), as shown in Figure 1C. The number of convalescent HCWs with a positive IFN‐γ+ CD4 T‐cell response after peptide stimulation was higher compared to uninfected HCWs for peptides M and N (P = .012 and P = .024, respectively). On the other hand, the number of convalescent HCWs with positive production of soluble IFN‐γ after cell stimulation was higher for each of the peptides S, M and N (P = .027, P < .001 and P < .001, respectively).
When different combinations of viral epitopes (proteins S, M and N) of positive response was analysed, no significant differences were found among the response to a unique epitope (only S, M or N) or a combination of two epitopes (S + M, S + N, or M + N) between convalescents and uninfected HCWs in either IFN‐γ+ CD8 T‐cell response, IFN‐γ+ CD4 T‐cell response or soluble IFN‐γ production, as shown in Table 2. Triple‐positive response (epitopes S + M + N at the same time) was only higher for soluble IFN‐γ production found in convalescent HCW cells (P < .001). On the contrary, positive response for at least one epitope (only peptide S, M or N) was higher in the convalescent HCW group in either IFN‐γ+ CD8 T‐cell response, IFN‐γ+ CD4 T‐cell response or soluble IFN‐γ production (P = .003, P < .001 and P = .005, respectively). In this case, 94.1% of the convalescent HCWs had a positive response to at least one epitope in IFN‐γ+ CD8 T cells compared to 53.8% among uninfected HCWs.
TABLE 2.
Qualitative positive response according to different SARS‐CoV‐2 peptides combination
| Peptide combination | CD8‐IFN‐γ+ cells (N, %) | CD4‐IFN‐γ+ cells (N, %) | IFN‐γ release (N, %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | UI | p | Con | UI | p | Con | UI | p | ||||
| At least one peptide |
16 94.1% |
21 53.8% |
0.003 |
15 88.2% |
14 35.9% |
<0.001 |
15 88.2% |
19 48.7% |
0.005 | |||
| S + M + N |
4 23.5% |
3 7.7% |
0.116 |
2 11.7% |
4 10.2% |
0.598 |
11 64.7% |
2 5.1% |
<0.001 | |||
| S + M |
6 35.3% |
8 20.5% |
0.199 | s |
3 7.7% |
0.116 |
1 5.9% |
2 5.1% |
0.670 | |||
| S + N |
0 0% |
0 0% |
1.000 |
2 11.7% |
0 0% |
0.088 |
0 0% |
2 5.1% |
0.481 | |||
| M + N |
3 17.6% |
3 7.7 |
0.254 |
1 5.9% |
1 2.5% |
0.519 |
1 5.9 |
0 0% |
0.304 | |||
| S only |
0 0% |
3 7.7% |
0.330 |
0 0% |
3 7.7% |
0.330 |
0 0% |
9 23.1% |
0.028 | |||
| M only |
2 11.7% |
4 10.2% |
0.598 |
2 11.7% |
0 0% |
0.088 |
1 5.9% |
2 5.1% |
0.670 | |||
| N only |
1 5.9% |
0 0% |
0.304 |
1 5.9% |
0 0% |
0.304 |
1 5.9% |
2 5.1% |
0.670 | |||
| Negative for all peptides |
1 5.9% |
18 46.1% |
0.003 |
2 11.7% |
25 64.1% |
<0.001 |
2 11.7% |
20 51.3% |
0.005 | |||
Chi‐square test with statistical significance when P <.005.
Abbreviations: Con, convalescent HCWs; M, SARS‐CoV‐2 membrane; N, SARS‐CoV‐2 nucleocapsid; S, SARS‐CoV‐2 spike; UI, uninfected HCWs.
Similar and high concordance between IFN‐γ+ CD8 T‐cell response and soluble IFN‐γ production was found between both groups, with 94.1% among convalescent HCWs and 89.7% among uninfected HCWs (P = .597) when comparing the response for at least one viral epitope (either peptide S, M or N), as shown in Table 3. This concordance was similar between both groups for the rest of the epitope combinations except for the response to at least peptide M, to the three epitopes at once and to epitope S only (P = .042, P = .005 and P = .043, respectively).
TABLE 3.
Concordance level between CD8‐IFN+ (CD4‐IFN+) cells and soluble IFN‐positive qualitative responses
| Peptide combination | CD8‐IFN‐γ+ cells vs soluble IFN‐γ (N, %) | CD4‐IFN‐γ+ cells vs soluble IFN‐γ (N, %) | ||||||
|---|---|---|---|---|---|---|---|---|
| Con | UI | p | Con | UI | p | |||
| At least one peptide (any) |
16 94.1% |
35 89.7% |
0.597 |
13 76.5% |
28 71.8% |
0.716 | ||
| At least S |
15 88.2% |
30 76.9% |
0.327 |
9 52.9% |
29 74.3% |
0.114 | ||
| At least M |
16 94.1% |
27 69.2% |
0.042 |
12 70.6% |
28 71.8% |
0.927 | ||
| At least N |
12 70.6% |
33 84.6% |
0.224 |
5 29.4% |
32 82.1% |
<0.001 | ||
| S + M + N |
9 52.9% |
34 87.2% |
0.005 |
6 35.3% |
35 89.7% |
<0.001 | ||
| S + M |
12 70.6% |
31 79.5% |
0.468 |
12 70.6% |
36 92.3% |
0.033 | ||
| S + N |
17 100% |
37 94.9% |
0.341 |
15 88.2% |
37 94.9% |
0.375 | ||
| M + N |
15 88.2% |
35 89.7% |
0.866 |
15 88.2% |
38 97.4% |
0.159 | ||
| S only |
17 100% |
31 79.5% |
0.043 |
17 100% |
31 79.5% |
0.043 | ||
| M only |
16 94.1% |
35 89.7% |
0.597 |
14 82.3% |
37 94.9% |
0.131 | ||
| N only |
17 100% |
37 94.9% |
0.341 |
17 100% |
37 94.9% |
0.342 | ||
| Negative for all peptides |
16 94.1% |
35 89.7% |
0.597 |
13 76.5% |
28 71.8% |
0.716 | ||
Chi‐square test with statistical significance when P <.005.
Abbreviations: Con, convalescent HCWs; M, SARS‐CoV‐2 membrane; N, SARS‐CoV‐2 nucleocapsid; S, SARS‐CoV‐2 spike; UI, uninfected HCWs.
In parallel, similar concordance between IFN‐γ+ CD4 T‐cell response and soluble IFN‐γ production was found, with 76.5% among convalescent HCWs and 71.8% among uninfected HCWs (P = .716) when comparing the response for at least one viral epitope. The concordance was similar between both groups for the rest of the epitope combinations except for the comparison for the response to at least epitope N (P < 0‐001), to the three epitopes at once (P < .001), to the combination of epitope S and M (P = .033) and epitope S only (P = .043).
4. DISCUSSION
The presence of the SARS‐CoV‐2 cellular immune response in the absence of specific antibodies could be very important for understanding immune protection in the general population. In this study, a high proportion of the uninfected HCWs (53.8%) had pre‐existing positive IFN‐γ+ CD8 T‐cell response after stimulation with at least one of the structural viral proteins S, M or N, and 35.9% had a pre‐existing positive IFN‐γ+ CD4 T‐cell response. Nevertheless and of note, both the proportion and the magnitude of this cellular response were significantly lower compared to the cellular response found in the convalescent HCWs. The data regarding the release of IFN‐γ after SARS. CoV‐2 stimulation was partially reported in a pre‐print format and included for the analysis of the concordance with the CD8/CD4 T‐cell response. 14
Interestingly, the concordance between CD8 T‐cell response and the release of IFN‐γ was high in both convalescent (94.1%) and uninfected HCWs (89.7%), while was around 75% between CD4 T‐cell response and the release of IFN‐γ in both groups, for the response to at least one peptide. On the other hand, the CD8 and CD4 T‐cell responses only to S peptide were completely concordant (100%) to the release of IFN‐γ in convalescent HCWs, while was around 80% in uninfected HCWs. This high concordance validates the use of the cytokine release assays when ELISPOT or flow cytometry, which require more complex laboratory equipment, is not available.
These findings are in agreement with several other studies that have reported the presence of specific T‐cell response, using either ELISPOT or flow cytometry, in almost all SARS‐CoV‐2‐infected and recovered patients. 10 , 11 , 12 In infected and recovered patients, the specific cellular response was directed mainly to structural and nonstructural proteins, while the response to nonstructural proteins was rarely detected in uninfected individuals, measured by the production of IFN‐γ and TNF‐α by CD4 and CD8 T cells. 15 , 16 , 17
Wide rate of response ranged from 30% to 60% has been reported in unexposed healthy individuals. 18 , 19 , 20 , 21 Nevertheless, other studies have reported no T‐cell response in healthy controls. 22 On the other hand, only a few studies have performed IFN‐γ release assays to detect specific T‐cell responses. 23 , 24 In these studies, no specific T‐cell response was detected in healthy unexposed individuals, in contrast to our study in healthy highly exposed individuals. Nevertheless, others reported up to 45% of unexposed subjects with T‐cell response analysed by cytokine release assay, in agreement with our results with 53.8% of T‐cell response, slightly higher rate since these individuals were highly exposed. 21 Besides, none of these studies compared IFN‐γ release with specific CD4/CD8 T‐cell responses, as we do in this study.
The reason why healthy subjects might have a specific SARS‐CoV‐2 T‐cell response remains unclear and is subject to speculation. Many studies have concluded that this response comes from prior contact with seasonal coronaviruses, previous exposure to flu and/or CMV viruses or other microbial agents. 18 , 25 , 26 , 27 In this study, the pre‐existing CD8 T‐cell response was directed mainly to peptides S and M (36.9% and 46.1%, respectively) among uninfected HCWs, which is in accordance with the higher release of IFN‐γ after peptide S stimulation (38.5%). This is important given that the main infective way for SARS‐CoV‐2 is through protein S and its interaction with the human receptor ACE‐2. Of note, the pre‐existing CD8 T‐cell response according to the different epitopes was similar in both groups of HCWs, being the proportion of CD8 T cells that respond to only one of the peptides S or M predominant (20.5%). On the other hand, the proportion of CD8 T cells that respond to the three peptides (S, M and N) together was higher in convalescents (23.5%), although not significant. In parallel, both IFN‐γ+ T‐cell response and soluble IFN‐γ released after stimulation only to peptide S was significantly higher in uninfected HCWs (23.1%) compared to the absence of response in convalescents. Although the response to a different combination of epitopes was less frequently found among uninfected HCWs, no statistical significance was found. The quantification of IFN‐γ after cellular stimulation could reflect in a good and easier way the proportion of IFN‐γ‐producing CD8/CD4 T cells.
Our study has some limitations including the cross‐sectional design that limits the association between exposure and the immune response. Additionally, the number of patients included was limited and there was a selection bias towards the inclusion of subjects including high and prolonged exposed HCWs with negative specific serology as the population of the study. Another limitation of the study was the impossibility of ascertaining that uninfected individuals were certainly never infected since they could have been asymptomatic and with no developing any specific serology, although we consider this unprovable.
In conclusions, this study confirms the high presence of SARS‐CoV‐2‐specific cellular immunity in seronegative highly exposed individuals (53.8%). This specific cellular immunity could be detected by the analysis of specific CD4+ and CD8+ T cells or by the quantification of IFN‐γ release upon SARS‐CoV‐2 stimulation since both methods showed a good concordance. Since IFN‐γ release is simple and can be easily performed in a basic laboratory, it might be widely implemented to quantify T‐cell response in the general population to determine the protective immunity to SARS‐CoV‐2 infection in future situations including post‐vaccine immune status. It is still unknown whether this pre‐existing level of cellular response would be sufficient to limit viral infection, attenuate the disease, or could enhance the response to SARS‐CoV‐2 vaccines.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
AV and JLC designed the study. JLC, PV, AM and CQ recruited the participants for this study. JLC, PV and AV collected data from participants to generate a database. AV performed all the laboratory work. AV and JLC analysed and interpreted the data. AV and JLC prepared the manuscript on the basis of comments from all authors. All authors provided data, reviewed and contributed to the final version of the manuscript and agreed to be accountable for the work.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
We thank all healthcare workers for their participation and Ana Abad for her assistance in database management.
Vallejo A, Vizcarra P, Quereda C, Moreno A, Luis Casado J. IFN‐γ+ cell response and IFN‐γ release concordance after in vitro SARS‐CoV‐2 stimulation. Eur J Clin Invest. 2021;51:e13636. 10.1111/eci.13636
Alejandro Vallejo and José Luis Casado contributed equally.
Funding information
This study was partially supported by Instituto de Salud Carlos III (grant number COV20‐1304)
DATA AVAILABILITY STATEMENT
Requests for materials or data should be addressed to corresponding authors upon request.
REFERENCES
- 1. World Health Organization . Coronavirus Disease 2019. Situation Reports. https://www.who.int/publications/m/item/weekly‐operational‐update‐on‐covid‐19‐‐‐14‐june‐2021 (Accessed May 20, 2021.)
- 2. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID‐19) in China. J Hosp Infect. 2020;105:100‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lahner E, Dilaghi E, Prestigiacomo C, et al. Prevalence of SARS‐CoV‐2 infection in health workers (HWs) and diagnostic test performance: The experience of a teaching Hospital in Central Italy. Int J Environ Res Public Health. 2020;17:4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers. A living rapid review. Ann Intern Med. 2020;M20:1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma H, Zeng W, He H, et al. Serum IgA, IgM, and IgG responses in COVID‐19. Cell Mol Immunol. 2020;17(7):773–775. 10.1101/2020.04.17.20064907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell. 2020;181:1489‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ni L, Ye F, Cheng M‐L, et al. Detection of SARS‐CoV‐2‐specific humoral and cellular immunity in COVID‐19 convalescent individuals. Immunity. 2020;52:971‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Bert N, Tan AT, Kunasegaran K, et al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature. 2020;584(7821):457‐462. [DOI] [PubMed] [Google Scholar]
- 10. Braun J, Loyal L, Frentsch M, et al. SARS‐CoV‐2‐reactive T cells in healthy donors and patients with COVID‐19. Nature. 2020;587(7833):270‐274. 10.1038/s41586-020-2598-9 [DOI] [PubMed] [Google Scholar]
- 11. Peng Y, Menzer AJ, Liu G, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS‐CoV‐2 in UK convalescent individuals following COVID‐19. Nature Immunol. 2020;21:1336‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183:158‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simera I, Moher D, Hoey J, et al. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 14. Vallejo A, Vizcarra P, Quereda C, Moreno A, Casado JL. SARS‐CoV‐2 cellular immune response in uninfected health care workers with prolonged and close exposure to COVID‐19 patients. Pre‐print Research Square, doi:10.21203/rs.3.rs‐55720/v1.
- 15. Bacher P, Rosati E, Esser D, et al. Low‐avidity CD4+ T cell responses to SARS‐CoV‐2 in unexposed individuals and humans with severe COVID‐19. Immunity. 2020;53(6):1258‐1271.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogbe A, Kronsteiner B, Skelly DT, et al. T cell assays differentiate clinical and subclinical SARS‐CoV‐2 infections from cross‐reactive antiviral responses. Nat Commun. 2021;12(1):2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen KW, Linderman SL, Moodie Z, et al. Longitudinal analysis shows durable and broad immune memory after SARS‐CoV‐2 infection with persisting antibody responses and memory B and T cells. medRxiv. 2021. 10.1101/2021.04.19.21255739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Vries RD. SARS‐CoV‐2‐specific T‐cells in unexposed humans: presence of cross‐reactive memory cells does not equal protective immunity. Sig Transduct Target Ther. 2020;5:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lipsitch M, Grad YH, Sette A, Crotty S. Cross‐reactive memory T cells and herd immunity to SARS‐CoV‐2. Nat Rev Immunol. 2020;20:709‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ansari A, Arya R, Sachan S, et al. Immune memory in mild COVID‐19 patients and unexposed donors reveals persistent T cell responses after SARS‐CoV‐2 infection. Front Immunol. 2021;11(12): 636768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Echeverría G, Guevara A, Coloma J, et al. Pre‐existing T‐cell immunity to SARS‐CoV‐2 in unexposed healthy controls in Ecuador, as detected with a COVID‐19 Interferon‐Gamma Release Assay. Int J Infect Dis. 2021;105:21‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell. 2020;183:996‐1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murugesan K, Jagannathan P, Pham TD, et al. Interferon‐γ release assay for accurate detection of severe acute respiratory syndrome coronavirus 2 T‐cell response. Clin Infect Dis. 2020;ciaa1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petrone L, Petruccioli E, Vanini V, et al. A whole blood test to measure SARS‐CoV‐2‐specific response in COVID‐19 patients. Clin Microb Infect. 2021;27:286.e7‐286.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lineburg KE, Grant EJ, Swaminathan S, et al. CD8 + T cells specific for an immunodominant SARS‐CoV‐2 nucleocapsid epitope cross‐react with selective seasonal coronaviruses. Immunity. 2021;54(5):1055‐1065.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sette A, Crotty S. Pre‐existing immunity to SARS‐CoV‐2: the knowns and unknowns. Nature Rev Immunol. 2020;20:457‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan CCS, Owen CJ, Tham CYL, et al. Pre‐existing T cell‐mediated cross‐reactivity to SARS‐CoV‐2 cannot solely be explained by prior exposure to endemic human coronaviruses. bioRxiv. 2020. 10.1101/2020.12.08.415703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
Requests for materials or data should be addressed to corresponding authors upon request.


