Abstract
Background
In 2020, early U.S. COVID‐19 testing sites offered diagnostic capacity to patients and were important sources of epidemiological data about the spread of the novel pandemic disease. However, little research has comprehensively described American testing sites’ distribution by race/ethnicity and sought to identify any relation to known disparities in COVID‐19 outcomes.
Methods
Locations of U.S. COVID‐19 testing sites were gathered from 16 April to 28 May 2020. Geographic testing disparities were evaluated with comparisons of the demographic makeup of zip codes around each testing site versus Monte Carlo simulations, aggregated to statewide and nationwide levels. State testing disparities were compared with statewide disparities in mortality observed one to 3 weeks later using multivariable regression, controlling for confounding disparities and characteristics.
Results
Nationwide, COVID‐19 testing sites geographically overrepresented White residents on 7 May, underrepresented Hispanic residents on 16 April, 7 May and 28 May and overrepresented Black residents on 28 May compared with random distribution within counties, with new sites added over time exhibiting inconsistent disparities for Black and Hispanic populations. For every 1 percentage point increase in underrepresentation of Hispanic populations in zip codes with testing, mortality among the state's Hispanic population was 1.04 percentage points more over‐representative (SE = 0.415, p = .01).
Conclusions
American testing sites were not distributed equitably by race during this analysis, often underrepresenting minority populations who bear a disproportionate burden of COVID‐19 cases and deaths. With an easy‐to‐implement measure of geographic disparity, these results provide empirical support for the consideration of access when distributing preventive resources.
Abbreviation
- SE
standard error
1. INTRODUCTION
As of 16 May 2021, the COVID‐19 pandemic has taken the lives of more than five hundred thousand Americans. 1 In the United States, the disease has had an outsized impact on Black and Hispanic populations and in geographic areas with more Black and Hispanic residents. 2 , 3 , 4 , 5 , 6
In the COVID‐19 pandemic, diagnostic testing is a central tool of controlling outbreaks because it provides information about individual and community spread. 7 , 8 , 9 However, analyses of New York City, Philadelphia and Chicago have shown that early in the outbreak, disadvantaged communities had lower incidence of testing, and those same areas continued to have higher test positivity rates. 10 , 11 , 12 The geographic distribution of preventive resources is of particular concern. Limitations to transportation already mean that millions of American adults cannot obtain medical care every year, disproportionately affecting minority populations. 13 Additionally, unequal distribution of healthcare resources means that minority neighbourhoods often have fewer clinics and providers, and the initial placement of COVID‐19 testing sites was concentrated in this existing health infrastructure, possibly compounding disparities. 14 , 15 Nationwide analysis has shown that travel time from a county to a COVID‐19 testing facility increases with the proportion of residents of colour in the county. 16
In previous pandemics, inequities in access to health care have been related to disparities in outcomes, especially in Hispanic populations in the United States. 17 Testing access might be related to disease outcomes if more accessible testing means earlier or more consistent detection, leading to better self‐isolation and earlier presentation for care. 7 , 8 , 9 Simply, testing arms individuals and communities with information, which they can use to reduce untreated disease and unmitigated spread. In Massachusetts, testing site accessibility, based on driving time, was negatively associated with COVID‐19 incidence, although such an effect has not been further generalized. 18 If testing access was in fact instrumental to reducing incidence, it is important to understand the performance of localities in distributing COVID‐19 testing sites equitably. By gaining a better understanding of the effectiveness of testing policies now, in future public health emergencies, resource distribution can be improved. As of writing, the authors know of no research that has compared U.S. testing distribution by race and ethnicity across individual states and nationally.
The goal of this study was to examine the racial and ethnic composition of neighbourhoods surrounding testing sites for the SARS‐CoV‐2 virus (COVID‐19 testing) and associated impacts on disease‐related morality. Our first objective was to evaluate whether COVID‐19 testing sites in each state were located in neighbourhoods (defined by zip code) that were demographically differentiable from neighbourhoods of simulated sites when they are placed at random. These disparities were calculated for the nation as a whole and each state, and changes in the testing distribution over time were described. We hypothesize that these disparities underrepresent Black and Hispanic populations but even out in later dates of analysis. Our second objective was to examine the correlation between such testing site disparities and racial/ethnic disparities in disease‐specific mortality. We hypothesize that racial and ethnic groups that are underrepresented in neighbourhoods near testing sites will have disproportionately high COVID‐19 mortality.
2. METHODS
2.1. Testing disparity analysis
2.1.1. Data and variables
Data on the locations of COVID‐19 testing centres were gathered from www.FindCovidTesting.com 19 and the COVID‐19 Testing Site Finder from Castlight Health Inc. (‘Castlight data’). 20 Both data sources have community input, so site operators and users can submit information on local sites, and the Castlight data incorporate locations directly from some testing providers. These sources were combined and duplicates removed to create a combined data set that represents the most accurate available count of nationwide testing with granular location data. To validate the inclusivity of the data set, we audited random counties in comparison with state public health websites. In every investigated county, all of the testing sites from the state websites were included in at least one of the two data sources (see Supplemental Section 1).
We conducted our analysis on data from 16 April, 7 May and 28 May 2020 (3‐week gaps). The 16 April analysis included 2821 testing sites. The 7 May analysis added 6081 new sites and dropped 556 from the data set (possible site closure or address changes) for a total of 8346 sites. The 28 May analysis added 3821 new sites and dropped 1886 for a total of 10,281 sites. Zip codes were approximated for demographic purposes using ZIP Code Tabulation Areas. 5 , 10 , 11
For demographic data, we used the US Census Bureau's 2014–2018 American Community Survey 5‐year (ACS). Variables included total population, Hispanic or Latino of any race (Hispanic), non‐Hispanic White alone (White), and non‐Hispanic Black or African American alone (Black).
2.1.2. Statistical analysis
To create a baseline for our analysis, we employed Monte Carlo simulation of 10,000 randomized testing site distributions for each date of analysis. Some states have high intra‐state and inter‐county variation in the demographic characteristics that are risk factors for high COVID‐19 mortality. 21 The need for testing may be more acute in those high‐risk areas. A completely random distribution of testing sites across the country or state would be naïve to this fact, so we only moved testing sites within their county. Simulated testing sites were proportionally likely to be located within each of the zip codes in the county based on the population of each zip code.
Testing disparities were calculated in each investigated jurisdiction (nationwide, aggregating all testing sites gathered, and in each state, DC, and Puerto Rico) for each demographic group (Black, Hispanic, White). For each group, aggregate proportion in a jurisdiction was the average population percentage in each zip code surrounding each of the testing sites within the jurisdiction (Figure 1) (see Supplemental Section 2 for sensitivity testing and discussion of this measure). Disparities were the difference between the aggregate proportion of the group for the empirical testing sites and the mean aggregate proportion of the group in the Monte Carlo simulations. Thus, they show whether, on aggregate, testing sites were placed in zip codes that evenly represented the group compared with random placement in their counties (disparity of zero), overrepresented the group compared with random placement in their counties (positive disparity) or underrepresented the group compared with random placement in their counties (negative disparity).
FIGURE 1.
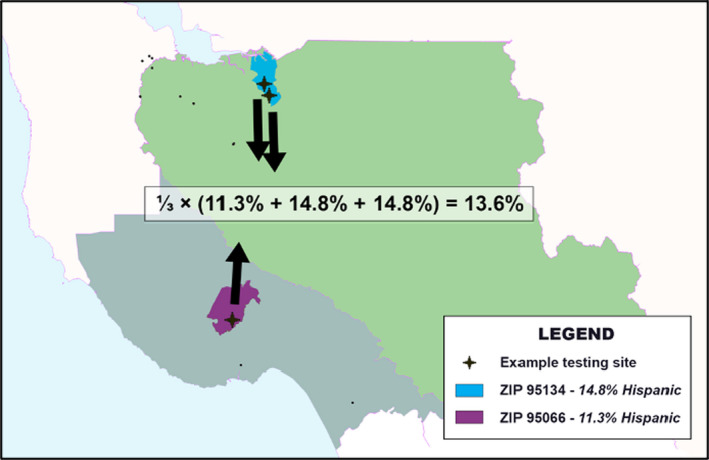
Demonstration of aggregate proportion calculation with three arbitrary testing sites. Three testing sites in California were arbitrarily chosen to demonstrate how the aggregate proportion in ZCTAs around testing sites is calculated. The Hispanic proportion was calculated for just these three sites. One site is in zip code 95066 (purple), which is 11.3% Hispanic. The other two sites are in zip code 95134 (blue), which is 14.8% Hispanic. For these three sites, the aggregate proportion is 13.6 per cent. In this study, all testing sites in the state or the country would be aggregated in this way for both the empirical and simulated testing distributions. The empirical aggregate proportion is compared to the mean‐simulated aggregate proportion to calculate the testing disparity
p‐values for disparities are empirical p‐values, and two‐tailed hypothesis tests were used with an a priori significance level of p < .05. Our null hypothesis was that, within counties, testing sites would be distributed randomly with respect to race, so we would not be able to differentiate the demographics of zip codes surrounding the empirical testing sites from demographics of zip codes surrounding simulated random testing site distributions.
2.2. Mortality disparity analysis
2.2.1. Data and variables
While perhaps still biased by unidentified infections, mortality is a more apt measure for determining effects of testing than total COVID‐19 cases because its identification is less dependent on testing. 22 Mortality data were gathered from The COVID Tracking Project, which reports COVID‐19 deaths by race/ethnicity in each reporting state. 23 For each date of testing disparity analysis, we gathered deaths from the 2‐week range approximately 1 to 3 weeks after testing analysis, when death for COVID‐19 patients who became symptomatic on the date of testing analysis is most likely. 24 While lags in mortality data reporting could be a concern, empirical estimates of the time from an uptick of new cases to the resulting uptick in reported deaths have been similar to this range, giving us confidence that this time window will capture much of the related mortality. 25 Given data availability, the best ranges were 22 April to 6 May, 13 May to 27 May and 3 June to 17 June for testing analyses on 16 April, 6 May and 28 May, respectively. States and territories individually report deaths related to COVID‐19 in their jurisdiction. Data availability increased in later date ranges as more states began to report race and/or ethnicity with COVID‐19 deaths. For Black and Hispanic populations, we calculated statewide mortality disparities for each range as the difference between the proportion of deaths from COVID‐19 in the range of dates that were of people of that race/ethnicity and the proportion of the state's population that was of that race/ethnicity.
2.2.2. Regression analysis and controls
We employed multivariable regression analyses to evaluate the associations between statewide disparities in the distribution of testing and statewide disparities in COVID‐19 mortality. Regressions included analytical weights for the number of total deaths in the date range, and within‐effects are reported for the three sets of state disparities defined by date.
Confounding variables identified in a directed acyclic graph (DAG) were included as controls in regression analysis (see Supplementary Figure). These included Black and Hispanic populations (%), 26 poverty rate disparity versus the general population, 27 medical comorbidity disparity versus the general population, health access disparity versus the general population, age disparity in proportion of the group who were elderly versus the general population and segregation.
Population and poverty rate data were from the U.S. Census Bureau, as reported by the Kaiser Family Foundation [KFF], and age data were from the 2018 ACS. 26 , 27 Medical comorbidity disparity controls included the difference in prevalence for the associated group versus the general population of three leading comorbidities: 28 diabetes (from United Health Foundation), 29 hypertension (United Health Foundation) 29 and obesity (Centers for Disease Control and Prevention [CDC]). 30 For both Black and Hispanic populations, to provide a summary of the three comorbidities, we extracted the first component from a principal component analysis (PCA) to generate a comorbidity disparity variable with loadings of each of the disparities (see Supplemental Table S3a for PCA loadings). Health access disparity was the first component extracted from a PCA to generate a health access disparity variable with loadings of differences in responses to three self‐reported health access questions from the CDC’s Behavioral Risk Factor Surveillance System Survey (see Supplemental Table S3b for questions and PCA loadings). 31 We included a dissimilarity index for Black and Hispanic populations (Frey) as our measure of residential segregation, choosing it for its independence from underlying racial composition. This index indicates what percentage of the corresponding minority population would have to relocate in order to be distributed exactly like the White population in the state. 32
3. RESULTS
3.1. Nationwide disparities in testing site locations
We described the demographic differences between the zip codes with testing and their corresponding counties. The national differences in neighbourhood racial and ethnic composition for the empirical testing site distribution and the mean random‐simulated distribution are shown over time in Table 1. The change in mean randomly simulated distribution over time shows the changing distribution of counties with testing sites.
TABLE 1.
Nationwide aggregate demographic proportions in zip codes around empirical testing sites compared to random placement
| Date | Tranche (Sites analysed of new sites) | Geography | White | Hispanic | Black | |||
|---|---|---|---|---|---|---|---|---|
| Aggregate proportion (standard deviation) | p‐value of disparity | Aggregate proportion (standard deviation) | p‐value of disparity | Aggregate proportion (standard deviation) | p‐value of disparity | |||
| Apr 16 | CUMULATIVE (2821 sites) | Empirical zip codes with testing sites | 63.03% | .32 | 14.76% | .002* | 11.99% | .20 |
| Mean for randomly distributed sites | 63.25% (0.32%) | 15.39% (0.22%) | 11.79% (0.24%) | |||||
| May 7 |
NEW SITES (6081 sites) |
Empirical zip codes with testing sites | 63.39% | <.001* | 17.46% | <.001* | 10.49% | .28 |
| Mean for randomly distributed sites | 62.59% (0.21%) | 18.59% (0.15%) | 10.58% (0.15%) | |||||
|
CUMULATIVE (8346 total sites) |
Empirical zip codes with testing sites | 63.46% | .001* | 16.64% | <.001* | 10.81% | .32 | |
| Mean for randomly distributed sites | 62.89% (0.18%) | 17.63% (0.13%) | 10.87% (0.13%) | |||||
| May 28 |
NEW SITES (3821 sites) |
Empirical zip codes with testing sites | 61.03 | <.001* | 15.99% | .22 | 15.21% | <.001* |
| Mean for randomly distributed sites | 62.30% (0.28%) | 15.85% (0.18%) | 14.17% (0.22%) | |||||
|
CUMULATIVE (10,281 total sites) |
Empirical zip codes with testing sites | 62.81% | .06 | 16.00% | <.001* | 12.53% | .002* | |
| Mean for randomly distributed sites | 63.07% (0.17%) | 16.44% (0.11%) | 12.17% (0.13%) | |||||
*Indicates significance at p < .05.
Aggregate proportion is the average demographic proportion of a given race in zip codes, either those around empirical testing sites or those around random simulations of testing site distributions. From the random Monte Carlo Simulations, the mean aggregate proportion is reported along with standard deviation.
3.1.1. Testing disparities, 16 April 2020
In the testing site location set on 16 April 2020 (n = 2821), empirical testing sites were in zip codes that had significantly lower Hispanic populations (14.76%) than would be expected in a random testing site distribution (15.39%, SD = 0.22%) (p = .002). The aggregate representations of Black and White populations in zip codes with testing sites were not significantly different from simulated distributions on 16 April.
3.1.2. Testing disparities, 7 May 2020
The new testing sites (n = 6081) added between 16 April and 7 May significantly overrepresented White populations in zip codes with testing (63.39% in zips vs. 62.59% expected, p < .001) and underrepresented Hispanic residents (17.46% in zips vs 18.59% expected, p < .001). However, these new testing sites were in counties with a higher Hispanic population than the baseline in April, so the absolute representation of Hispanic populations in zip codes with testing increased. Concurrently, these new testing sites were in counties with a lower Black population than the baseline in April, so the absolute representation of Black populations in zip codes with testing decreased from 16 April to 7 May.
3.1.3. Testing disparities, 28 May 2020
In a reversal from the previous testing sites, the new testing sites (n = 3821) added between 7 May and 28 May significantly underrepresented White populations in zip codes with testing (61.03% in zips vs. 62.30% expected, p < .001) and overrepresented Black populations in zip codes with testing (15.21% in zips vs 14.17% expected, p < .001) compared with the counties. Compared to the baseline of the cumulative testing sites as of 7 May, these new sites were in counties with higher Black populations (p < .001) and lower White populations and Hispanic populations (p = .04 and p < .001, respectively).
3.2. Total demographic representation near testing sites
We also examined differences between the aggregate racial makeup of the neighbourhoods/counties with testing sites and the nation as a whole. While 12.3% of the overall U.S. population recorded in the data source was Black, Black residents made up as little as 10.49% of the population of zip codes with testing sites on 7 May. While 17.8% of the US population was Hispanic, Hispanic residents had their lowest representation in the 16 April data set, when the population made up as little as 14.76% of people living in zip codes with testing on 16 April.
3.3. State‐by‐state disparities in testing site location
We sought to identify disparities in individual states and territories, as testing policies have varied by locality and the virus has not had a uniform effect. 33 At each date of analysis, states with statistically significant disparities for Hispanic and/or Black populations are graphed in Figure 2. (For detailed data by jurisdiction, see Supplemental Tables S4a–c.). On the final date of analysis (28 May), the states/territories with the largest disparities that underrepresented Black populations were DC, PA and VA. Throughout the analysis, the disparity for Black residents in DC was the largest of any group. On 28 May, the states with the largest disparities that underrepresented Hispanic populations were in AZ, CA and TX.
FIGURE 2.
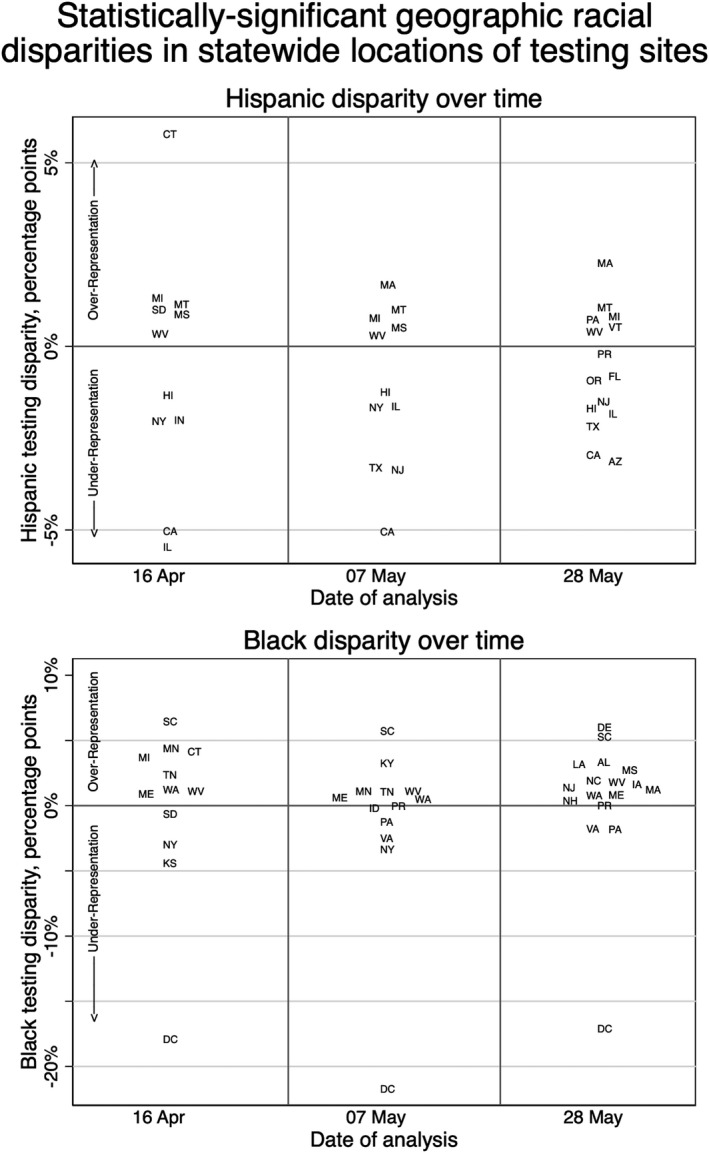
Statistically significant statewide racial and ethnic disparities in testing geography. For both Hispanic and non‐Hispanic Black, states with significant disparities (p‐value <.05) are distributed along the y‐axis based on the percentage point disparity between the per cent makeup of the zip codes containing testing sites and per cent makeup of surrounding counties. Positive (negative) testing disparities indicate that the racial/ethnic group is overrepresented (underrepresented) in the zip codes with testing compared to the surrounding counties. Horizontal separation is only to make state abbreviations legible and does not convey meaning
3.4. Association of disparities in testing site distribution and COVID‐19–related mortality
We sought to investigate the role of COVID‐19 testing on outcomes by describing the relationship between disparities in testing location and eventual disparities in disease‐specific mortality. The simple linear relationship between testing disparity and mortality disparity is negative for both groups and all dates expect for Black populations on the last date of analysis, 28 May (Figure 3). Controlled for statewide confounding variables as described in the Methods, the relationship between testing disparities and mortality disparities was significant in Hispanic populations (b = −1.04, SE = 0.415, p = .01). These results mean that, ceteris paribus, a one percentage point decrease in the representation of Hispanic people near testing sites leads to a 1.04 percentage point increase in Hispanic mortality disparity. The relationship for Black testing disparity and mortality disparity did not meet statistical significance with these controls. (Supplemental Table S5).
FIGURE 3.
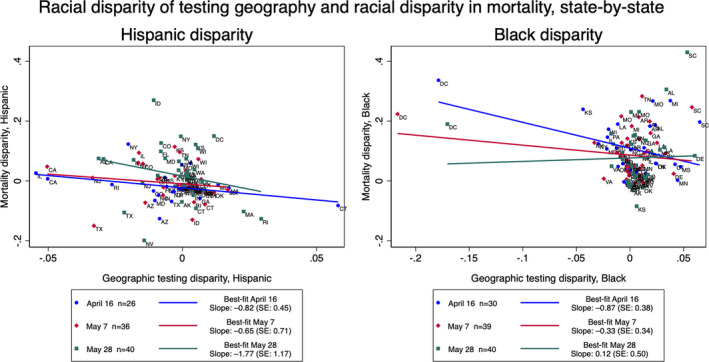
Disparity of testing geography and disparity in mortality, state‐by‐state. For Hispanic and non‐Hispanic Black and all dates of analysis, statewide disparities in the makeup of zip codes and what would be expected from random distribution are plotted against the statewide disparity in mortality, comparing the group's proportion of total state population to its proportion of COVID‐19 deaths 1 to 3 weeks later with known race/ethnicity. Positive (negative) testing disparities indicate that the racial/ethnic group is overrepresented (underrepresented) in the zip codes with testing compared with the surrounding counties. Positive (negative) mortality disparities indicate that members of the racial/ethnic group are overrepresented (underrepresented) among deaths compared to what would be expected given the proportion of the racial/ethnic group in the state. Best‐fit lines show simple linear regression between the two disparities for each date of analysis. A version of this figure without DC and CT, the two notable outliers, is available in the supplement (Supplemental Figure 2)
4. DISCUSSION
This study sought to describe the racial and ethnic disparities in the distribution of COVID‐19 testing sites in the United States and to examine potential effects of the distribution of testing sites on disease outcomes. By comparing the demographics of the neighbourhoods with testing sites to simulated random distributions of testing sites, we found disparities in the nationwide distribution of COVID‐19 testing sites. While there were changes over the timeframe with the addition of new testing sites, they do not show a consistent trend of remediation nor expansion of disparities.
Nationally, Hispanic populations were consistently underrepresented in neighbourhoods with testing compared what would be expected with a random distribution at all dates of analysis (16 April, 7 May, 28 May). Testing sites added between 16 April and 7 May were in counties with higher Hispanic populations than before, but within those counties, the testing sites were in zip codes with lower Hispanic populations than would be expected with random distribution. After 7 May, the testing sites added were in counties with lower Hispanic populations. Cumulatively, however, the counties with testing sites on 28 May had significantly higher Hispanic representation than the original testing sites on 16 April.
The new testing sites’ representation of Black populations was different, perhaps suggesting disparities for Black and Hispanic populations are not naturally resolved in tandem. New testing sites added from 16 April to 7 May were in counties with lower Black population than existing sites. Conversely, new testing sites from 7 May to 28 May were in counties with higher Black populations than existing sites, and within those counties, sites were in zip codes that overrepresented the Black population compared to what would be expected with random distribution. The cumulative testing sites on 28 May were in zip codes with higher Black populations would be expected with random distribution, and compared to original testing sites on 16 April, testing sites were in counties that had slightly greater Black representation.
This study also examined testing distribution in each state, Puerto Rico and DC. To our knowledge, this is the first study to compare racial and ethnic disparities in the distribution of COVID‐19 resources in the states. This granularity may be more suitable for policy action, given that the responsibility for testing strategy and resource distribution falls largely on states and localities in the United States. 33 On the final date of analysis (28 May), the states/territories with the largest disparities that underrepresented Black populations in neighbourhoods with testing were DC, PA and VA, and the largest disparities that underrepresented Hispanic populations were in AZ, CA and TX. These states are all in the top half of states by population percentage of the noted demographic group, meaning policies in these states have larger populations to affect. However, this does not explain the entire effect: some states with large minority populations had small disparities or overrepresented minority groups near testing. Further research should examine how specific policies or characteristics of states affected COVID‐19 testing site distribution by race/ethnicity.
To assess associations with testing disparities, we used regression analyses to examine the relationship between the testing disparities affecting Black and Hispanic populations and the eventual disparity in COVID‐19 mortality. State testing disparity was negatively associated with state mortality disparity, meaning states with testing in neighbourhoods that underrepresented one racial population on a date of analysis were more likely to report a higher rate of mortality in the underrepresented population over the next few weeks. We found that for Hispanic populations, when controlling for confounding disparities and state characteristics, underrepresentation of Hispanic individuals in zip codes with testing sites was significantly associated with higher mortality among that group compared with their population size (b = –1.04, SE = 0.415, p = .01).
These results provide further evidence supporting efforts to increase testing availability in underserved neighbourhoods. 34 It may be prudent to aim for a disparity in the opposite direction, where minority residents are overrepresented near testing sites, as Black and Hispanic populations are overrepresented in the burden of the disease. Performing need‐based assessment based on racial composition of the county/state prior to establishing testing sites, so as to provide resources to communities that are most in need, may help reduce disparities in access to testing and mortality related to COVID‐19. Other policies may include improving testing availability at existing community centres that have previously improved healthcare access for Hispanic patients, expanding culturally competent testing to ensure language and fear of stigmatization or deportation are not barriers to care and reducing non‐geographical barriers to testing, such as eliminating out‐of‐pocket costs, given people of colour are less likely to report confidence in their ability to overcome the negative effects of COVID‐19 infection, including being able to afford testing. 35 , 36 , 37 While the most effective point of change is likely at the first rollout of testing, any remaining testing disparities should be ameliorated. In order to guide this improvement, more state‐ and county‐level racial and ethnic data should be made available on COVID‐19 tests administered (positive and negative).
This study had multiple limitations. First, it relied on the accuracy of the underlying data on COVID‐19 testing locations, although we used two data sources to try to maximize coverage, and we audited for accuracy. It is possible community input varies with the demographic makeup of the area and that missing data are thus biased by race. Additionally, testing site data did not have the necessary detail to assess capacity or temporary closures, another possible source of bias. Finally, medical settings that offered testing to only inpatient populations may have acted as a testing site of last resort if individuals admitted themselves to be tested, but these may not have been included in the data set. Future studies should examine the distribution of testing sites at more granular levels for increased precision. Second, we were only able to use the demographics of the neighbourhoods around testing locations and not data about the actual tests administered because too few states report demographic data on testing incidence. Third, zip codes are of varying shape and size and of course can be crossed, so their actual predictive power for testing access may be different in different regions, although we did employ sensitivity tests. Fourth, testing access disparities may have varied within counties or states, which may give more insight into the effect of testing, but our analysis does not investigate beyond averages. Finally, the observed correlation between testing site disparities and rates of mortality does not infer causality, but rather a relationship that warrants further investigation and validation in future studies.
5. CONCLUSIONS
Across the United States and in many states, testing sites for COVID‐19 are not equitably distributed, often underrepresenting minority populations who have the highest need for testing. During the course of this study, even after new sites were added with improved Hispanic representation, testing sites nationwide were in neighbourhoods that underrepresented the Hispanic population of their county. States with distributions of testing sites that underrepresented Hispanic residents were more likely to report higher mortality rates among the Hispanic population.
How an institution acts under stress reveals its weaknesses and fracture lines. The early period of the COVID‐19 pandemic investigated here was one of systemic stress and fear, and the response to distribute testing sites was, in many places, not equitable. Disparities in testing during this pandemic should be a consideration for future epidemiological responses and should inform how the healthcare system prepares for emergencies. Most tactically, this study offers a framework through which to expand equitable access and an easy‐to‐implement measure for distribution disparities: new (testing) site placement should be informed by the racial and ethnic makeup of the immediate neighbourhood in the context of the wider county, and existing (testing) sites should be examined for remaining inequity in geographic distribution. For simplified use, this measure can be implemented by comparing the aggregated demographic makeup of the zip codes surrounding (testing) sites to the total demographic makeup of the county. More broadly, we should work to build systems to ensure that, even under great stress, emergency responses do not amplify those disparities. For example, the initial placement of COVID‐19 testing sites often began with existing health infrastructure, 15 and there are known structural disadvantages in access to healthcare infrastructures such as clinics and providers in minority neighbourhoods. 14 Long‐term change should aim to reduce disparities in access to healthcare resources among Black and Hispanic communities.
ACKNOWLEDGEMENTS AND DISCLOSURE
The authors would like to thank the many groups whose publicly available data were essential to this study. We would also like to thank the editors and reviewers who improved this work. Author ND was employed by Detect, a biotechnology company with a COVID‐19 test in development, following the completion of the analysis and discussion contained here. Detect played no role in the completion of this work.
Supporting information
Supplementary Material
Dalva‐Baird NP, Alobuia WM, Bendavid E, Bhattacharya J. Racial and ethnic inequities in the early distribution of U.S. COVID‐19 testing sites and mortality. Eur J Clin Invest. 2021;51:e13669. 10.1111/eci.13669
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
REFERENCES
- 1. Centers for Disease Control and Prevention . Cases of Coronavirus Disease (COVID‐19) in the U.S. Published online 2020. https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/cases‐in‐us.html
- 2. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID‐19 on black communities. Ann Epidemiol. 2020;47:37‐44. 10.1016/j.annepidem.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gross CP, Essien UR, Pasha S, Gross JR, Wang S‐Y, Nunez‐Smith M. Racial and ethnic disparities in population‐level Covid‐19 mortality. J Gen Intern Med. 2020;35(10):3097‐3099. 10.1007/s11606-020-06081-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahajan UV, Larkins‐Pettigrew M. Racial demographics and COVID‐19 confirmed cases and deaths: a correlational analysis of 2886 US counties. J Public Health. 2020;42(3):445‐447. 10.1093/pubmed/fdaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maroko AR, Nash D, Pavilonis BT. COVID‐19 and inequity: a comparative spatial analysis of New York city and Chicago hot spots. J Urban Heal. 2020;97(4):461‐470. 10.1007/s11524-020-00468-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez‐Diaz CE, Guilamo‐Ramos V, Mena L, et al. Risk for COVID‐19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics. Ann Epidemiol. 2020;52:46‐53.e2. 10.1016/j.annepidem.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marcel S, Althaus CL, Neher R, et al. COVID‐19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly. 2020;150:w20225. 10.4414/smw.2020.20225 [DOI] [PubMed] [Google Scholar]
- 8. Rosenthal PJ. The importance of diagnostic testing during a viral pandemic: early lessons from novel Coronavirus Disease (COVID‐19). Am J Trop Med Hyg. 2020;102(5):915‐916. 10.4269/AJTMH.20-0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z. Considerations for diagnostic COVID‐19 tests. Nat Rev Microbiol. 2020;19(3):171‐183. 10.1038/s41579-020-00461-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bilal U, Tabb LP, Barber S, Diez Roux AV. Spatial inequities in COVID‐19 testing, positivity, confirmed cases, and mortality in 3 U.S. cities. Annals of Internal Medicine. 2021;174(7):936. –944. 10.7326/m20-3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borjas GJ. Demographic determinants of testing incidence and COVID‐19 infections in New York city neighborhoods. SSRN Electron J. 2020. HKS Working Paper No. RWP20‐008. 10.2139/ssrn.3572329 [DOI] [Google Scholar]
- 12. Lieberman‐Cribbin W, Tuminello S, Flores RM, Taioli E. Disparities in COVID‐19 testing and positivity in New York City. Am J Prev Med. 2020;59(3):326‐332. 10.1016/j.amepre.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wallace R, Hughes‐Cromwick P, Mull H, Khasnabis S. Access to health care and nonemergency medical transportation: two missing links. Transp Res Rec. 2005;1924:76‐84. 10.3141/1924-10 [DOI] [Google Scholar]
- 14. Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404‐416. 10.1016/S0033-3549(04)50068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen J. ‘We’re behind the curve’: U.S. hospitals confront the challenges of large‐scale coronavirus testing. Science (80). 2020. 10.1126/science.abb6856 [DOI] [Google Scholar]
- 16. Rader B, Astley CM, Sy KTL, et al. Geographic access to United States SARS‐CoV‐2 testing sites s healthcare disparities and may bias transmission estimates. J Travel Med. 2020;27(7):1–4. 10.1093/jtm/taaa076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quinn SC, Kumar S, Freimuth VS, Musa D, Casteneda‐Angarita N, Kidwell K. Racial disparities in exposure, susceptibility, and access to health care in the US H1N1 influenza pandemic. Am J Public Health. 2011;101(2):285‐293. 10.2105/AJPH.2009.188029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu T, Yue H, Wang C, et al. Racial segregation, testing site access, and COVID‐19 incidence rate in Massachusetts, USA. International Journal of Environmental Research and Public Health. 2020;17(24):9528. 10.3390/ijerph17249528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caballero J, Kemendo A, DiMarco A, Coders Against COVID. FindCovidTesting.com. Published 2020. Accessed May 28, 2020. www.findcovidtesting.com
- 20. Castlight Health Inc. Castlight COVID‐19 Test Site Finder. Published 2020. Accessed May 28, 2020. https://my.castlighthealth.com/corona‐virus‐testing‐sites/
- 21. Chin T, Kahn R, Li R, et al. US‐county level variation in intersecting individual, household and community characteristics relevant to COVID‐19 and planning an equitable response: a cross‐sectional analysis. BMJ Open. 2020;10(9):e039886. 10.1136/bmjopen-2020-039886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bialek S, Bowen V, Chow N, et al. Geographic differences in COVID‐19 cases, deaths, and incidence — United States, February 12–April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465‐471. 10.15585/mmwr.mm6915e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madrigal A, Meyer R. The COVID Tracking Project. The COVID Tracking Project.
- 24. Chen Y, Li T, Ye Y, Chen Y, Pan J. Impact of fundamental diseases on patients with COVID‐19. Disaster Med Public Health Prep. 2020;14(6):776‐781. 10.1017/dmp.2020.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin R. The lag between daily reported Covid‐19 cases and deaths and its relationship to age. J Public Health Res. 2021. 10.4081/jphr.2021.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaiser Family Foundation . Population Distribution by Race/Ethnicity. Published online 2019. https://www.kff.org/other/state‐indicator/distribution‐by‐raceethnicity/
- 27. Kaiser Family Foundation . Poverty Rate by Race/Ethnicity. Published online 2019. https://www.kff.org/other/state‐indicator/poverty‐rate‐by‐raceethnicity/
- 28. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York city area. J Am Med Assoc. 2020;323(20):2052–2059. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. United Health Foundation . America’s health rankings analysis of CDC, behavioral risk factor surveillance system. AmericasHealthRankings.org
- 30. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Nutrition, Physical Activity, and Obesity . Data, Trend and Maps [online].
- 31. Centers for Disease Control and Prevention . Behavioral risk factor surveillance system survey questionnaire.
- 32. Frey WH. New Racial Segregation Measures for Large Metropolitan Areas: Analysis of the 1990‐2010 Decennial Censuses. University of Michigan Social Science Data Analysis Network. [Google Scholar]
- 33. Kettl DF. States divided: the implications of American federalism for COVID‐19. Public Admin Rev. 2020;80(4):595‐602. 10.1111/puar.13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tromberg BJ, Schwetz TA, Pérez‐Stable EJ, et al. Rapid scaling up of Covid‐19 diagnostic testing in the United States — the NIH RADx initiative. N Engl J Med. 2020;383(11):1071‐1077. 10.1056/NEJMsr2022263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pérez‐Escamilla R, Garcia J, Song D. Health care access among hispanic immigrants: ¿alguien está escuchando?[is anybody listening?]. NAPA Bulletin. 2010;34(1):47‐67. 10.1111/j.1556-4797.2010.01051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown IM, Khan A, Slocum J, Campbell LF, Lacey JR, Landry AM. COVID‐19 disparities and the black community: a health equity‐informed rapid response is needed. Am J Public Health. 2020;110(9):1350‐1351. 10.2105/AJPH.2020.305804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alobuia WM, Dalva‐Baird NP, Forrester JD, Bendavid E, Bhattacharya J, Kebebew E. Racial disparities in knowledge, attitudes and practices related to COVID‐19 in the USA. J Public Health. 2020;42(3):470‐478. 10.1093/pubmed/fdaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


