Abstract
During the COVID‐19 pandemic, schools around the world rapidly transitioned from in‐person to remote learning, providing an opportunity to examine the impact of in‐person vs remote learning on sleep, circadian timing, and mood. We assessed sleep‐wake timing using wrist actigraphy and sleep diaries over 1‐2 weeks during in‐person learning (n = 28) and remote learning (n = 58, where n = 27 were repeat assessments) in adolescents (age M ± SD = 12.79 ± 0.42 years). Circadian timing was measured under a single condition in each individual using salivary melatonin (Dim Light Melatonin Onset; DLMO). Online surveys assessed mood (PROMIS Pediatric Anxiety and Depressive Symptoms) and sleepiness (Epworth Sleepiness Scale – Child and Adolescent) in each condition. During remote (vs in‐person) learning: (i) on school days, students went to sleep 26 minutes later and woke 49 minutes later, resulting in 22 minutes longer sleep duration (all P < .0001); (ii) DLMO time did not differ significantly between conditions, although participants woke at a later circadian phase (43 minutes, P = .03) during remote learning; and (iii) participants reported significantly lower sleepiness (P = .048) and lower anxiety symptoms (P = .006). Depressive symptoms did not differ between conditions. Changes in mood symptoms were not mediated by sleep. Although remote learning continued to have fixed school start times, removing morning commutes likely enabled adolescents to sleep longer, wake later, and to wake at a later circadian phase. These results indicate that remote learning, or later school start times, may extend sleep and improve some subjective symptoms in adolescents.
Keywords: adolescence, circadian rhythms, COVID‐19, melatonin, remote learning, school start times, sleep
1. INTRODUCTION
Adolescents often obtain less than the recommended 9 hours of sleep on school nights,1 resulting in chronic sleep restriction.2 One reason for this sleep loss is that the circadian clock that regulates the timing of sleep shifts later during adolescence,3 resulting in later subjective sleepiness and truncated sleep duration when paired with early school start times.2 Sleep restriction is associated with reduced cognitive function,4 daytime sleepiness,5 poor emotion regulation,6 and negative mood.7, 8 Not surprisingly, later school start times increase the amount of time available for sleep (ie, sleep opportunity9, 10, 11) and improve school performance, daytime functioning, mood, and school attendance.12, 13, 14
The COVID‐19 pandemic and associated mitigation strategies led to widespread restrictions to behavior and movement, including changes in the mode of education delivery. These restrictions were particularly stringent in Melbourne, Australia, which underwent approximately 7 months of lockdown. Schools commenced remote learning during Term 2 (14 April 2020) and continued through to early Term 4 (approximately October 12, 2020), with students participating in schooling via online platforms. While schools continued to schedule set start times for secondary school students, the need to prepare for and commute to school was removed or reduced. This change provided a unique opportunity to examine whether remote learning would affect sleep duration, circadian timing, and mood.
A prepandemic study in the United States found that home‐schooled students had later self‐reported sleep timing and longer sleep duration compared to students attending schools in person.15 Survey studies indicate that children and adolescents report sleeping longer during COVID‐related lockdown.16, 17, 18, 19 These studies did not include objective measures of sleep or circadian timing, and only two reported prepandemic assessments.16, 19 Studies in young adults and adults also report significant mood disturbances during the COVID‐19 pandemic,20, 21 though research in adolescents is limited. In this study, we used both self‐report and objective measures to examine whether the COVID‐19 induced change in school mode (in‐person vs remote learning) was associated with changes in sleep, circadian timing, and mood in early adolescents aged 12‐13 years. Sleep onset, wake time, and sleep duration were also examined as potential mediators of changes in mood and daytime sleepiness.
2. METHODS
2.1. Participants
Participants were in the first year of secondary schooling (Year 7; N = 59; 33 females, age M ± SD = 12.8 ± 0.4 years) in Melbourne, Australia, who were part of an ongoing longitudinal study examining sleep and academic performance (the Circadian Light in Adolescence, Sleep and School [CLASS] Study). Participants were recruited via online advertisements and recruitment sessions at local secondary schools. Inclusion criteria were: enrolled in Year 7 in Melbourne, access to a smartphone with internet access, and ability to communicate in English. Exclusion criteria for the broader longitudinal study were: a parent reported current sleep disorder other than delayed sleep‐wake phase disorder (DSWPD) or insomnia, and severe psychiatric (depressive, bipolar, schizophrenia/psychotic, substance related, obsessive‐compulsive, feeding and eating, and trauma‐ and stressor‐related disorders) and neurodevelopmental disorders (autism spectrum disorder, attention‐deficit/hyperactivity disorder, intellectual disabilities or neurocognitive disorders such as traumatic brain injury), diagnosed by a medical professional (Figure S1). No participants included in this analysis had a medical diagnosis of insomnia or DSWPD. No participant, or members of their household, reported testing positive for COVID‐19 either before or during data collection. Participants and their parent/legal guardian provided written informed consent prior to participating. All procedures were approved by the Monash University Human Research Ethics Committee and were performed in compliance with the Declaration of Helsinki. Participants were reimbursed for their time.
2.2. Design
Participants completed measurements for one to two weeks during in‐person learning (November 2019 to March 2020) before changes in school mode (n = 28) and again during remote learning (April 2020 to August 2020) (n = 27, one participant withdrew). For a subset (n = 15), we measured sleep for one week during in‐person learning, with the second week collected during school vacation (23 to 27 March 2020), which was moved earlier than anticipated as part of the state government COVID‐19 response. Another sample (n = 31) were enrolled during remote learning (14 April to 14 August 2020), and so have no in‐person baseline.
Each timepoint consisted of one to two weeks of at‐home daily measurements of sleep‐wake timing and school attendance, and an online set of questionnaires. Due to the design of the broader longitudinal study, circadian phase (salivary dim light melatonin onset; DLMO) was measured in the first wave of data collection for each group of participants (for n = 12 during in‐person school, n = 15 during vacation, n = 30 during remote learning) (Figure 1).
FIGURE 1.
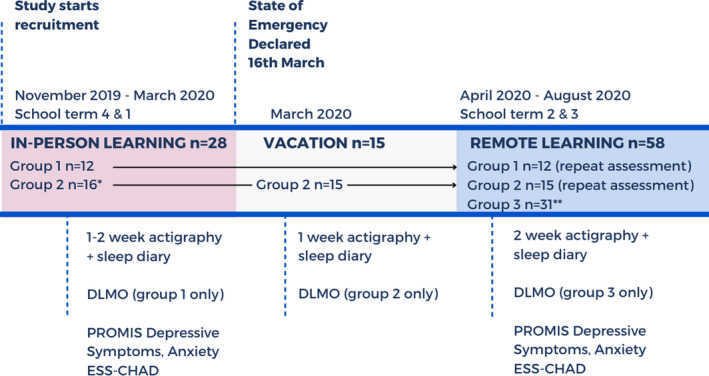
Protocol and data collection timing. Data were collected as part of an existing longitudinal cohort trial in Australia, with repeated assessments in 6‐month waves. Group 1 was monitored during in‐person school and during remote learning. Group 2 commenced data collection during in‐person school in March 2020, with their second week of at‐home monitoring and circadian phase assessment conducted during vacation. Group 3 was monitored during remote learning only. Due to the design of the longitudinal study, circadian phase (salivary dim light melatonin onset; DLMO) was measured in the first wave of data collection for each Group. DLMO was measured for Group 1 during in‐person learning, for Group 2 during vacation, and Group 3 during remote learning. Abbreviations: DLMO, dim light melatonin onset; ESS‐CHAD, Epworth Sleepiness Scale Child and Adolescent version. Note: * one participant in Group 2 was monitored during in‐person and remote learning, but not during vacation. Another participant withdrew from the study prior to the remote learning assessment. ** one participant in Group 3 did not complete the DLMO assessment
2.3. Sleep‐wake timing
Sleep was measured using actigraphy (GENEActiv Original, Activinsights), with the device worn on the nondominant wrist. Sleep episodes were confirmed using sleep diaries administered via mobile phone app (Metricwire Inc). These sleep diaries were completed daily, reporting on the previous night of sleep, and were available for completion from 06:00 hours to 23:59 hours each day. Notifications and SMS reminders were used to improve compliance.
2.4. Circadian phase
Circadian phase was measured using salivary DLMO. Participants collected hourly saliva samples from 4 hours before to 2 hours after their average sleep onset time, calculated from one week of sleep diaries. For 12 participants, samples were collected in the laboratory during in‐person learning, under dim light conditions (<3 lux). For the remaining 45 participants, due to lockdown restrictions that prevented bringing participants into the laboratory, samples were collected in the participants' homes (n = 15 during vacation, n = 30 during remote learning). The at‐home collections were facilitated by videoconference where researchers prompted the collection of every sample and ensured protocol compliance, including the continuous dim light conditions. Participants were provided with plastic welder's goggles fit with neutral density filters (209 Neutral Density Filter, LEE; measured incoming light <1 lux in room lighting of 500 lux) to ensure dim light was maintained during toilet visits. Participants remained seated, without food or drink, for 20 minutes prior to each sample. During internal testing in a dark room, we found that to maintain photopic illuminance at eye‐level <3 lux, devices in the room must be kept at least 1 meter away, with a blue light filter installed, and brightness set to the lowest setting. This set up was confirmed with participants, and compliance monitored via videoconference throughout the collections. Samples were stored in the participant's home freezer overnight and transported the following day to be stored in the laboratory at −20°C.
Melatonin concentration was assayed via radioimmunoassay, conducted using procedures developed at the University of Adelaide22 and reagents provided by Buhlmann Laboratories (Allschwil). Limit of detection of the assay is 1 pg/mL.
2.5. Questionnaires
Depression and anxiety symptoms were assessed using the PROMIS Pediatric Depressive Symptoms, and Anxiety scales (both CAT v2.0),23 respectively. Both PROMIS Pediatric scales provide a standardized t‐score, which have a population mean of 50, and standard deviation of 10. Daytime sleepiness was measured using the Epworth Sleepiness Scale for Children and Adolescents (ESS‐CHAD).24 Perceived stress was measured using the 10‐item Perceived Stress Scale (PSS).25 The School Sleep Habits Survey26 measured sleep habits, diurnal preference, and school behaviors, including commute to and from school. A COVID‐impact survey was used to monitor lockdown restrictions, symptoms, and whether any household members had been tested or diagnosed with COVID‐19. Surveys were administered online via REDCap.27, 28
2.6. School attendance
Each day, participants reported whether they attended school or participated in remote learning. If in remote learning, participants reported any differences in school hours compared to their usual in‐person school schedule. Daily school attendance was used to categorize daily sleep data into the following conditions: in‐person learning, remote learning, or vacation. Daily data for in‐person and remote learning groups were further sub‐categorized as either a school day or free day (including weekends and administrative/student‐free days).
2.7. Data analysis
Sleep onset and wake times were derived using R Version 3.6.129 with the package GENEActiv and GENEA Data (GGIR30), using the sleep diary as a guide. When a sleep diary was not available (n = 93 days, 7.5%), the sleep episode was detected using the HDCZA algorithm.31 When there were discrepancies of >30 minutes between GGIR and the sleep diary, two independent researchers visually inspected raw activity and light data. Sleep onset time was modified if reported sleep onset time was >30 minutes before or after a sustained substantial reduction in activity and light levels, and wake time was modified if reported wake time was >30 minutes before or after a sustained substantial increase in activity and light levels. Self‐reported naps were not included in this analysis due to the low nap frequency (1.5% of all days) and lack of substantial differences in nap frequency between conditions (in person: n = 7 naps from 7 participants, mean duration = 0.84 hours; remote: n = 11 naps from 9 participants, mean duration: 1.14 hours).
DLMO time was calculated using linear interpolation across timepoints before and after salivary melatonin concentration increased to, and remained above, a fixed threshold of 4 pg/mL.32 Phase angle on school days was calculated as the time between DLMO and average sleep onset time (sleep onset phase angle) and between DLMO and average wake time (wake phase angle).
2.7.1. Primary analyses
Visual checks of q‐q plots for normality were conducted for all models to ensure no major violations of normality. Linear mixed models were used as they are robust to violations of normality, differences in sample size and missing data.33 Linear mixed models (“fitlme” function, MATAB R2018b) were used to examine the effect of school mode on daily sleep onset time, wake time, and sleep duration. Each model included a fixed effect for school mode, incorporating school days vs free days (5‐factor variable: in‐person school days, in‐person free days, remote‐learning school days, remote‐learning free days, and vacation), with participant as a random effect. Post hoc comparisons examined differences between each fixed effect, with Bonferroni corrections for multiple comparisons applied to each outcome variable. Linear mixed models were used to examine the effect of school mode (in‐person vs remote) on mood (depressive symptoms and anxiety) and daytime sleepiness (ESS), with participants as a random effect.
2.7.2. Secondary analyses
Mediation analysis (R “lavaan” package34) was used to explore whether sleep variables (sleep onset, wake time, sleep duration) mediated the effects of school mode on mood outcomes that differed significantly between school modes. Between‐subjects ANOVAs (“anova1” function, MATLAB R2018b) were used to compare DLMO time and phase angles across school modes (in‐person, remote learning, vacation). Power analyses for these between‐subjects comparisons indicated that we were well powered to detect a 30‐minute difference in DLMO time between school conditions (80% power). Pearson's correlations were used to examine the associations of DLMO time with average sleep onset and wake times on school days and free days, during in‐person learning, remote learning, and vacation, respectively.
3. RESULTS
Participant characteristics are reported in Table 1. In our sample, school started on average at 8:49 ± 0:18 (range 8:00 to 9:35) hh:mm and ended at 15:12 ± 0:13 (range 14:30 to 15:50) hh:mm. 98% (57 of 58) of students reported no change in their usual scheduled school times during remote learning.
TABLE 1.
Participant characteristics by school mode
| In‐person learning | Remote learning | Vacation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Min | Max | n | Mean | SD | Min | Max | n | Mean | SD | Min | Max | |
| Sex (F, M) | 18, 10 | 33, 25 | 9, 6 | ||||||||||||
| Age (years) | 28 | 12.9 | 0.5 | 12.0 | 13.7 | 58 | 12.8 | 0.4 | 12.0 | 13.7 | 15 | 12.6 | 0.4 | 12.0 | 13.1 |
| Sleep onset time (hh:mm) | 28 | 22:16 | 0:40 | 20:53 | 23:44 | 58 | 22:44 | 1:03 | 21:15 | 2:05 | 15 | 23:01 | 1:18 | 20:49 | 1:05 |
| Wake time (hh:mm) | 28 | 7:12 | 0:34 | 6:00 | 8:18 | 58 | 7:55 | 0:41 | 6:19 | 9:43 | 15 | 8:20 | 1:02 | 6:37 | 10:31 |
| Sleep duration (hh:mm) | 28 | 8:55 | 0:35 | 7:36 | 10:01 | 58 | 9:10 | 0:45 | 7:29 | 11:14 | 15 | 9:18 | 0:59 | 7:26 | 10:55 |
| DLMO (hh:mm) | 12 | 20:24 | 1:03 | 19:07 | 22:21 | 30 | 20:30 | 0:52 | 18:45 | 22:40 | 15 | 20:43 | 1:21 | 18:09 | 22:45 |
| Sleep onset phase angle (hh:mm) | 12 | −1:44 | 1:19 | −3:04 | 0:32 | 30 | −2:03 | 0:49 | −3:33 | −0:04 | 15 | −2:18 | 1:20 | −5:03 | −0:04 |
| Wake time phase angle (hh:mm) | 12 | 10:19 | 1:12 | 8:14 | 11:51 | 30 | 11:03 | 0:50 | 9:14 | 13:00 | 15 | 11:37 | 1:19 | 9:32 | 13:43 |
| Depressive symptoms (PROMIS) | 28 | 51.4 | 8.2 | 35.2 | 66.0 | 56 | 52.9 | 7.6 | 31.8 | 67.5 | — | — | — | — | — |
| Anxiety (PROMIS) | 26 | 49.5 | 6.3 | 35.7 | 62.9 | 55 | 46.1 | 8.8 | 31.9 | 76.6 | — | — | — | — | — |
| Daytime sleepiness (ESS‐CHAD) | 28 | 5.1 | 3.0 | 0.0 | 12.0 | 56 | 4.1 | 3.2 | 0.0 | 13.0 | — | — | — | — | — |
| Perceived stress (PSS) | 27 | 15.1 | 5.9 | 3.0 | 28.0 | 55 | 15.3 | 5.9 | 5.0 | 29.0 | — | — | — | — | — |
n = 27 participants had repeated assessments during in‐person and remote learning. n = 15 participants had repeated assessments during in‐person, remote learning, and during vacation. Sleep onset, wake time, and sleep duration represent the average sleep timing across the two‐week monitoring phase, measured via actigraphy. Phase angle is calculated for school days only.
Abbreviations: DLMO, dim light melatonin onset; ESS‐CHAD, Epworth Sleepiness Scale – Child and Adolescent version; PROMIS, Patient Reported Outcomes Measurement Information System; PSS, Perceived Stress Scale; SD, standard deviation.
3.1. Sleep is later and longer during remote learning compared to in‐person learning
On school days during remote learning, students on average went to sleep 26 minutes later (P < .0001) and woke 49 minutes later (P < .0001), resulting in a 22 minutes increase in sleep duration (P < .0001), compared to in‐person school days (measured via actigraphy; Figure 2, Table 2). These findings remained consistent when we restricted the sample to only participants who provided longitudinal data from both in‐person and remote learning, with sleep‐wake timing delaying, and sleep duration increasing during remote learning (n = 27; Table S1).
FIGURE 2.
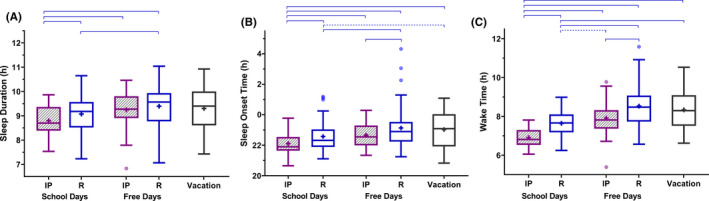
Differences in sleep duration and timing during in‐person and remote learning. Box plots for sleep duration (A), sleep onset time (B), and wake time (C) are shown for school days and free days during in‐person (IP; n = 28) and remote (R; n = 58) learning, and during school vacation (n = 15), with Tukey‐style whiskers. Linear mixed models were used to compare conditions, with participant as a random factor. Horizontal blue lines indicate significant group differences: dashed lines indicate P < .05, solid lines indicate P < .001. Group mean is indicated by a plus symbol. See also Table S2
TABLE 2.
Sleep outcomes assessed each day on school days and free days during in‐person, remote learning, and vacation (fixed effects), with participant (n = 59) as random effect. Data for other conditions are presented as relative to the mean during in‐person school days (“Reference”)
| β | Lower CI | Upper CI | P | |
|---|---|---|---|---|
| Sleep onset time (hh:mm) | ||||
| Reference: In‐person school day | 22:08 | 21:51 | 22:24 | |
| In‐person free day | 0:35 | 0:23 | 0:46 | <.0001 |
| Remote school day | 0:26 | 0:17 | 0:34 | <.0001 |
| Remote free day | 0:59 | 0:49 | 1:08 | <.0001 |
| Vacation | 0:57 | 0:40 | 1:13 | <.0001 |
| Wake time (hh:mm) | ||||
| Reference: In‐person school day | 6:50 | 6:39 | 7:02 | |
| In‐person free day | 1:05 | 0:54 | 1:16 | <.0001 |
| Remote school day | 0:49 | 0:41 | 0:57 | <.0001 |
| Remote free day | 1:40 | 1:31 | 1:50 | <.0001 |
| Vacation | 1:20 | 1:04 | 1:36 | <.0001 |
| Sleep duration (hh:mm) | ||||
| Reference: In‐person school day | 8:42 | 8:29 | 8:55 | |
| In‐person free day | 0:30 | 0:16 | 0:43 | .0002 |
| Remote school day | 0:22 | 0:12 | 0:32 | <.0001 |
| Remote free day | 0:41 | 0:29 | 0:52 | <.0001 |
| Vacation | 0:23 | 0:04 | 0:43 | .18 |
Table shows unstandardized coefficients (β) in h:mm time, lower and upper 95% confidence intervals in h:mm time, and P‐values, with significant values in bold. Comparisons are made to the in‐person school day mean. Multiple post hoc comparisons were run to examine differences between each fixed effect (see also Table S2). Daily sleep timing was measured using wrist actigraphy. 1196 sleep entries were included in the model, with 5 fixed effects coefficients, and participant as a random effect. P‐values indicate whether the metric is significantly different from zero.
3.2. Sleep on school days was earlier and shorter compared to free days in both remote and in‐person learning
As expected, students went to sleep later, woke later, and slept longer during free (nonschool) days compared to school days, in both learning conditions (In‐person: slept 35 minutes later, woke 65 minutes later, slept 30 minutes longer on free days; Remote: slept 33 minutes later, woke 51 minutes later, slept 18 minutes longer on free days, all P < .0001; Figure 2, Table S2).
3.3. Sleep during vacation was similar to free days
There were no significant differences in sleep onset, wake onset, and sleep duration during vacation compared to free days during both in‐person and remote learning (Table S2). Sleep onset and wake onset times were significantly later, with moderate to large effect sizes, but sleep duration was not significantly changed during vacation compared to school days during both in‐person and remote learning (Figure 2).
3.4. Students woke at a later circadian phase during remote learning
There was large inter‐individual variability in DLMO time in all conditions (range 19:07‐22:21 hours during in‐person learning, 18:45‐22:40 hours during remote learning, and 18:09‐22:45 hours during vacation). Average DLMO time did not differ significantly between school modes (in‐person, remote, vacation; F[2,55] = 0.28, P = .76), with small pairwise effect sizes (in‐person vs remote learning d = 0.11, in‐person vs remote learning d = 0.26, remote vs vacation d = 0.18).
The time between DLMO and wake time (ie, phase angle) on school days, however, was significantly shorter during in‐person learning (M ± SD = 10:19 ± 1:12 h:mm), compared to remote learning (M ± SD = 11:16 ± 0:45 h:mm, d = 0.9) and vacation (M ± SD = 11:37 ± 1:19 h:mm, d = 1.02) with large effect sizes, showing that students woke at a significantly later relative circadian phase during remote learning and vacation, compared to during in‐person learning (Figure 3B). The phase angle between DLMO and sleep onset did not differ significantly between conditions (in‐person, remote, vacation: F[2, 54] = 2.57, P = .41).
FIGURE 3.
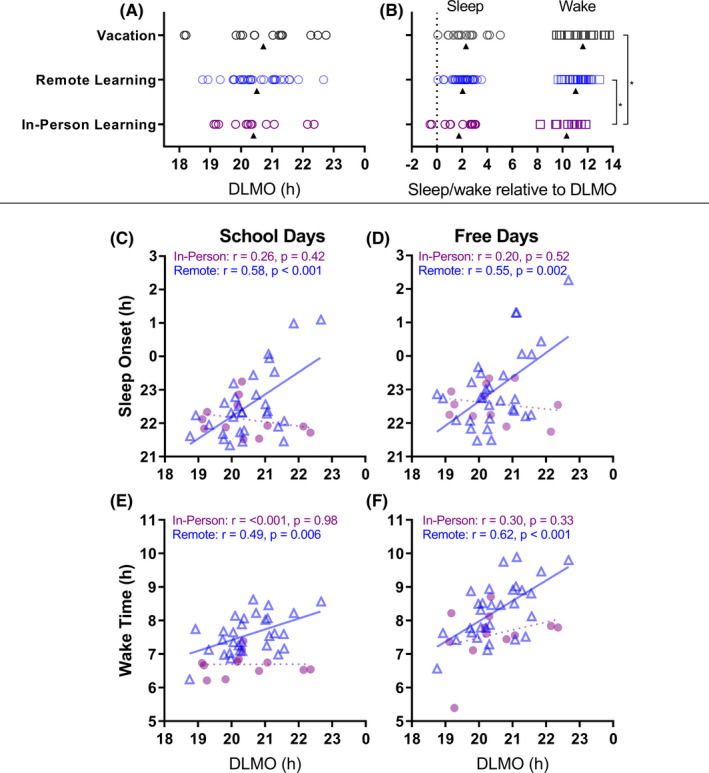
Timing of circadian phase (DLMO, h) and sleep. Top panel: Timing of DLMO (A); and timing of sleep (circles) and wake (squares) on school days relative to DLMO time (phase angle, 0 = DLMO time) (B) during in‐person learning (n = 12), remote learning (n = 30), and vacation (n = 15). Open circles represent individual participants, and triangles represent the group mean. * indicates P < .05. See also Figure S1. Lower panels: Relationships between circadian phase (DLMO, h) and sleep‐wake timing during school days (C and E) and free days (D and F). The in‐person learning group (n = 12) is represented as closed circles, and the remote learning group (n = 30) in open triangles. Straight solid lines indicate a significant linear relationship, whereas dashed lines indicate a nonsignificant linear relationship
Later DLMO was significantly correlated with later sleep onset and wake times on both school nights and free days during remote learning (Figure 3C‐F). DLMO was not associated with sleep time or wake time during in‐person learning (all P > .33); there was a nonsignificant trend in the vacation group for the association of DLMO with sleep times (r [95% CI] = 0.49 [−0.03, 0.80], P = .06) and wake times (r [95% CI] = 0.41 [−0.13, 0.76], P = .13).
3.5. Lower anxiety symptoms and less daytime sleepiness during remote learning compared to in‐person learning
Self‐reported anxiety and depressive symptoms were within the normal to mild symptom range on average in both in‐person and remote learning conditions35 (Table 1). Anxiety symptoms were significantly lower during remote learning compared to in‐person learning, with a moderate effect size (Figure 4A) that exceeds established thresholds for a minimally important difference.36 This was observed using a linear mixed model, with school mode as a fixed effect and participant as random effect (β [95% CI] = −3.52 [−6.04, −1.00], P = .007, d = 0.44), and in the repeated measures sample using a paired samples t test (t [22] = −3.059, 95% CIs [−6.74, −1.29], P = .006, d = 0.64). We further examined the change in anxiety symptoms by testing mediation models for each sleep and sleepiness parameter (ie, sleep duration, sleep onset, wake time) in the repeated measures dataset (n = 27). In each case, the indirect effect was null (all P > .15), indicating that changes in anxiety symptoms were improved due to not being at school and learning at home, rather than changes in sleep or daytime sleepiness.
FIGURE 4.
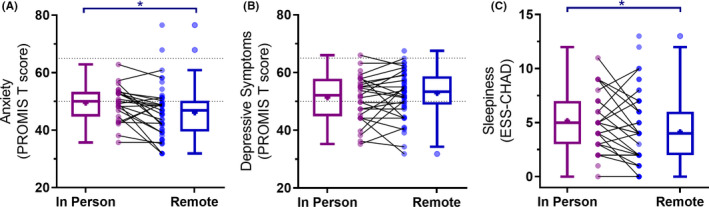
Changes between in‐person and remote learning in (A) anxiety symptoms, (B) depressive symptoms, and (C) daytime sleepiness. Box plots with Tukey‐style whiskers are shown for each group. Group mean is indicated by a plus symbol. Individual datapoints are shown as circles for each group, with a line connecting participants who had repeated measurements. Blue lines indicate significant group differences, where * signifies P < .05
Higher anxiety symptoms were associated with higher perceived stress during both school modes (in‐person n = 28, r [95% CI] = 0.66 [0.38, 0.83], P < .001; remote n = 55, r [95% CI] = 0.68 [0.51, 0.80], P < .001), and the observed change in anxiety was associated with the observed change in perceived stress (n = 24, r [95% CI] = 0.63 [0.30, 0.82], P = .02). No significant differences in reported depressive symptoms were observed between in‐person learning and remote learning conditions (β [95% CI] = 1.28 [−1.04, 3.59], P = .28, d = 0.19; Figure 4B), with the average scores for both conditions similar to population norms (Table 1).
Daytime sleepiness (ESS) was significantly lower during remote learning (β [95% CI] = −0.97 [−1.86, −0.09], P = .03, d = 0.31; Figure 4C). We observed a small, significant reduction in ESS score during remote learning compared to in‐person in the repeated measures sample (t (25) = −2.08, P = .048, d = 0.3). No sleep variable (sleep duration, sleep onset, wake time) mediated the change in daytime sleepiness between in‐person and remote learning conditions (all P > .27).
4. DISCUSSION
Multiple beneficial changes were observed in sleep and mood measures on school days when early adolescents engaged in remote learning during the COVID‐19 pandemic lockdown, compared to in‐person learning before the lockdown. While school start and end times generally did not change, participants required less time to prepare and did not have commute to and from school during remote learning. Our findings support the hypothesis, which has been a topic of theoretical debate,37 that if more time is provided before school in the morning, adolescents will use some of it for sleep. Sleep occurred at a later circadian phase during remote learning; this phase was at a more appropriate phase angle with the later circadian timing observed in teenagers.38 We also observed a moderate reduction in anxiety symptoms associated with the change to remote learning, which was related to reduced perceived stress but not specifically to changes in sleep.
During remote learning, sleep and wake times were later, and sleep duration was longer, with largest effect sizes observed for differences in wake time. This is consistent with survey studies of adolescent sleep during COVID‐19 in the United States and Italy,16, 19 and with studies examining home‐schooled children,15 or delayed school start times,11, 39 and in adults during similar COVID‐19 lockdowns.40, 41 Adding to this literature, we found that on school days during remote learning, compared to in‐person learning, adolescents slept in closer alignment with their endogenous circadian rhythms. In particular, the phase angle between DLMO and wake time was longer during remote learning compared to in‐person learning, meaning adolescents woke at a later phase in the melatonin rhythm. Waking at an earlier circadian phase, closer to the circadian nadir, is associated with poorer alertness and cognitive performance.42, 43 During remote learning (unlike during in‐person learning), sleep timing was significantly associated with DLMO, indicating that sleep times were more influenced by an individual's circadian system than the imposed schedules. We suggest, therefore, that later school start times or shorter commutes would have positive effects on sleep timing and duration, by permitting student to sleep at a more appropriate circadian time. We note that our average sleep onset time on school days during in‐person learning was consistent with that observed in the same age group in the United States.44 Later (or flexible) school times may be expected to primarily benefit older adolescents, who have substantially delayed circadian timing.44, 45 Yet, our findings indicate that there are also benefits of extending sleep opportunity on school days for early adolescents, who may be less delayed in their circadian timing.
Our findings suggest potential benefits of learning from home for adolescent mental health during lockdown. Self‐reported anxiety symptoms were reduced during remote learning compared to (prepandemic) baseline, which was not mediated by changes in sleep. The magnitude of the reduction in anxiety symptoms exceeded the threshold for a clinically important difference,36 suggesting the change would have been noticeable to the students. Given that the state of emergency associated with COVID‐19 would be anticipated to increase anxiety in general, the observed change in the opposite direction is probably attributable to changes in behaviors associated with lockdown, including the mode of education and interactions with family and peers. This finding is contrary to reported increases in negative and anxious mood symptoms in young adult and adult populations during the pandemic, for example,20, 21 potentially due to differences in the experiences of adults and young adolescents, in recruitment approaches, in geographical location, and instruments used to measure anxiety. Remote learning may have contributed to reduced daily social stressors, including reduced social and extracurricular activities, leading to reduced stress and anxiety.46 More time with caregivers could also have reduced anxiety symptoms, via increased social support and resilience.47, 48
Limitations of the study should be noted. The study may have lacked sufficient power to detect associations between sleep timing and circadian phase during in‐person learning. Nevertheless, findings are consistent with previous work showing that relationships between sleep and circadian timing are stronger on free schedules compared to school term.49 There were no repeated assessments of circadian timing, limiting interpretations of potential changes in circadian alignment with sleep. Nevertheless, this study was well powered to detect changes in DLMO time of 30 minutes or more between conditions. Repeated measurements of sleep timing and duration, as well as mood outcomes, were only available in a subsample of participants (n = 27) who had been tested prior to the COVID‐19 pandemic. This limited our statistical power to observe small changes in some outcomes (eg, depression). In addition, while our sample experienced strict and prolonged periods of lockdown, they were from moderate‐to‐high socioeconomic backgrounds with adequate resources to support learning from home, and we excluded participants with a diagnosed psychiatric or neurodevelopmental disorder. The sample may not be representative of adolescents in families experiencing more substantial economic and health impacts of the COVID‐19 pandemic, or adolescents with neurodevelopmental or psychiatric disorders. Most participants were within the normal range of anxiety symptoms, meaning our findings may not generalize to individuals at higher risk of psychological distress. While this study showed positive outcomes for sleep and mood associated with remote learning during lockdown, we acknowledge this does not provide a holistic view of remote education. We did not, for example, measure stress or burden on parents associated with remote learning. It is unclear how this period of remote learning during the pandemic may relate to long‐term sleep habits and academic outcomes, or how the reduced interpersonal interactions may impact social development. Longitudinal studies are needed to investigate the long‐term impacts of remote learning.
Our findings point to a silver lining of remote learning during lockdown related to the COVID‐19 pandemic: Adolescents had more sleep and less self‐reported anxiety. When the need to travel to/from school was removed, adolescents slept later and longer and in closer alignment with their circadian rhythms. These results suggest that there should be greater focus on delaying the timing of activities in the morning, such as school commuting time and school start times, as potential targets for interventions to increase sleep duration in adolescents.
CONFLICT OF INTEREST
JES, EC, AJH, SL, JFW, MAC, and BB have no conflicts to declare. AJKP was an investigator on projects supported by the CRC for Alertness, Safety and Productivity, and he has received research funding from Versalux and Delos. EBK reports (nongovernment, nonuniversity): Travel support from World Conference of Chronobiology, Gordon Research Conference, Sleep Research Society, Santa Fe Institute, German Sleep Society (DGSM); Consulting/grant reviews income from Puerto Rice Science, Technology, and Research Trust, National Sleep Foundation, Sanofi‐Genzyme; Family member owns Chronsulting. SWL has had a number of commercial interests in the last 2 years (2019‐21). His interests were reviewed and managed by Mass General Brigham in accordance with their conflict of interest policies. No interests are directly related to the research or topic reported in this paper but, in the interests of full disclosure, are outlined below. SWL has received consulting fees from the EyeJust Inc, Rec Room, Six Senses, and Stantec; and has current consulting contracts with Akili Interactive; Apex 2100 Ltd.; Consumer Sleep Solutions; Hintsa Performance AG; KBR Wyle Services, Light Cognitive; Lighting Science Group Corporation/HealthE; Look Optic; Mental Workout/Timeshifter and View Inc He has received honoraria and travel or accommodation expenses from MIT, Roxbury Latin School, and University of Toronto, and travel or accommodation expenses (no honoraria) from Wiley; and royalties from Oxford University Press. He holds equity in iSleep Pty. He has received an unrestricted equipment gift from F. Lux Software LLC and holds an investigator‐initiated grant from F. Lux Software LLC. He has a Clinical Research Support Agreement and a Clinical Trials Agreement with Vanda Pharmaceuticals Inc He is an unpaid Board Member of the Midwest Lighting Institute (nonprofit). He was a Program Leader for the CRC for Alertness, Safety and Productivity, Australia, through an adjunct professor position at Monash University (2015‐2019). He is currently a part‐time faculty member at the University of Surrey. He has served as a paid expert in legal proceedings related to light, sleep, and health. SWMR was a Program Leader for the CRC for Alertness, Safety and Productivity, Australia, and currently serves as the Chair of the Sleep Health Foundation. SWMR reports grants from Vanda Pharmaceuticals, Philips Respironics, Cephalon, Rio Tinto and Shell and receiving equipment support and consultancy fees through his institution from Vanda, Circadian Therapeutics, Optalert, Tyco Healthcare, Compumedics, Mental Health Professionals Network, and Teva Pharmaceuticals, which are not related to this paper.
AUTHOR CONTRIBUTIONS
JES, AJKP, MAC, EBK, SWL, JFW, BB, and SMWR designed the study; JES, EC, AJH, and SL performed research; JES, AJKP, and JFW analyzed data; and all wrote the paper.
Supporting information
Supplementary Material
Figure S1
ACKNOWLEDGEMENTS
We thank the research participants for their dedication and efforts, and the CLASS Study research staff and students at the Monash University Sleep and Circadian Rhythms Laboratory. We give special thanks to Dr Megan Mulhall and Dr Monika Raniti for assistance adapting procedures to meet social distancing requirements during the pandemic, and the Adelaide Research Assay Facility for conducting the melatonin assays.
Stone JE, Phillips AJK, Chachos E, et al. In‐person vs home schooling during the COVID‐19 pandemic: Differences in sleep, circadian timing, and mood in early adolescence. J Pineal Res. 2021;71:e12757. 10.1111/jpi.12757
Bei Bei and Shantha M. W. Rajaratnam contributed equally to the manuscript.
Funding information
This study was funded by an Australian Research Council Discovery Project Grant (DP190103444) and supported by an equipment grant from Vanda Pharmaceuticals. Wiley (1178487) and Bei (1140299) were supported by NHMRC fellowships. Klerman was supported by a NIH K24 award.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Galland BC, Short MA, Terrill P, et al. Establishing normal values for pediatric nighttime sleep measured by actigraphy: a systematic review and meta‐analysis. Sleep. 2018;41(4):zsy017. [DOI] [PubMed] [Google Scholar]
- 2.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta‐analysis of age, region, and sleep. Sleep Med. 2011;12(2):110‐118. [DOI] [PubMed] [Google Scholar]
- 3.Carskadon MA, Tarokh L. Developmental changes in sleep biology and potential effects on adolescent behavior and caffeine use. Nutr Rev. 2014;72(Suppl_1):60‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo JC, Ong JL, Leong RL, Gooley JJ, Chee MW. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2016;39(3):687‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiol. 1981;18(2):107‐113. [DOI] [PubMed] [Google Scholar]
- 6.Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 2014;55(2):180‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen L, van Schie J, Ditchburn G, Brook L, Bei B. Positive and negative emotions: differential associations with sleep duration and quality in adolescents. J Youth Adol. 2018;47(12):2584‐2595. [DOI] [PubMed] [Google Scholar]
- 8.Yeo SC, Jos AM, Erwin C, et al. Associations of sleep duration on school nights with self‐rated health, overweight, and depression symptoms in adolescents: problems and possible solutions. Sleep Med. 2019;60:96‐108. [DOI] [PubMed] [Google Scholar]
- 9.Lo JC, Lee SM, Lee XK, et al. Sustained benefits of delaying school start time on adolescent sleep and well‐being. Sleep. 2018;41(6):zsy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gariépy G, Janssen I, Sentenac M, Elgar FJ. School start time and sleep in Canadian adolescents. J Sleep Res. 2017;26(2):195‐201. [DOI] [PubMed] [Google Scholar]
- 11.Minges KE, Redeker NS. Delayed school start times and adolescent sleep: a systematic review of the experimental evidence. Sleep Med Rev. 2016;28:86‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boergers J, Gable CJ, Owens JA. Later school start time is associated with improved sleep and daytime functioning in adolescents. J Dev Behav Pediatr. 2014;35(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 13.Owens JA, Belon K, Moss P. Impact of delaying school start time on adolescent sleep, mood, and behavior. Arch Pediatr Adolesc Med. 2010;164(7):608‐614. [DOI] [PubMed] [Google Scholar]
- 14.Kelley P, Lockley SW, Kelley J, Evans MD. Is 8: 30 am still too early to start school? A 10: 00 am school start time improves health and performance of students aged 13–16. Front Hum Neurosci. 2017;11:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meltzer LJ, Shaheed K, Ambler D. Start later, sleep later: school start times and adolescent sleep in homeschool versus public/private school students. Behav Sleep Med. 2016;14(2):140‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrobelli A, Pecoraro L, Ferruzzi A, et al. Effects of COVID‐19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity. 2020;28(8):1382‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore SA, Faulkner G, Rhodes RE, et al. Impact of the COVID‐19 virus outbreak on movement and play behaviours of Canadian children and youth: a national survey. Int J Behav Nutr Phys Act. 2020;17(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliviero B, Emanuela M, Mattia D, et al. Changes in sleep patterns and disturbances in children and adolescents in Italy during the Covid‐19 outbreak. Sleep Med. 2021. [Epub ahead of print]. 10.1016/j.sleep.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker SP, Dvorsky MR, Breaux R, Cusick CN, Taylor KP, Langberg JM. Prospective examination of adolescent sleep patterns and behaviors before and during COVID‐19. Sleep. 2021. [Epub ahead of print]. 10.1093/sleep/zsab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terry PC, Parsons‐Smith RL, Terry VR. Mood responses associated with COVID–19 restrictions. Front Psychol. 2020;11:3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varma P, Junge M, Meaklim H, Jackson ML. Younger people are more vulnerable to stress, anxiety and depression during COVID‐19 pandemic: a global cross‐sectional survey. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:e110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12(5):457‐466. [DOI] [PubMed] [Google Scholar]
- 23.Quinn H, Thissen D, Liu Y, et al. Using item response theory to enrich and expand the PROMIS® pediatric self report banks. Health Qual Life Outcomes. 2014;12(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen KC, Phillipson S, O'Connor J, Johns MW. Validation of the Epworth Sleepiness Scale for children and adolescents using Rasch analysis. Sleep Med. 2017;33:30‐35. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;385‐396. [PubMed] [Google Scholar]
- 26.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875‐887. [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:e103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Team RC . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 30.Migueles JH, Rowlands AV, Huber F, Sabia S, van Hees VT. GGIR: a research community‐driven open source R package for generating physical activity and sleep outcomes from multi‐day raw accelerometer data. J Meas Phys Behav. 2019;2(3):188‐196. [Google Scholar]
- 31.Van Hees VT, Sabia S, Jones SE, et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci Rep. 2018;8(1):12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12(3):278‐289. [DOI] [PubMed] [Google Scholar]
- 33.Schielzeth H, Dingemanse NJ, Nakagawa S, et al. Robustness of linear mixed‐effects models to violations of distributional assumptions. Methods Ecol Evol. 2020;11(9):1141‐1152. [Google Scholar]
- 34.Lavaan RY. An R package for structural equation modeling and more. Version 0.5–12 (BETA). J Stat Softw. 2012;48(2):1‐36. [Google Scholar]
- 35.Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI‐II, CES‐D, and PHQ‐9 to PROMIS depression. Psychol Assess. 2014;26(2):513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thissen D, Liu Y, Magnus B, et al. Estimating minimally important difference (MID) in PROMIS pediatric measures using the scale‐judgment method. Qual Life Res. 2016;25(1):13‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skeldon AC, Phillips AJ, Dijk D‐J. The effects of self‐selected light‐dark cycles and social constraints on human sleep and circadian timing: a modeling approach. Sci Rep. 2017;7:e45158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu LJ, Acebo C, Seifer R, Carskadon MA. Sleepiness and cognitive performance among younger and older adolescents across a 28‐hour forced desynchrony protocol. Sleep. 2015;38(12):1965‐1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meltzer LJ, Wahlstrom KL, Plog AE, Strand MJ. Changing school start times: impact on sleep in primary and secondary school students. Sleep. 2021;44(7):zsab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright KP Jr, Linton SK, Withrow D, et al. Sleep in university students prior to and during COVID‐19 stay‐at‐home orders. Current Biol. 2020;30(14):R797‐R798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blume C, Schmidt MH, Cajochen C. Effects of the COVID‐19 lockdown on human sleep and rest‐activity rhythms. Current Biol. 2020;30(14):R795‐R797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright KP, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283(6):R1370‐R1377. [DOI] [PubMed] [Google Scholar]
- 43.Lockley SW, Dijk DJ, Kosti O, Skene DJ, Arendt J. Alertness, mood and performance rhythm disturbances associated with circadian sleep disorders in the blind. J Sleep Res. 2008;17(2):207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crowley SJ, Van Reen E, LeBourgeois MK, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep. Ann NY Acad Sci. 2004;1021:276‐291. [DOI] [PubMed] [Google Scholar]
- 46.Becker SP, Gregory AM. Editorial perspective: perils and promise for child and adolescent sleep and associated psychopathology during the COVID‐19 pandemic. J Child Psychol Psychiatry. 2020;61(7):757‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stice E, Ragan J, Randall P. Prospective relations between social support and depression: Differential direction of effects for parent and peer support? J Abnorm Psychol. 2004;113(1):155. [DOI] [PubMed] [Google Scholar]
- 48.Ozbay F, Johnson DC, Dimoulas E, Morgan C III, Charney D, Southwick S. Social support and resilience to stress: from neurobiology to clinical practice. Psychiatry (Edgmont). 2007;4(5):35. [PMC free article] [PubMed] [Google Scholar]
- 49.Crowley SJ, Acebo C, Fallone G, Carskadon MA. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school‐year sleep/wake schedules. Sleep. 2006;29(12):1632‐1641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Figure S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


