Abstract
Grain-based ingredients are replaced in part by pulse ingredients in grain-free pet foods. Pulse ingredients are lower in methionine and cysteine, amino acid (AA) precursors to taurine synthesis in dogs. Although recent work has investigated plasma and whole blood taurine concentrations when feeding grain-free diets, supplementation of a grain-free diet with various nutrients involved in the biosynthesis of taurine has not been evaluated. This study aimed to investigate the effects of supplementing a complete grain-free dry dog food with either methionine (MET), taurine (TAU), or methyl donors (choline) and methyl receivers (creatine and carnitine; CCC) on postprandial AA concentrations. Eight healthy Beagle dogs were fed one of the three treatments or the control grain-free diet (CON) for 7 d in a 4 × 4 Latin square design. On day 7, cephalic catheters were placed and one fasted sample (0 min) and a series of nine post-meal blood samples were collected at 15, 30, 60, 90, 120, 180, 240, 300, and 360 min. Data were analyzed as repeated measures using the PROC GLIMMIX function in SAS (Version 9.4). Dogs fed MET had greater plasma and whole blood methionine concentrations from 30 to 360 min after a meal (P < 0.0001) and greater plasma homocysteine concentrations from 60 to 360 min after a meal (P < 0.0001) compared with dogs fed CON, TAU, and CCC. Dogs fed TAU had greater plasma taurine concentrations over time compared with dogs fed CON (P = 0.02) but were not different than dogs fed MET and CCC (P > 0.05). In addition, most AAs remained significantly elevated at 6 h post-meal compared with fasted samples across all treatments. Supplementation of creatine, carnitine, and choline in grain-free diets may play a role in sparing the methionine requirement without increasing homocysteine concentrations. Supplementing these nutrients could also aid in the treatment of disease that causes metabolic or oxidative stress, including cardiac disease in dogs, but future research is required.
Keywords: amino acids, canine, grain-free, meal response
Introduction
Grain-free pet foods have been popular in the pet food industry for more than a decade. In 2017, grain-free dog foods comprised more than 40% of all dog foods available (Plantz, 2017). Grain-free diets typically replace grain ingredients with pulse and tuber ingredients, including field peas, chickpeas, lentils, and beans. Compared with the amino acid (AA) composition of protein from grain ingredients, such as corn gluten meal, which is high in methionine and cysteine and deficient in lysine, pulse ingredients are typically low in methionine and cysteine and high in lysine (NRC, 2006). Pulse ingredients have been used in pet foods for more than 25 yr (Butterwick et al., 1994) but likely at much lower inclusion rates than they are currently estimated to be at (some greater than 40%) in grain-free diets (Mansilla et al., 2019).
In 2018, the U.S. Food and Drug Administration (FDA) released a statement that warned dog owners of a possible link between grain-free diets, commonly those with large amounts of field peas and/or lentils (FDA, 2019a), and canine dilated cardiomyopathy (DCM). Taurine deficiency has been reported as a cause of DCM in cats (Pion et al., 1987), the American Cocker Spaniel (Kittleson et al., 1997), and more recently in large breed dogs, possibly due to a lower taurine biosynthesis rate (Ko et al., 2007; Mansilla et al., 2020). Taurine is not incorporated in protein but is found at very high concentrations in cardiac, skeletal, brain, and retinal tissues and is one of the most abundant free AAs in the body (Hayes, 1988). Taurine also plays a major role in maintaining contractibility of cardiac tissue (Galler et al., 1990) and maintaining osmotic conditions in cardiac cells (Thurston et al., 1981; Rasmusson et al., 1993). Dogs have the ability to synthesize taurine from methionine, an indispensable AA (IDAA), and cysteine, a conditionally IDAA, through transmethylation and transsulfuration pathways.
Although methionine and cysteine are considered the major precursors of taurine, this biosynthetic pathway is composed of multiple reactions and can be impacted by other dietary components (i.e., methyl donors and receivers). Transmethylation occurs when methionine is converted to S-adenosylmethionine (SAM), the major methyl (-CH3) donor in cells. SAM can donate a methyl group to guanidinoacetate to produce creatine (Mudd and Poole, 1975) and to phosphatidylethanolamine to produce phosphatidylcholine (Vance and Ridgway, 1988). These two reactions are estimated to make up approximately 80% of all methylation reactions in human adults (Stead et al., 2006). SAM is also responsible for providing the methyl groups needed to produce carnitine, a metabolite that is also found in high concentrations in the heart (Rebouche and Engel, 1983), is involved in generating energy (Gilbert, 1985), and, when deficient, is also related to nutritionally mediated DCM (Fascetti et al., 2003; Kaplan et al., 2018). After donation of methyl groups, SAM is converted to S-adenosylhomocysteine, which can go on to produce homocysteine. Homocysteine is either remethylated to methionine or enters the irreversible transsulfuration pathway to produce cysteine and can then be metabolized to taurine.
The dietary supplementation of choline can increase methionine availability through remethylation of homocysteine in piglets, when fed a methionine-restricted diet (Robinson et al., 2016) and in rats when fed a folate-deficient diet (Shinohara et al., 2006). Thus, the dietary provision of these nutrients may impact methionine and homocysteine status, and consequently, the production of taurine. However, to our knowledge, no published studies have investigated how the provision of methyl donors impacts taurine status in dogs. Additionally, while some recent studies have evaluated plasma and whole blood taurine in dogs consuming a grain-free diet (Donadelli et al., 2020; Pezzali et al., 2020), to the authors’ knowledge, no one has evaluated the effects of supplementing other nutrients on taurine status.
It is important to not only understand how dietary micronutrients affect taurine status, but also how to assess these outcomes in dogs. To date, whole blood taurine has been shown to be a better predictor of skeletal taurine concentrations during taurine depletion in cats, and plasma taurine has been shown to be a better predictor of skeletal taurine concentrations during taurine repletion (Pacioretty et al., 2001). Therefore, current recommendations suggest taking both plasma and whole blood samples from dogs suspected to have DCM in order to assess whole body taurine status, assuming that the same pattern holds true for dogs as well. In addition, whole blood taurine has been shown to be more stable over time in dogs (Gray et al., 2016). However, there are very limited data on the effect a meal may have on postprandial plasma and whole blood AA concentrations in dogs. While some dogs reported to the FDA had normal taurine levels and others were taurine deficient, sampling time in relation to a meal was not disclosed (FDA, 2019b). Regardless of whether an animal is affected by DCM or not, to our knowledge, there are no data published on the postprandial changes in plasma and whole blood AA concentration in healthy dogs.
Therefore, the objectives of this study were, first, to investigate the effects of 1) methionine, 2) taurine, or 3) methyl donor (choline) and receiver (creatine and carnitine) supplementation on plasma and whole blood AA concentrations in dogs fed a commercial grain-free diet and, second, to quantify the postprandial response of both plasma and whole blood AA concentrations in dogs. We hypothesized that dogs supplemented with methionine would have greater plasma and whole blood methionine, greater plasma cysteine, homocysteine, and glutathione, and no change in plasma and whole blood taurine concentrations; dogs supplemented with taurine would have greater plasma and whole blood taurine, but no change in plasma and whole blood methionine and plasma cysteine, homocysteine, and glutathione concentrations; dogs supplemented with creatine, carnitine, and choline would have greater plasma and whole blood methionine and greater plasma cysteine but no change in plasma or whole blood taurine or plasma homocysteine and glutathione concentrations, compared with dogs fed the control grain-free diet. Finally, we hypothesized that both plasma and whole blood AA concentrations would increase after a meal.
Materials and Methods
Animals
All experimental procedures for this study were approved by the University of Guelph Animal Care Committee (AUP #4250). Five spayed female and three intact male Beagle dogs housed at the Central Animal Facility at the University of Guelph were used in this study. The mean (±SD) age of the dogs was 1.6 ± 0.04 yr, and the mean (±SD) body weight of the dogs was 7.8 ± 1.5 kg (range 6.4 to 10.2 kg) at the start of the study. Body condition scores for the dogs were between 4 and 7 on a 9-point scale, in which 4 to 5 is considered ideal (Laflamme, 1997). All dogs were deemed healthy before the start of the study based on complete blood count and serum biochemistry profiles.
Dogs were pair-housed according to body weight and, as such, the average body weight among each pair of dogs was similar. Dogs were housed in a temperature-controlled room maintained at approximately 22 °C, and humidity maintained between 40% and 60% with a 12:12 (L:D) h cycle. Dogs were exercised both indoors and outdoors for 30 min to 1 h each day. Prior to the start of the study, the dogs were transitioned to the control diet (Nutrience Grain-Free Pork, Lamb and Duck Formula, single batch, Rolf C. Hagen Inc., QC, Canada) and to being fed once a day over a 14-d adaption period. During this adaptation period, dogs were also habituated to all study procedures. Dogs were fed individually, once daily between 0730 and 0830 hours to maintain body weight. Dogs were weighed weekly prior to the morning meal, and body condition score was assessed weekly according to Laflamme (1997).
Dietary treatments and study design
Four dietary treatments were tested: control (CON), control + methionine (MET), control + taurine (TAU), and control + creatine, choline, and carnitine (CCC). A proximate analysis and analysis of all AAs, choline, and carnitine was done for the CON diet by Midwest Laboratories (Omaha, NE). The creatine content of the control diet was not analyzed; however, it can be assumed that it was negligible as creatine was not supplemented in the control diet and animal-derived ingredients may experience creatine degradation during extrusion (Dobenecker and Braun, 2015). dl-Methionine (99%, Evonik Corporation, Parsippany, NJ) was supplemented at 2.6 g/kg dry matter (DM) with the goal of being a total of three times the canine NRC (2006) recommended allowance (RA) according to formulated values (9.4 g/kg DM). Taurine (98.5%, Hubei Grand Life Science & Technology Co., LTD., Hubei, China) was supplemented at 0.7 g/kg DM with the goal of being a total of 2 g/kg DM, according to formulated values and based on a veterinary early cardiac food (Royal Canin Early Cardiac Dry Dog Food, Mars Pet Care, St. Charles, MO). Creatine monohydrate (99.5%, Shanghai Baosui Chemical Co., LTD., Shanghai, China) was supplemented at 9.6 g/kg DM based on McBreairty et al.’s (2015) study in pigs. Choline chloride (60%, Balchem Corporation, New Hampton, NY) was supplemented at 2.13 g/kg DM with the goal of being two times the canine NRC (2006) RA (3.2 g/kg DM) according to formulated values. l-Carnitine (50%, Lonza Group LTD., Kourim, Czech Republic) was supplemented at 0.24 g/kg DM, which is above the current 0.10 to 0.20 g/kg DM that is commonly supplemented in food intended for dogs (Sunvold et al., 2000).
Methionine and taurine were dissolved in water and given as a solution on top of the dry kibble and allowed to soak into the food for 15 min each day. For dogs on CON or CCC, an equal amount of water was added to the kibble and allowed to soak for 15 min to ensure consistency. The creatine, carnitine, and choline were weighed each day and given in a canned food meatball (Nutrience Grain-Free Pork, Lamb and Venison Pâté, Rolf C. Hagen Inc., QC, Canada). Every dog received a canned food meatball that was 4% of their daily caloric requirement to ensure consistency among all treatments. All dogs were provided with tap water that was refreshed daily, ad libitum.
The study was a complete, randomized, 4 × 4 Latin Square design with each pair of dogs receiving each of the four treatments in random order for 7 d with no washout period in between because of the common base diet (CON). Dogs that were pair housed together received the same dietary treatment each week. Dogs were split into two cohorts of four dogs that were matched for body weight and comprised one dog from each treatment group. Each dog was considered an experimental unit totaling eight experimental units per dietary treatment.
Meal response
Cohort 2 started their 7-d period 1 d after cohort 1, and the meal response was done on day 7; therefore, the two cohorts were tested on consecutive days. On day 7, the four dogs making up one cohort were weighed and brought to a separate treatment room that the dogs were familiar with at 0630 hours for catheter placement. Each dogs’ forearm was shaved and topical anesthetic (EMLA cream [2.5% lidocaine and 2.5% prilocaine], Astra Pharmaceuticals, L.P. Wayne, PA) was applied. After 20 min, each dogs’ front leg was cleaned with 4% chlorhexidine, 70% alcohol, and 0.5% chlorhexidine in that order, and then 20 G cephalic catheters (Insyte-W 20 G × 1.1, Becton Dickinson Canada Inc., Mississauga, ON) were placed and a 3-mL fasted sample (time 0) was taken immediately after placement. A three-way stopcock (Cardinal Health Canada, Vaughan, ON) was attached to each catheter and flushed with 0.5 mL of 125 United States Pharmacopeia (USP) heparinized saline and locked with 0.5 mL of 495 USP heparinized saline (Sandoz Canada Inc., Boucherville, QC). If a catheter could not be placed, a fasted sample was taken via cephalic or jugular vein. Once catheters were placed, volunteers who the dogs were familiar with sat with each dog to prevent the dog from licking or chewing at the catheter throughout the day. Dogs were fed 5 min apart from one another starting at approximately 0745 hours and time started and time finished eating were recorded. Immediately after the dog started eating, the timer was started and samples were collected at 15, 30, 60, 90, 120, 180, 240, 300, and 360 min after starting to eat the meal. All dogs ate all of their food on every sample day. For the first post-meal sample, 0.25 mL of blood was discarded as it was contaminated with heparin flush. For all other samples, flush was done after a sample was collected using the same port on the three-way stopcock, and blood was taken from the other port, so no blood was discarded. For every sample, 3 mL of blood was taken, placed in a 4-mL sodium heparin tube (Becton Dickinson Canada Inc., Mississauga, ON), and placed on ice. After every sample was taken, the catheter was flushed with 0.5 mL of 125 USP heparinized saline and locked with 0.5 mL of 495 USP heparinized saline via the same port on the three-way stopcock. If a catheter could not be placed, one fasted and three post-meal samples (30, 60, and 120 min post-meal) were collected via cephalic, jugular, or saphenous vein. Once all four samples were obtained at each collection, 1 mL of whole blood was separated and stored at −18 °C. The other 2 mL of blood was centrifuged at 4 °C at 12,000 × g for 10 min. Plasma was separated and stored at −18 °C. At the end of each sampling day, all samples were moved to a −80 °C freezer until analysis.
Plasma and whole blood AA analysis
Free AA concentrations in plasma and whole blood were analyzed using ultra-performance liquid chromatography (UPLC) (adapted from Bidlingmeyer et al., 1984; Waters Corporation, Milford, MA). Briefly, 100 μL of 10% sulfosalicylic acid (Sigma-Aldrich, St. Louis, MO) was used to deproteinate 100 μL of plasma or whole blood. Before deproteinization, whole blood was frozen at −80 °C and thawed twice to lyse open red blood cells, releasing taurine. Samples were then centrifuged at 14,000 × g for 5 min. AA standards and deproteinized samples were derivatized by an AccQ-Tag Ultra derivatization kit (Waters Corporation). The derivatized AAs (1 μL injection) were separated in an AccQ-Tag Ultra RP Column (2.1 × 100 mm, 1.7 μm; Waters Corporation) that was maintained at 55 °C using UPLC with UV detection (260 nm). AA peak areas were compared with known standards and analyzed with Waters Empower 2 Software (Waters Corporation).
Total plasma cysteine, homocysteine, and glutathione were analyzed using UPLC using an adapted method from Vester and Rasmussen (1991) and Pfeiffer et al. (1999). Briefly, 50 μL of 8-aminonaphthalene-1,3,6-trisulfonic acid, disodium salt (Thermo Fisher Scientific, Waltham, MA) in 0.1 M K-borate (pH 9.5) + 2 mM ethylenediaminetetraacetic acid (EDTA) solution (Sigma-Aldrich) and 30 μL of the reducing agent, tris(2-carboxyethyl)phosphine (Sigma-Aldrich) in 1X phosphate-buffered saline (Thermo Fisher Scientific) were added to 75 μL of plasma and 75 μL of the internal standard, N-acetyl-l-cysteine (Sigma-Aldrich) in 0.1 M K-borate (pH 9.5) + 2 mM EDTA. The samples were placed in the fridge for 30 min and then 125 μL of the derivatizing agent, 70% perchloric acid (Sigma-Aldrich), was added, and samples were allowed to sit for 10 min before being centrifuged at 12,000 × g for 1 min. In a light-sensitive centrifuge tube, 50 μL of supernatant was added to 100 μL of 2 M K-borate (pH 10.5) + 5 mM EDTA and 50 μL of the fluorescent thiol-specific dye, 7-fluorobenzofurazan-4-sulfonic acid ammonium salt (Sigma-Aldrich) in 0.1 M K-borate (pH 9.5) + 2 mM EDTA. Samples were then incubated in a water bath maintained at 60 °C for 60 min and then immediately after, put on ice for 5 min. Samples were centrifuged again at 12,000 × g for 1 min and then 100 μL was transferred to a UPLC vial for analysis. The derivatized thiols (1 μL injection) were separated in an Acquity UPLC BEH C18 Column (2.1 × 50 mm, 1.7 μm; Waters Corporation) that was maintained at 28 °C using UPLC with fluorescence detection at 515 nm emission and 385 nm excitation. Peak areas were compared with known standards and analyzed with Waters Empower 2 Software (Waters Corporation).
Statistical analysis
Body weight between pairs and over time was analyzed using the PROC GLIMMIX procedure in SAS (SAS version 9.4, SAS Inst., Inc., Cary, NC). Pair and period were treated as fixed effects, and the effect of pair, period, and their interaction was evaluated. Means were separated using the Tukey–Kramer adjustment, and results were deemed significant at P ≤ 0.05 and trends at 0.05 < P < 0.10.
Concentrations of all AA, total AA, total IDAA and total dispensable amino acids (DAA), and glutathione in plasma and whole blood over time were analyzed as repeated measures using the PROC GLIMMIX procedure in SAS (SAS version 9.4, SAS Inst., Inc., Cary, NC). Period and dog were treated as random effects and dietary treatment and time were treated as fixed effects. In the statistical model, the effect of dietary treatment, time, and their interaction was evaluated. For each variable, model assumptions were assessed through residual analysis. Four data points were much higher than the upper reference values reported by Delaney et al. (2003) and were more than 10 SD from the mean. Thus, these values were assumed to be a result of machine error and were removed. Means were separated using the Tukey–Kramer adjustment, and results were deemed significant at P ≤ 0.05 and trends at 0.05 < P < 0.10.
Results
All animals consumed all food and supplement and remained healthy throughout the study. Body weight between pairs was not different (P = 0.4581) and did not change during the course of the study (P = 1.0000). Not all blood samples were collected due to difficulties with catheters and, as such, number of observations varied for each treatment and time point.
Analyzed methionine content (Midwest Laboratories, Omaha, NE) in the control diet was approximately 1.48 times lower than predicted values, making total methionine content for MET treatment only 2.20 times the NRC (2006) RA (7.2 g/kg DM). Analyzed taurine content in the CON diet was approximately 1.44 times lower than predicted values, making total taurine content for the TAU treatment 1.6 g/kg DM. Analyzed choline content in the control diet was 1.33 times higher than predicted values, making the total choline content of the CCC treatment 2.30 times the canine NRC (2006) RA (3.8 g/kg of DM). Analyzed carnitine content in the control diet was 1.32 times higher than predicted values, making total carnitine content for the CCC treatment 0.33 g/kg DM (Table 1).
Table 1.
Analyzed nutrient composition of the control diet (CON; Nutrience Grain-Free Pork, Lamb and Duck Formula, Rolf C. Hagen Inc., QC, Canada1) and three test diets: control + methionine (MET), control + taurine (TAU), and control + methyl donors (choline) and methyl acceptors (creatine and carnitine) (CCC)2
| Nutrient | CON | MET | TAU | CCC |
|---|---|---|---|---|
| Proximate analysis, % | ||||
| Moisture | 6.93 | |||
| Crude protein | 29.60 | |||
| Fat | 16.63 | |||
| Crude Fiber | 3.31 | |||
| Nitrogen-free extract (calculated) | 34.55 | |||
| Ash | 8.98 | |||
| Calculated metabolizable energy,3 kcal/kg | 3,659 | |||
| Indispensable amino acids, % | ||||
| Arginine | 2.06 | |||
| Histidine | 0.61 | |||
| Isoleucine | 1.04 | |||
| Leucine | 1.97 | |||
| Lysine | 1.77 | |||
| Methionine | 0.43 | 0.67 | ||
| Cystine | 0.31 | |||
| Phenylalanine | 1.15 | |||
| Threonine | 0.83 | |||
| Tryptophan | 0.23 | |||
| Valine | 1.37 | |||
| Dispensable amino acids, % | ||||
| Alanine | 1.64 | |||
| Aspartic acid | 2.62 | |||
| Glutamic acid | 4.03 | |||
| Glycine | 2.32 | |||
| Proline | 1.67 | |||
| Serine | 1.24 | |||
| Taurine | 0.08 | 0.15 | ||
| Tyrosine | 0.76 | |||
| Other, mg/kg | ||||
| Choline | 2,393 | 3,534 | ||
| l-Carnitine | 80.67 | 306.90 | ||
| Creatine | 8,928 |
1Ingredients: Pork, lamb, deboned duck, green peas, pork meal, lentils, pork fat (preserved with mixed tocopherols), sweet potatoes, lamb meal, natural pork flavor, sun-cured alfalfa meal, salmon oil (source of docosahexaenoic acid), dicalcium phosphate (pumpkin, butternut squash, carrots, spinach, broccoli, apples, blueberries, cranberries, pomegranate, juniper berry extract, ginger, fennel, chamomile, peppermint leaf, licorice root, turmeric), salt, coconut oil, potassium chloride, calcium propionate, lecithin, choline chloride, dl-methionine, vitamins (vitamin E supplement, vitamin A supplement, niacin, calcium pantothenate, riboflavin, pyridoxine hydrochloride, thiamine mononitrate, biotin, vitamin B12 supplement, vitamin D3 supplement, folic acid), minerals (zinc sulfate, zinc proteinate, iron proteinate, ferrous sulfate, copper proteinate, copper sulfate, manganese proteinate, manganous oxide, calcium iodate, sodium selenite), yeast extract, chicory root extract, dried kelp, Yucca schidigera extract, glucosamine hydrochloride, l-lysine, taurine, chondroitin sulfate, rosemary extract (dried Lactobacillus acidophilus fermentation product, dried Lactobacillus casei fermentation product, dried Bifidobacterium bifidum fermentation product, dried Enterococcus faecium fermentation product), and l-carnitine.
2Values are presented as a percentage on an as-fed basis unless otherwise specified.
3Metabolizable energy = 8.5 kcal metabolizable energy (ME)/g crude fat + 3.5 kcal ME/g crude protein + 3.5 kcal ME/g nitrogen-free extract.
Plasma AA concentrations
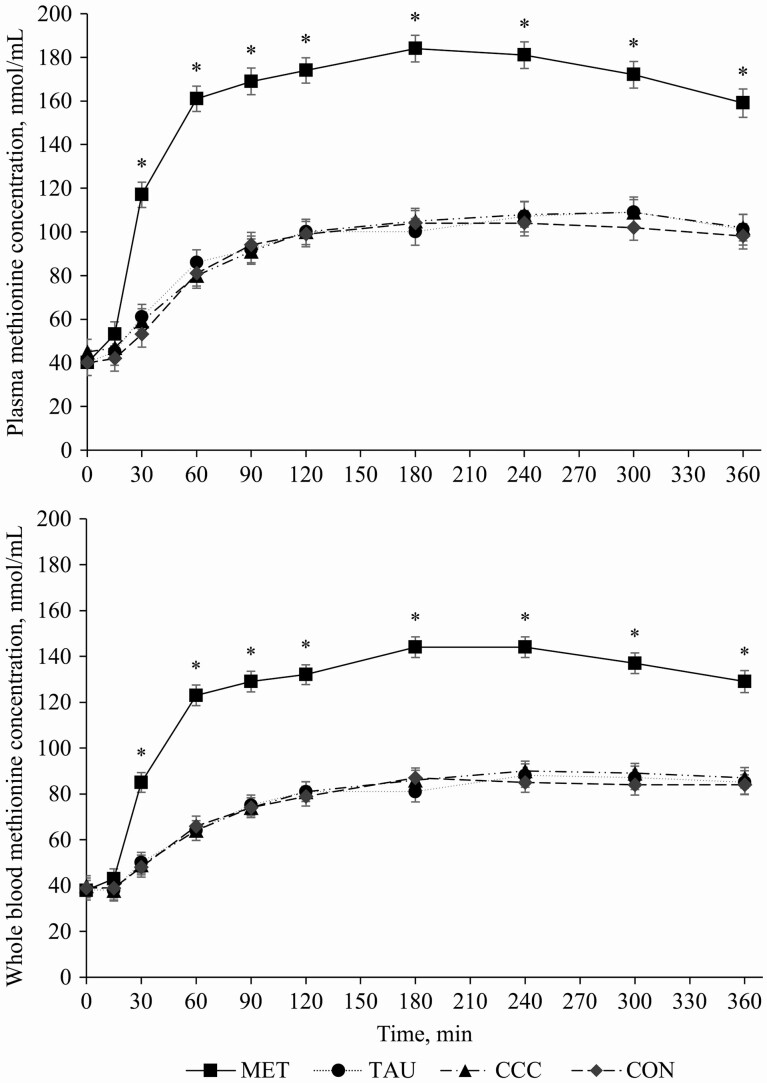
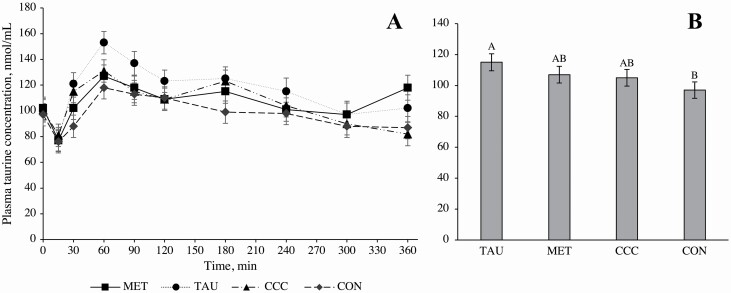
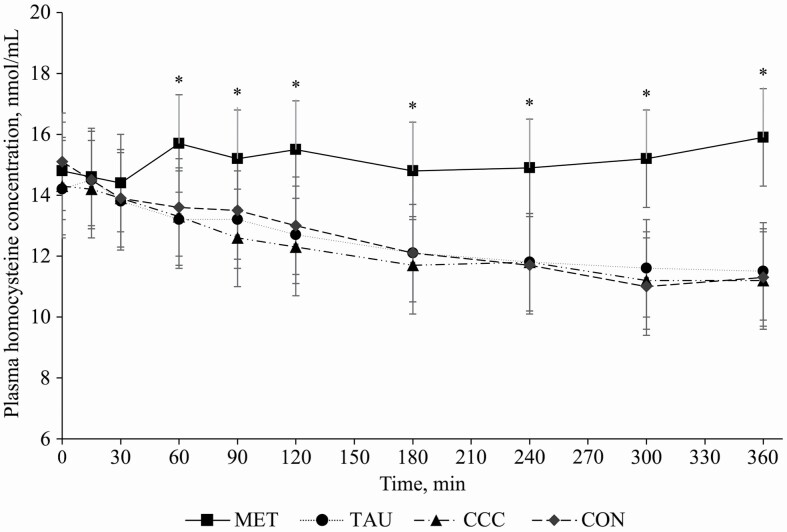
A time by treatment interaction effect was observed for plasma methionine concentrations (P < 0.0001), wherein dogs fed MET had greater plasma methionine concentrations at 30, 60, 90, 120, 180, 240, 300, and 360 min post-meal compared with dogs fed CON, TAU, and CCC (Figure 1). There was no difference in plasma methionine concentrations among dogs fed CON, TAU, or CCC at any time point (P > 0.05). A time effect (P < 0.0001) and treatment effect (P = 0.0162) were observed for plasma taurine concentrations. Taurine concentrations decreased at 15 min (P < 0.05), returned to fasted concentrations at 30 min (P > 0.05), increased from 60 to 90 min (P < 0.05), and then returned to fasted concentrations from 120 to 360 min (P > 0.05; Table 2). The plasma taurine concentration across time points in dogs fed TAU was 18.5% greater than dogs fed control but not different from dogs fed MET or CCC (P = 0.0162, Figure 2). There was also a time by treatment interaction effect for plasma homocysteine, where dogs fed MET had greater plasma homocysteine concentrations at 60, 90, 120, 180, 240, 300, and 360 min than dogs fed TAU, CCC, or CON (P < 0.0001), except at 90 min, where CON only tended to be different from MET (P = 0.0769; Figure 3). There was no time, treatment, or interaction effect for total plasma cysteine; however, there was a time effect for plasma cystine, where concentrations at 15 and 30 min were greater than concentrations at 90 and 120 min and concentrations at 360 min were greater than concentrations at 90, 120, 180, and 240 min (P < 0.05). Similarly, there was a time effect but no treatment or interaction effect for plasma glutathione, which increased from 60 to 360 min and did not return to fasted concentrations (P < 0.05; Table 2).
Figure 1.
Mean plasma (top) and whole blood (bottom) methionine concentrations (nmol/mL) in dogs from fasted (0 min) to 360 min after a meal on either control (CON), methionine (MET), taurine (TAU), or creatine, carnitine, and choline (CCC) supplementation. Values are presented as least squares means (lsmeans) ± SEM. The asterisk indicates a significant time by treatment interaction effect (P < 0.05) at each time point.
Table 2.
Concentration (lsmeans ± SEM nmol/mL) of plasma amino acids and glutathione that did not have a significant interaction effect in dogs across all treatments from fasted (0 min) to 360 min after a meal
| Time, min | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (Fasted) | 15 | 30 | 60 | 90 | 120 | 180 | 240 | 300 | 360 | |
| n | 32 | 31 | 32 | 32 | 30 | 32 | 30 | 28 | 28 | 26 |
| Total cysteine1 | 183 ± 9 | 183 ± 9 | 176 ± 9 | 172 ± 9 | 165 ± 9 | 164 ± 9 | 160 ± 9 | 158 ± 9 | 156 ± 9 | 158 ± 9 |
| Cystine | 21 ± 2 | 22 ± 2 | 22 ± 2 | 21 ± 2 | 18 ± 2 | 18 ± 2 | 18 ± 2 | 19 ± 2 | 22 ± 2 | 23 ± 2 |
| Glutathione1 | 13 ± 1 | 13 ± 1 | 14 ± 1 | 15 ± 1* | 15 ± 1* | 16 ± 1* | 16 ± 1* | 16 ± 1* | 16 ± 1* | 16 ± 1* |
| Taurine | 100 ± 6 | 78 ± 6* | 106 ± 6 | 132 ± 6* | 121 ± 6* | 113 ± 6 | 115 ± 6 | 104 ± 6 | 93 ± 6 | 97 ± 6 |
| Alanine | 265 ± 12 | 242 ± 12 | 335 ± 12* | 391 ± 12* | 366 ± 12* | 344 ± 12* | 318 ± 12 | 316 ± 13** | 326 ± 13* | 324 ± 13* |
| Arginine | 153 ± 9 | 145 ± 10 | 209 ± 9* | 273 ± 9* | 260 ± 10* | 243 ± 9* | 213 ± 10* | 208 ± 10** | 209 ± 10** | 205 ± 10** |
| Asparagine | 45 ± 4 | 44 ± 4 | 74 ± 4* | 105 ± 4* | 103 ± 4* | 101 ± 4* | 102 ± 4* | 109 ± 4* | 119 ± 4* | 121 ± 4* |
| Aspartic acid | 9 ± 2 | 10 ± 2 | 9 ± 2 | 11 ± 2 | 13 ± 2 | 15 ± 2* | 16 ± 2* | 16 ± 2* | 15 ± 2** | 15 ± 2 |
| Glutamine | 1,021 ± 28 | 984 ± 28 | 1,016 ± 28 | 952 ± 28* | 854 ± 28* | 808 ± 28* | 794 ± 28* | 801 ± 28* | 840 ± 28* | 890 ± 28* |
| Glutamic acid | 71 ± 7 | 69 ± 7 | 73 ± 7 | 82 ± 7 | 88 ± 7* | 94 ± 7* | 89 ± 7* | 89 ± 7* | 85 ± 7 | 85 ± 7 |
| Histidine | 74 ± 4 | 70 ± 4 | 85 ± 4* | 99 ± 4* | 100 ± 4* | 104 ± 4* | 108 ± 4* | 110 ± 4* | 110 ± 4* | 108 ± 4* |
| Isoleucine | 44 ± 5 | 46 ± 5 | 68 ± 5* | 98 ± 5* | 106 ± 5* | 118 ± 5* | 116 ± 5* | 113 ± 6* | 107 ± 6* | 94 ± 6* |
| Leucine | 96 ± 11 | 94 ± 11 | 131 ± 11* | 190 ± 11* | 211 ± 11* | 224 ± 11* | 219 ± 11* | 213 ± 11* | 199 ± 11* | 177 ± 12* |
| Lysine | 154 ± 10 | 145 ± 11 | 212 ± 10* | 285 ± 10* | 281 ± 11* | 265 ± 10* | 246 ± 11* | 242 ± 11* | 232 ± 11* | 228 ± 11* |
| Phenylalanine | 48 ± 3 | 48 ± 3 | 61 ± 3* | 77 ± 3* | 82 ± 3* | 86 ± 3* | 83 ± 3* | 80 ± 3* | 77 ± 3* | 71 ± 3* |
| Proline | 126 ± 11 | 123 ± 11 | 206 ± 11* | 295 ± 11* | 303 ± 11* | 303 ± 11* | 301 ± 11* | 325 ± 11* | 346 ± 11* | 349 ± 11* |
| Serine | 139 ± 11 | 134 ± 12 | 157 ± 11 | 194 ± 11* | 182 ± 12** | 179 ± 11** | 178 ± 12 | 211 ± 12* | 209 ± 12* | 225 ± 12* |
| Threonine | 141 ± 12 | 135 ± 12 | 166 ± 12* | 215 ± 12* | 224 ± 12* | 228 ± 12* | 230 ± 12* | 245 ± 12* | 263 ± 12* | 271 ± 12* |
| Tryptophan | 68 ± 6 | 80 ± 6* | 95 ± 6* | 108 ± 6* | 106 ± 6* | 109 ± 6* | 114 ± 6* | 116 ± 6* | 116 ± 6* | 111 ± 6* |
| Tyrosine | 34 ± 3 | 33 ± 3 | 47 ± 3* | 68 ± 3* | 70 ± 3* | 73 ± 3* | 68 ± 3* | 66 ± 3* | 63 ± 3* | 62 ± 3* |
| Valine | 130 ± 13 | 126 ± 13 | 163 ± 13 | 254 ± 13* | 282 ± 13* | 321 ± 13* | 332 ± 13* | 332 ± 14* | 320 ± 14* | 290 ± 14* |
| Total AA | 2,982 ± 87 | 2,859 ± 87 | 3,595 ± 87* | 4,324 ± 87* | 4,241 ± 88* | 4,199 ± 87* | 4,121 ± 88* | 4,227 ± 90* | 4,293 ± 90* | 4,307 ± 92* |
| Total IDAA2 | 951 ± 44 | 932 ± 44 | 1,262 ± 44* | 1,699 ± 44* | 1,763 ± 44* | 1,806 ± 44* | 1,779 ± 44* | 1,785 ± 45* | 1,756 ± 45* | 1,671 ± 46* |
| Total DAA3 | 2,031 ± 65 | 1,926 ± 65 | 2,332 ± 65* | 2,625 ± 65* | 2,478 ± 65* | 2,393 ± 65* | 2,341 ± 65* | 2,442 ± 67* | 2,537 ± 67* | 2,640 ± 68* |
1Values were computed in a separate analysis from the other amino acids (AAs).
2Indispensable amino acids.
3Dispensable amino acids.
*Indicates concentration is significantly different from fasted sample (time = 0, P ≤ 0.05).
**Indicates concentration tends to be different from fasted sample (between P > 0.05 and P ≤ 0.10).
Figure 2.
Mean plasma taurine concentrations (nmol/mL) in dogs from fasted (0 min) to 360 min after a meal on either control (CON), methionine (MET), taurine (TAU), or creatine, carnitine, and choline (CCC) supplementation (A). Values are presented as lsmeans ± SEM. The time by treatment interaction effect is not significant at each time point (P > 0.05). Mean plasma taurine concentrations (nmol/mL) pooled across time in dogs on either CON, MET, TAU, or CCC supplementation (B). Values are presented as lsmeans ± SEM, n = 68 for TAU, n = 74 for MET, n = 79 for CCC, and n = 80 for CON. Treatments that do not share a common letter are significantly different from each other (P ≤ 0.05).
Figure 3.
Mean plasma homocysteine concentrations (nmol/mL) in dogs from fasted (0 min) to 360 min after a meal on either control (CON), methionine (MET), taurine (TAU), or creatine, carnitine, and choline (CCC) supplementation. Values are presented as lsmeans ± SEM. The asterisk indicates a significant time by treatment interaction effect (P < 0.05) at each time point.
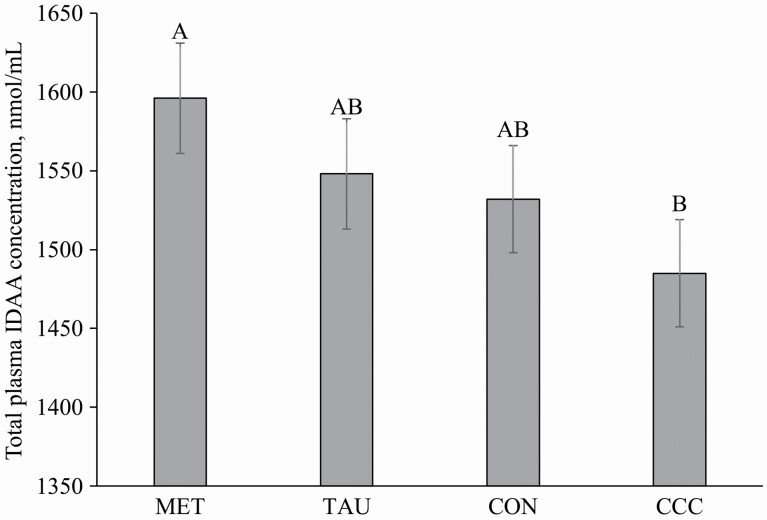
A time effect (P < 0.05) was observed for all other plasma AAs (Table 2). Plasma alanine increased from 30 to 120 min (P < 0.05), returned to fasted concentrations from 180 to 240 min (P > 0.05), and then increased again from 300 to 360 min (P < 0.05). Plasma arginine increased from 30 to 180 min (P < 0.05) and then returned to fasted concentrations from 240 to 360 min (P > 0.05). Plasma asparagine, histidine, isoleucine, leucine, lysine, phenylalanine, proline, threonine, and tyrosine all increased from 30 to 360 min and did not return to fasted concentrations (P < 0.05). Plasma aspartic acid increased from 120 to 240 min (P < 0.05) and then returned to fasted concentrations from 300 to 360 min (P > 0.05). Plasma glutamine decreased from 60 to 360 min and did not return to fasted concentrations (P < 0.05). Plasma glutamic acid increased from 90 to 240 min (P < 0.05) and then returned to fasted concentrations from 300 to 360 min (P > 0.05). Plasma serine increased at 60 min (P < 0.05), returned to fasted concentrations from 90 to 180 min (P > 0.05), and then increased again from 240 to 360 min (P < 0.05). Plasma tryptophan increased from 15 to 360 min and did not return to fasted concentrations (P < 0.05). Finally, plasma valine increased from 60 to 360 min and did not return to fasted concentrations (P < 0.05). Interestingly, a time by treatment interaction effect was observed for plasma glycine concentrations (P = 0.0444), where at 240 min, glycine concentrations on CCC treatment were lower than CON. The total plasma AA, total IDAA, and total DAA all increased from 30 to 360 min compared with fasted concentrations (P < 0.05). There was also a treatment effect for total IDAA (P = 0.0361), where the concentration of total IDAA for dogs fed MET was 7.5% greater than dogs fed CCC (Figure 4).
Figure 4.
Mean total plasma indispensable amino acid concentrations (IDAA) (nmol/mL) pooled across time in dogs on either control (CON), methionine (MET), taurine (TAU), or creatine, carnitine, and choline (CCC) supplementation. Values are presented as lsmeans ± SEM. Treatments that do not share a common letter are significantly different from each other (P ≤ 0.05).
Whole blood AA concentrations
A time by treatment interaction effect was also observed for whole blood methionine concentrations (P < 0.0001), wherein dogs fed MET had greater whole blood methionine concentrations at 30, 60, 90, 120, 180, 240, 300, and 360 min post-meal compared with dogs fed CON, TAU, and CCC (Figure 1). There was no difference in whole blood methionine concentrations among dogs fed CON, TAU, or CCC at any time point (P > 0.05). Whole blood taurine concentrations for dogs fed TAU were 5.8% greater than dogs fed CCC; however, this was only a trend in the data (P = 0.0558). There was also a trend toward significance for whole blood taurine concentrations over time (P = 0.0713) where concentrations were only significantly greater at 60 vs. 15 min (P = 0.0198). In addition, whole blood cysteine concentrations were 17% greater in dogs fed TAU compared with dogs fed CON, but this was only a trend in the data (P = 0.0605).
A time effect (P < 0.05) was also observed for all whole blood AA concentrations with the exception of glutamic acid and cystine (Table 3). Although the null hypothesis was rejected in the F-test (P = 0.0452), no significant differences were observed between time points for whole blood glutamic acid when pairwise comparisons were analyzed using the Tukey–Kramer adjustment. Whole blood alanine, asparagine, glycine, isoleucine, leucine, lysine, phenylalanine, proline, tryptophan, and valine concentrations all increased from 30 to 360 min and did not return to fasted concentrations (P < 0.05). In addition, whole blood valine was 5% greater in dogs fed MET compared with dogs fed CCC, but this was only a trend in the data (P = 0.0795). Whole blood arginine concentrations increased from 30 to 180 min (P < 0.05), returned to fasted from 240 to 300 (P > 0.05), and then increased at 360 min (P < 0.05). Whole blood aspartic acid, threonine, and tyrosine concentrations increased from 60 to 360 min and did not return to fasted concentrations (P < 0.05). Whole blood glutamine and histidine concentrations increased from 90 to 360 min and did not return to fasted concentrations (P < 0.05). Whole blood serine concentrations increased at 15 min (P < 0.05), returned to fasted concentrations at 30 min (P > 0.05), increased from 60 to 120 min (P < 0.05), returned to fasted concentrations at 180 min (P > 0.05), and then increased from 240 to 360 min (P < 0.05). Finally, total AA, total IDAA, and total DAA all increased over time, compared with fasted concentrations, from 30 to 360 min for total AA and IDAA (P < 0.05). The total DAA significantly decreased at 15 min and increased from 30 to 360 min compared with fasted concentrations (P < 0.05). Whole blood total IDAA concentration was 5.2% greater in dogs fed MET compared with dogs fed CCC, but this was only a trend (P = 0.060).
Table 3.
Concentration (lsmeans ± SEM nmol/mL) of all whole blood amino acids that did not have a significant interaction effect in dogs across all treatments from fasted (0 min) to 360 min after a meal
| Time, min | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (Fasted) | 15 | 30 | 60 | 90 | 120 | 180 | 240 | 300 | 360 | |
| n | 32 | 30 | 31 | 31 | 30 | 32 | 30 | 28 | 27 | 26 |
| Cystine | 15 ± 0.7 | 15 ± 0.8 | 15 ± 0.7 | 16 ± 0.7 | 15 ± 0.8 | 15 ± 0.7 | 15 ± 0.8 | 15 ± 0.8 | 16 ± 0.8 | 15 ± 0.8 |
| Taurine | 238 ± 6 | 227 ± 6 | 245 ± 6 | 262 ± 6 | 263 ± 6 | 259 ± 6 | 255 ± 6 | 252 ± 6 | 237 ± 6 | 238 ± 7 |
| Alanine | 264 ± 10 | 242 ± 10 | 308 ± 10* | 362 ± 10* | 354 ± 10* | 341 ± 10* | 315 ± 10* | 318 ± 11* | 322 ± 11* | 324 ± 11* |
| Arginine | 237 ± 6 | 230 ± 6 | 263 ± 6* | 296 ± 6* | 293 ± 6* | 284 ± 6* | 268 ± 6* | 267 ± 6** | 266 ± 7 | 270 ± 7* |
| Asparagine | 50 ± 3 | 47 ± 3 | 63 ± 3* | 84 ± 3* | 86 ± 3* | 85 ± 3* | 89 ± 3* | 94 ± 3* | 104 ± 3* | 105 ± 3* |
| Aspartic acid | 37 ± 2 | 37 ± 2 | 40 ± 2 | 45 ± 2* | 46 ± 2* | 46 ± 2* | 45 ± 2* | 49 ± 2* | 47 ± 2* | 47 ± 2* |
| Glutamine | 700 ± 28 | 678 ± 30 | 695 ± 34 | 671 ± 34 | 629 ± 30* | 597 ± 27* | 584 ± 27* | 574 ± 28* | 591 ± 28* | 614 ± 31* |
| Glutamic acid | 92 ± 7 | 88 ± 7 | 90 ± 7 | 92 ± 7 | 91 ± 7 | 100 ± 7 | 93 ± 7 | 102 ± 7 | 90 ± 7 | 95 ± 7 |
| Glycine | 217 ± 12 | 192 ± 12** | 265 ± 12* | 346 ± 12* | 350 ± 12* | 332 ± 12* | 333 ± 12* | 368 ± 12* | 402 ± 12* | 423 ± 13* |
| Histidine | 92 ± 3 | 85 ± 3 | 93 ± 3 | 101 ± 3** | 103 ± 3* | 106 ± 3* | 109 ± 3* | 113 ± 3* | 112 ± 3* | 113 ± 3* |
| Isoleucine | 53 ± 4 | 51 ± 4 | 63 ± 4* | 86 ± 4* | 98 ± 4* | 108 ± 4* | 112 ± 4* | 112 ± 4* | 109 ± 4* | 100 ± 4* |
| Leucine | 99 ± 7 | 96 ± 7 | 123 ± 7* | 166 ± 7* | 184 ± 7* | 197 ± 7* | 198 ± 7* | 194 ± 7* | 186 ± 7* | 169 ± 7* |
| Lysine | 269 ± 12 | 269 ± 12 | 305 ± 12* | 349 ± 12* | 349 ± 12* | 340 ± 12* | 333 ± 12* | 326 ± 12* | 326 ± 12* | 321 ± 12* |
| Phenylalanine | 50 ± 2 | 49 ± 2 | 63 ± 2* | 69 ± 2* | 75 ± 2* | 80 ± 2* | 78 ± 2* | 80 ± 2* | 74 ± 3* | 72 ± 3* |
| Proline | 132 ± 9 | 124 ± 9 | 173 ± 9* | 240 ± 9* | 259 ± 9* | 265 ± 9* | 276 ± 9* | 297 ± 9* | 316 ± 9* | 326 ± 9* |
| Serine | 156 ± 5 | 140 ± 6* | 158 ± 6 | 175 ± 5* | 180 ± 6* | 174 ± 6* | 172 ± 6 | 190 ± 6* | 206 ± 6* | 219 ± 6* |
| Threonine | 154 ± 10 | 145 ± 10 | 163 ± 10 | 192 ± 10* | 203 ± 10* | 207 ± 10* | 213 ± 10* | 225 ± 10* | 240 ± 10* | 250 ± 10* |
| Tryptophan | 40 ± 2 | 45 ± 2 | 54 ± 2* | 54 ± 2* | 55 ± 2* | 59 ± 2* | 61 ± 2* | 67 ± 2* | 65 ± 3* | 64 ± 3* |
| Tyrosine | 64 ± 2 | 61 ± 2 | 69 ± 2 | 78 ± 2* | 82 ± 2* | 84 ± 2* | 82 ± 2* | 83 ± 2* | 81 ± 2* | 83 ± 2* |
| Valine | 132 ± 9 | 125 ± 9 | 148 ± 9* | 201 ± 9* | 236 ± 9* | 264 ± 9* | 290 ± 9* | 297 ± 9* | 296 ± 9* | 276 ± 9* |
| Total AA | 3,131 ± 72 | 2,986 ± 73 | 3,455 ± 72* | 3,965 ± 72* | 4,038 ± 72* | 4,032 ± 72* | 4,023 ± 73* | 4,127 ± 74* | 4,186 ± 74* | 4,223 ± 75* |
| Total IDAA1 | 1,165 ± 27 | 1,134 ± 27 | 1,334 ± 27* | 1,593 ± 27* | 1,683 ± 27* | 1,733 ± 27* | 1,762 ± 27* | 1,783 ± 28* | 1,775 ± 28* | 1,734 ± 29* |
| Total DAA2 | 1,965 ± 64 | 1,852 ± 64* | 2,121 ± 64* | 2,372 ± 64* | 2,355 ± 64* | 2,299 ± 64* | 2,260 ± 64* | 2,342 ± 65* | 2,410 ± 65* | 2,487 ± 65* |
1Indispensable amino acids.
2Dispensable amino acids.
*Indicates concentration is significantly different from fasted sample (time = 0, P ≤ 0.05).
**Indicates concentration tends to be different from fasted sample (between P > 0.05 and P ≤ 0.10).
Plasma–whole blood comparison
When fasted concentrations of each AA were compared in plasma vs. whole blood, arginine, asparagine, aspartic acid, glutamic acid, glycine, histidine, isoleucine, lysine, phenylalanine, taurine, threonine, and tyrosine concentrations were all greater in whole blood compared with plasma (P < 0.05; Table 4). In contrast, glutamine, methionine, and tryptophan concentrations were all greater in plasma compared with whole blood (P < 0.05; Table 4). Alanine, proline, leucine, and serine concentrations were not statistically different in whole blood compared with plasma (P > 0.05; Table 4). Finally, the total IDAA and total AA were greater in whole blood compared with plasma (P < 0.05), whereas total DAAs were not different in whole blood or plasma (P > 0.05; Table 4).
Table 4.
Concentration (lsmeans ± SEM nmol/mL) of fasted plasma and whole blood amino acids in dogs across all treatments
| Fasted1 concentration, nmol/mL | |||
|---|---|---|---|
| Plasma | Whole blood | P-value | |
| n | 32 | 32 | |
| Alanine | 265 ± 10 | 264 ± 10 | 0.8854 |
| Arginine | 153 ± 5 | 237 ± 5* | <0.0001 |
| Asparagine | 45 ± 3 | 50 ± 3* | 0.0085 |
| Aspartic acid | 9 ± 2 | 37 ± 2* | <0.0001 |
| Glutamine | 1,021 ± 38* | 700 ± 38 | <0.0001 |
| Glutamic acid | 71 ± 6 | 92 ± 6* | <0.0001 |
| Glycine | 202 ± 8 | 217 ± 2* | 0.0352 |
| Histidine | 74 ± 2 | 92 ± 2* | <0.0001 |
| Isoleucine | 44 ± 3 | 53 ± 3* | <0.0001 |
| Leucine | 96 ± 6 | 99 ± 6 | 0.6163 |
| Lysine | 154 ± 13 | 269 ± 13* | <0.0001 |
| Methionine | 42 ± 2* | 39 ± 2 | 0.0411 |
| Phenylalanine | 48 ± 2 | 50 ± 2* | 0.0444 |
| Proline | 126 ± 7 | 132 ± 7 | 0.1096 |
| Serine | 139 ± 8 | 156 ± 8 | 0.0545 |
| Taurine | 100 ± 6 | 238 ± 6* | <0.0001 |
| Threonine | 141 ± 12 | 154 ± 12* | 0.0011 |
| Tryptophan | 68 ± 4* | 40 ± 4 | <0.0001 |
| Tyrosine | 34 ± 2 | 64 ± 2* | <0.0001 |
| Valine | 130 ± 8 | 132 ± 8 | 0.6542 |
| Total AA | 2,982 ± 57 | 3,131 ± 57* | 0.0049 |
| Total IDAA2 | 951 ± 36 | 1,165 ± 36* | <0.0001 |
| Total DAA3 | 2,031 ± 44 | 1,965 ± 44 | 0.0890 |
1Fasted samples were taken at time 0, after approximately 23 h of fast.
2Indispensable amino acids.
3Dispensable amino acids.
*Indicates amino acid concentrations are significantly different (P ≤ 0.05).
Discussion
The goal of this study was 2-fold; first, to investigate the effects of supplementing either methionine, taurine, or creatine, carnitine, and choline on plasma and whole blood AA concentrations in dogs consuming a grain-free diet and, second, to quantify the post-meal AA response in both plasma and whole blood. According to the NRC (2006) and AAFCO (2019), the RA of methionine for adult dogs at maintenance is 0.33% DM and the methionine + cystine RA is 0.65% DM. Since this diet was formulated for all life stages, it exceeded the methionine RA, at 0.46% DM, and the methionine + cystine RA, at 0.80%. After supplementation, the MET diet was at 0.72% DM methionine, more than double the RA, and at 1.05% methionine + cystine. In addition, crystalline taurine was already added to the CON diet resulting in 0.086% DM of taurine. After supplementation, the taurine content in the TAU diet was 0.16% DM.
As hypothesized, dogs that were supplemented with methionine had greater plasma and whole blood methionine concentrations from 30 to 360 min post-meal compared with dogs fed CON, TAU, or CCC. This also led to greater concentration of total IDAA in dogs fed MET compared with dogs fed CCC. Plasma methionine concentrations were positively correlated with plasma taurine concentrations in dogs (Delaney et al., 2003; Backus et al., 2006) when these AAs are provided at greater levels than the requirement. However, the study by Delaney et al (2003) used client-owned dogs, did not control the amount fed, and noted that dogs were fed 3 to 5 h before blood collection, which likely resulted in greater variability. Our results show that methionine concentrations increase from approximately 60 min (30 for dogs on MET) to 360 min and do not return to baseline. However, plasma taurine concentrations returned to fasted levels by 120 min, suggesting that the methionine levels may be elevated compared with the taurine levels at 3 to 5 h after a meal.
In the methionine-supplemented group, we expected to see an increase in cysteine and glutathione concentrations, but this was not the case. There is limited work on methionine supplementation above the requirement in dogs and cats. Chen et al. (2014) found that after 7 d of supplementing methionine to piglets, both cysteine and glutathione concentrations increased compared with the control group. However, the control diet was deficient in methionine, and the “methionine-supplemented” group was only supplemented to meet the requirement of a growing piglet. Additionally, neither plasma methionine nor taurine concentrations were elevated compared with the control group (Chen et al., 2014). Similarly, when Newfoundland dogs were supplemented with 3 g of methionine per kilogram of diet (as-fed), they had increased fasted plasma taurine, methionine, and cysteine concentrations after 30 d (Backus et al., 2003). However, these dogs were clinically diagnosed as taurine deficient and had plasma taurine concentrations below 40 nmol/mL at the start of the trial. In the present study, all dogs had plasma taurine concentrations above 40 nmol/mL throughout the trial. Perhaps, the effects of methionine supplementation on primary and secondary metabolites of sulfur AA metabolism are more prominent when the metabolic requirement of methionine is increased (i.e., oxidative stress; Green et al., 2012) or where the basal diet is deficient in sulfur AAs, which was not the case in the present study.
As hypothesized, supplementation of taurine did not increase methionine concentrations and supplementation with methionine did not increase taurine concentrations compared with control. Many factors can influence the requirement of AAs and one limitation of only measuring plasma and whole blood AA concentrations is the difficulty in inferring AA metabolism or flux from these data. Plasma AAs respond as a function of their peripheral use for protein synthesis and secondary metabolite synthesis. When below the individual animals’ requirement, they will remain low. Only after the requirement is met will they increase in the central blood pool (Zello et al., 1993). The methionine content in the control diet in the present study was already above the RA (NRC, 2006) and was also supplemented with taurine. Furthermore, the dogs used in the present study were all deemed healthy. However, supplementation of these nutrients in animals with pathological conditions may be beneficial. For example, the sulfur AA requirements (methionine and methionine + cystine) in piglets increase when piglets are challenged with a bacterial load (Litvak et al., 2013; Rakhshandeh et al., 2014; Rodrigues et al., 2021), likely due to the increase in demand for cysteine to be used in the synthesis of glutathione, an antioxidant (Malmezat et al., 2000). Taurine also has antioxidant activity in the mitochondria of cardiomyocytes (Jong et al., 2012); therefore, it is likely that when the heart is under oxidative stress, the metabolic requirement of taurine may be higher, which is partially supported by taurine deficiency being linked with DCM in dogs (Kittleson et al., 1997). Given that healthy dogs were used in this study, the control diet contained an excess of methionine and was also supplemented with taurine, there was no increase in demand for methionine or taurine, and this may explain the results observed in the present study in comparison to the previous studies. Future studies should consider the investigation of the methionine and total sulfur AA requirements in dogs under metabolic or immune stress as the requirements for these nutrients may be higher and increase the risk of secondary nutritional diseases.
In contrast to our hypotheses, plasma total cysteine was not affected by dietary treatment, and methionine concentrations did not increase in dogs fed the CCC diet compared with the CON diet. It was hypothesized that methionine would increase in dogs fed CCC since choline acts as a methyl donor via betaine, and the supplementation of creatine and carnitine would decrease the need for SAM methylation to produce these compounds (Stead et al., 2006). An alternative explanation is that the CCC treatment did increase methionine concentration, but it was immediately oxidized and was, therefore, not captured in the plasma and whole blood data. Much of the work that investigates the remethylation capacity of choline (via betaine) and folate are done using a methionine-restricted diet, whereas even the CON diet in this study was in excess, which could help explain our lack of findings in the CCC group. However, Robinson et al. (2018) also reported no change in plasma methionine concentrations in piglets after a methionine-restricted diet that was deficient in choline, betaine, and folate was then supplemented with betaine and/or folate. In fact, both remethylation and transmethylation significantly increased after supplementation with betaine and/or folate, leading to a significant decrease in plasma homocysteine, but no change in methionine, cysteine, or taurine (Robinson et al., 2018). These results are similar to ours and highlight the important interplay between AA used for protein and metabolite synthesis. However, we saw no difference in plasma homocysteine in the CCC group compared with the CON group and this is likely due to the fact that none of our diets were deficient in methionine or methyl donors. This again highlights the limitations to only measuring plasma and whole blood AA concentrations and perhaps we would have seen increases in remethylation and transmethylation in the CCC group in the present study.
Although supplementation with CCC did not cause any change in homocysteine concentrations compared with control, supplementation with methionine did increase homocysteine concentrations. In a long-term feeding trial conducted by Harrison et al. (2020), adult Labrador Retrievers were fed semi-purified diets with differing levels of methionine for 32 wk. Their control diet contained ~0.55% DM methionine, exceeding the current NRC recommendation and their two test diets were both below the current minimum requirement (test diet 1: ~0.22% DM and test diet 2: ~0.28% DM). Their data for plasma homocysteine paralleled ours, in that over 32 wk, the dogs on control showed no difference in homocysteine concentrations, but dogs on the methionine-restricted diets showed decreasing homocysteine concentrations (Harrison et al., 2020). In the 6 h after a meal in the current study, we saw a similar pattern, and, together, these results suggest both immediate and lasting effects of methionine supplementation/restriction.
Elevated levels of homocysteine, a condition known as hyperhomocysteinemia, have been linked to cardiovascular disease in humans (Clarke et al., 1991) and possibly in dogs (Rossi et al., 2008; Lee et al., 2017). One model that is often used in hyperhomocysteinemia research is the rodent. However, in contrast to dogs, where methionine restriction decreased plasma homocysteine concentrations (Harrison et al., 2020), in rats, methionine restriction has been shown to elevate homocysteine concentrations (Elshorbagy et al., 2010). Interestingly, despite methionine restriction causing hyperhomocysteinemia and a larger heart to body weight ratio in mice, it did not affect cardiac function according to electrocardiogram (Ables et al., 2015). Instead, methionine-restricted mice showed an increase in both adiponectin and fibroblast growth factor 21, two hormones that are involved in cardioprotection (Ables et al., 2015). In dogs, a serum homocysteine reference range has been established by one study as 5.0 to 22.1 µmol/L (Grützner et al., 2013) and has been shown to be positively correlated with heart disease (Lee et al., 2017); however, more research is needed to establish what is considered hyperhomocysteinemia in different dog breeds and at what levels homocysteine starts to impair cardiac function. In the present study, even dogs fed supplemental methionine were below the upper range defined by Grützner et al. (2013). Therefore, supplementation of CCC may be able to help maintain lower homocysteine concentrations in dogs with cardiac conditions, compared with methionine supplementation. This avenue of research should be explored as nutritionists and veterinarians continue to investigate the causes of nutritionally mediated DCM related to grain-free diets, especially since there is currently contradictory information surrounding homocysteine’s role in the development of cardiac dysfunction in dogs.
Together, our findings suggest a role for CCC supplementation in dogs with pathological conditions caused by increased oxidative stress or in grain-free diets that lack sufficient methyl donors or bioavailable sulfur AAs. As mentioned earlier, cardiomyocytes under oxidative stress require taurine to act as an antioxidant (Jong et al., 2012) and pigs challenged with bacteria require more sulfur AAs, likely to produce glutathione (Malmezat et al., 2000; Litvak et al., 2013; Rakhshandeh et al., 2014). If a diet is deficient in methyl donors, CCC could increase remethylation, as seen in methionine-restricted diets (Robinson et al., 2018), resulting in more methionine available for the aforementioned metabolic needs without the corresponding increase in homocysteine when methionine is supplemented. Additionally, if a dog is under oxidative stress, but the diet is meeting methionine requirements, additional CCC could spare available cysteine to produce taurine and/or glutathione. In fact, in adult healthy women undergoing regular moderate weekly exercise, supplementation of both choline and carnitine significantly decreased a measure of oxidative stress and increased the antioxidants measured (retinol and α-tocopherol; Sachan et al., 2005).
A secondary outcome of the current study was post-meal AA concentration data, which is limited in dogs to date. The majority of plasma and whole blood AAs had a significant overall time effect, with most of them increasing following a meal. While there are no complete data sets to compare these results to in dogs, some postprandial AA concentrations have been reported. Gray et al (2016) found that plasma taurine measured 5 h after a meal did not decrease until 47 h post-meal and increased 1 h after re-feeding in adult Labrador Retrievers. Although they compared the post-meal time points to a sample that was taken 5 h after a meal, this sample was not significantly different from the 24 h post-meal sample, which would have been equivalent to our fasted sample. In contrast, in the present study, plasma taurine decreased at 15 min post-meal, returned to fasted levels at 30 min, increased from 60 to 90 min post-meal, and then returned to fasted levels from 120 to 360 min post-meal, and whole blood taurine concentrations increased from 60 to 180 min post-meal and then returned to fasted levels from 240 to 360 min. One major difference in design that distinguishes the present study from others is the more frequent sampling times. We sampled at 15, 30, 60, and 90 min post-meal and found significant changes at both 15 and 90 min for plasma taurine, whereas Gray et al. (2016) only sampled at 1 and 2 h, missing some of the changes that we observed.
Another study done by Söder et al. (2019) measured plasma AA concentrations at 1, 2, 3, and 4 h after a meal in both lean and obese Labrador Retrievers and found a significant time effect for all AAs they measured. They reported similar trends in AA concentration over time, compared with the present study, for alanine, arginine, glycine, isoleucine, leucine, proline, valine, and glutamic acid. Söder et al. (2019) reported that plasma lysine increased at 1 h after a meal and then returned to fasted levels from 2 to 4 h after a meal. In contrast, in the present study, plasma lysine increased from 30 min to 6 h after a meal. Additionally, in the present study, majority of the AAs measured, including total AA, total IDAA, and total DAA, increased after a meal and did not return to fasted levels by 6 h post-meal. Interestingly, this aligns with a greater postprandial increase in several AA concentrations in cats fed once daily (Camara et al., 2020). Although it was not a direct outcome of this study, the Beagles were also fed once daily, supporting the hypothesis that less frequent feeding may promote greater protein synthesis (El-Kadi et al., 2018).
In conclusion, the results from this study highlight that additional supplementation of methionine on top of a complete and balanced grain-free dog diet led to increased plasma and whole blood methionine and plasma homocysteine concentrations, but no changes in plasma taurine compared with the control diet. When taurine was supplemented on top of a grain-free diet, only plasma taurine concentrations were elevated compared with control. When the methyl donors and receivers were supplemented, no changes in any of the AAs of interest were observed compared with control. In addition, almost all of the AAs showed significant changes in both plasma and whole blood after consumption of a meal, compared with fasted concentrations. In fact, many of them remained elevated 6 h after feeding. Therefore, based on the current study, it is recommended that when collecting blood to assess plasma and whole blood taurine concentrations, a minimum of 2 h between meal consumption and blood collection be achieved in order to prevent potentially elevated concentrations of taurine and possible misdiagnoses of secondary nutritionally mediated diseases where taurine is a key diagnostic metric. Furthermore, there may be a role for the supplementation of creatine, carnitine, and choline in grain-free dog diets in order to support methionine status without increasing homocysteine concentrations.
Acknowledgments
We would like to thank Dr. Renan Antunes Donadelli and Cuilan Zhu for their help with laboratory analysis and Michelle Cieplak and Tammy Buitenhuis for their help with catheterizing the dogs. This research was funded by Rolf C Hagen, Inc. (Baie d′Urfé, QC).
Glossary
Abbreviations
- AA
amino acid
- DAA
dispensable amino acids
- DCM
dilated cardiomyopathy
- DM
dry matter
- EDTA
ethylenediaminetetraacetic acid
- IDAA
indispensable amino acids
- RA
recommended allowance
- SAM
S-adenosylmethionine
- UPLC
ultra-performance liquid chromatography
Authors’ Contributions
S.B. and A.K.S. designed the experiment. S.B. and J.G.P. conducted the research, S.B. analyzed the data, and all authors contributed to the writing of the manuscript. A.K.S. had primary responsibility for the final content. All authors read and approved the final manuscript.
Conflict of interest statement
The authors S.B., J.G.P., M.B., and K.M.W. have no conflicts of interest. A.V. is the Royal Canin Veterinary Diets Endowed Chair in Canine and Feline Clinical Nutrition and declares that she serve on pet industry-related scientific advisory boards and have received honoraria and research funding from various pet food manufacturers and ingredient suppliers. A.K.S. declares that she serve on the Trouw Nutrition scientific advisory board and has received honoraria and research funding from various pet food manufacturers and ingredient suppliers.
Literature Cited
- Ables, G. P., Ouattara A., Hampton T. G., Cooke D., Perodin F., Augie I., and Orentreich D. S.. . 2015. Dietary methionine restriction in mice elicits an adaptive cardiovascular response to hyperhomocysteinemia. Sci. Rep. 5:8886. doi: 10.1038/srep08886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of American Feed Control Officials (AAFCO) . 2019. AAFCO manual. West Lafayette (IN): AAFCO Inc. [Google Scholar]
- Backus, R. C., Cohen G., Pion P. D., Good K. L., Rogers Q. R., and Fascetti A. J.. . 2003. Taurine deficiency in Newfoundlands fed commercially available complete and balanced diets. J. Am. Vet. Med. Assoc. 223:1130–1136. doi: 10.2460/javma.2003.223.1130 [DOI] [PubMed] [Google Scholar]
- Backus, R. C., Ko K. S., Fascetti A. J., Kittleson M. D., Macdonald K. A., Maggs D. J., Berg J. R., and Rogers Q. R.. . 2006. Low plasma taurine concentration in Newfoundland dogs is associated with low plasma methionine and cyst(e)ine concentrations and low taurine synthesis. J. Nutr. 136:2525–2533. doi: 10.1093/jn/136.10.2525 [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer, B. A., Cohen S. A., and Tarvin T. L.. . 1984. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. B Biomed. Appl. 336: 93–104. doi: 10.1016/S0378-4347(00)85133-6 [DOI] [PubMed] [Google Scholar]
- Butterwick, R. F., Markwell P. J., and Thorne C. J.. . 1994. Effect of level and source of dietary fiber on food intake in the dog. J. Nutr. 124(12 Suppl):2695S–2700S. doi: 10.1093/jn/124.suppl_12.2695S [DOI] [PubMed] [Google Scholar]
- Camara, A., Verbrugghe A., Cargo-Froom C., Hogan K., DeVries T. J., Sanchez A., Robinson L. E., and Shoveller A. K.. . 2020. The daytime feeding frequency affects appetite-regulating hormones, amino acids, physical activity, and respiratory quotient, but not energy expenditure, in adult cats fed regimens for 21 days. PLoS One. 15:e0238522. doi: 10.1371/journal.pone.0238522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Li D., Dai Z., Piao X., Wu Z., Wang B., Zhu Y., and Zeng Z.. . 2014. l-Methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids 46:1131–1142. doi: 10.1007/s00726-014-1675-5 [DOI] [PubMed] [Google Scholar]
- Clarke, R., Daly L., Robinson K., Naughten E., Cahalane S., Fowler B., and Graham I.. . 1991. Hyperhomocysteinemia: an independent risk factor for vascular disease. N. Engl. J. Med. 324:1149–1155. doi: 10.1056/NEJM199104253241701 [DOI] [PubMed] [Google Scholar]
- Delaney, S. J., Kass P. H., Rogers Q. R., and Fascetti A. J.. . 2003. Plasma and whole blood taurine in normal dogs of varying size fed commercially prepared food. J. Anim. Physiol. Anim. Nutr. (Berl). 87:236–244. doi: 10.1046/j.1439-0396.2003.00433.x [DOI] [PubMed] [Google Scholar]
- Dobenecker, B., and Braun U.. . 2015. Creatine and creatinine contents in different diet types for dogs—effects of source and processing. J. Anim. Physiol. Anim. Nutr. 99:1017–1024. doi: 10.1111/jpn.12383 [DOI] [PubMed] [Google Scholar]
- Donadelli, R. A., Pezzali J. G., Oba P. M., Swanson K. S., Coon C., Varney J., Pendlebury C., and Shoveller A. K.. . 2020. A commercial grain-free diet does not decrease plasma amino acids and taurine status but increases bile acid excretion when fed to Labrador Retrievers. Transl. Anim. Sci. 4:txaa141. doi: 10.1093/tas/txaa141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kadi, S. W., Boutry C., Suryawan A., Gazzaneo M. C., Orellana R. A., Srivastava N., Nguyen H. V., Kimball S. R., Fiorotto M. L., and Davis T. A.. . 2018. Intermittent bolus feeding promotes greater lean growth than continuous feeding in a neonatal piglet model. Am. J. Clin. Nutr. 108:830–841. doi: 10.1093/ajcn/nqy133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshorbagy, A. K., Valdivia-Garcia M., Refsum H., Smith A. D., Mattocks D. A., and Perrone C. E.. . 2010. Sulfur amino acids in methionine-restricted rats: hyperhomocysteinemia. Nutrition 26:1201–1204. doi: 10.1016/j.nut.2009.09.017 [DOI] [PubMed] [Google Scholar]
- Fascetti, A. J., Reed J. R., Rogers Q. R., and Backus R. C.. . 2003. Taurine deficiency in dogs with dilated cardiomyopathy: 12 cases (1997-2001). J. Am. Vet. Med. Assoc. 223:1137–1141. doi: 10.2460/javma.2003.223.1137 [DOI] [PubMed] [Google Scholar]
- FDA . 2019a. FDA Investigation into Potential Link between Certain Diets and Canine Dilated Cardiomyopathy. Available from https://www.fda.gov/animal-veterinary/outbreaks-and-advisories/fda-investigation-potential-link-between-certain-diets-and-canine-dilated-cardiomyopathy [updated June 27, 2019; accessed March 17, 2021].
- FDA . 2019b. Dilated Cardiomyopathy in Dogs & Cats: Complaints Submitted to FDA-CVM. Available from https://www.fda.gov/media/128303/download [updated April 30, 2019; accessed March 17, 2021].
- Galler, S., Hutzler C., and Haller T.. . 1990. Effects of taurine on Ca2+-dependent force development of skinned muscle fibre preparations. J. Exp. Biol. 152:255–264. doi: 10.1242/jeb.152.1.255 [DOI] [PubMed] [Google Scholar]
- Gilbert, E. F. 1985. Carnitine deficiency. Pathology. 17:161–171. doi: 10.3109/00313028509063752 [DOI] [PubMed] [Google Scholar]
- Gray, K., Alexander L. G., Staunton R., Colyer A., Watson A., and Fascetti A. J.. . 2016. The effect of 48-hour fasting on taurine status in healthy adult dogs. J. Anim. Physiol. Anim. Nutr. (Berl). 100:532–536. doi: 10.1111/jpn.12378 [DOI] [PubMed] [Google Scholar]
- Green, C. O., Badaloo A., Hsu J. W., Taylor-Bryan C., Reid M., Forrester T., and Jahoor F.. . 2012. Effects of methionine supplementation on cysteine and glutathione production in malnourished infants. FASEB J. 26:1013–1017. doi: 10.1096/fasebj.26.1_supplement.1013.17 [DOI] [Google Scholar]
- Grützner, N., Heilmann R. M., Stupka K. C., Rangachari V. R., Weber K., Holzenburg A., Suchodolski J. S., and Steiner J. M.. . 2013. Serum homocysteine and methylmalonic acid concentrations in Chinese Shar-Pei dogs with cobalamin deficiency. Vet. J. 197:420–426. doi: 10.1016/j.tvjl.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Harrison, M., Thomas G., Gilham M., Gray K., Colyer A., and Allaway D.. . 2020. Short-term determination and long-term evaluation of the dietary methionine requirement in adult dogs. Br. J. Nutr. 123:1333–1344. doi: 10.1017/S0007114520000690 [DOI] [PubMed] [Google Scholar]
- Hayes, K. C. 1988. Taurine nutrition. Nutr. Res. Rev. 1:99–113. doi: 10.1079/NRR19880009 [DOI] [PubMed] [Google Scholar]
- Jong, C. J., Azuma J., and Schaffer S.. . 2012. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids 42:2223–2232. doi: 10.1007/s00726-011-0962-7 [DOI] [PubMed] [Google Scholar]
- Kaplan, J. L., Stern J. A., Fascetti A. J., Larsen J. A., Skolnik H., Peddle G. D., Kienle R. D., Waxman A., Cocchiaro M., Gunther-Harrington C. T., . et al. 2018. Taurine deficiency and dilated cardiomyopathy in golden retrievers fed commercial diets. PLoS One. 13:e0210233. doi: 10.1371/journal.pone.0209112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittleson, M. D., Keene B., Pion P. D., and Loyer C. G.. . 1997. Results of the multicenter spaniel trial (MUST): taurine- and carnitine-responsive dilated cardiomyopathy in American cocker spaniels with decreased plasma taurine concentration. J. Vet. Intern. Med. 11:204–211. doi: 10.1111/j.1939-1676.1997.tb00092.x [DOI] [PubMed] [Google Scholar]
- Ko, K. S., Backus R. C., Berg J. R., Lame M. W., and Rogers Q. R.. . 2007. Differences in taurine synthesis rate among dogs relate to differences in their maintenance energy requirement. J. Nutr. 137:1171–1175. doi: 10.1093/jn/137.5.1171 [DOI] [PubMed] [Google Scholar]
- Laflamme, D. 1997. Development and validation of a body condition score system for dogs. Canine Pract. 22:10–15. ISSN: 0094-4904. [Google Scholar]
- Lee, C. M., Jeong D. M., Kang M. H., Kim S. G., Han J. I., and Park H. M.. . 2017. Correlation between serum homocysteine concentration and severity of mitral valve disease in dogs. Am. J. Vet. Res. 78:440–446. doi: 10.2460/ajvr.78.4.440 [DOI] [PubMed] [Google Scholar]
- Litvak, N., Rakhshandeh A., Htoo J. K., and de Lange C. F.. . 2013. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs. J. Anim. Sci. 91:4188–4196. doi: 10.2527/jas.2012-6160 [DOI] [PubMed] [Google Scholar]
- Malmezat, T., Breuillé D., Capitan P., Patureau Mirand P., and Obled C.. . 2000. Glutathione turnover is increased during the acute phase of sepsis in rats. J. Nutr. 130:1239–1246. doi: 10.1093/jn/130.5.1239 [DOI] [PubMed] [Google Scholar]
- Mansilla, W. D., Marinangeli C. P. F., Ekenstedt K. J., Larsen J. A., Aldrich G., Columbus D. A., Weber L., Abood S. K., and Shoveller A. K.. . 2019. Special Topic: The association between pulse ingredients and canine dilated cardiomyopathy: addressing the knowledge gaps before establishing causation. J. Anim. Sci. 97:983–997. doi: 10.1093/jas/sky488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla, W. D., Templeman J. R., Fortener L., and Shoveller A. K.. . 2020. Minimum dietary methionine requirements in Miniature Dachshund, Beagle, and Labrador Retriever adult dogs using the indicator amino acid oxidation technique. J. Anim. Sci. 98:1–10. doi: 10.1093/jas/skaa324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBreairty, L. E., Robinson J. L., Furlong K. R., Brunton J. A., and Bertolo R. F.. . 2015. Guanidinoacetate is more effective than creatine at enhancing tissue creatine stores while consequently limiting methionine availability in Yucatan miniature pigs. PLoS One. 10:e0131563. doi: 10.1371/journal.pone.0131563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd, S. H., and Poole J. R.. . 1975. Labile methyl balances for normal humans on various dietary regimens. Metabolism. 24:721–735. doi: 10.1016/0026-0495(75)90040-2 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) . 2006. Nutrient requirements of dogs and cats. 10th ed. Washington (DC): The National Academic Press. [Google Scholar]
- Pacioretty, L., Hickman M. A., Morris J. G., and Rogers Q. R.. . 2001. Kinetics of taurine depletion and repletion in plasma, serum, whole blood and skeletal muscle in cats. Amino Acids 21:417–427. doi: 10.1007/s007260170006 [DOI] [PubMed] [Google Scholar]
- Pezzali, J. G., Acuff H. L., Henry W., Alexander C., Swanson K. S., and Aldrich C. G.. . 2020. Effects of different carbohydrate sources on taurine status in healthy Beagle dogs. J. Anim. Sci. 98:skaa010. doi: 10.1093/jas/skaa010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer, C. M., Huff D. L., and Gunter E. W.. . 1999. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin. Chem. 45:290–292. doi: 10.1093/clinchem/45.2.290 [DOI] [PubMed] [Google Scholar]
- Pion, P. D., Kittleson M. D., Rogers Q. R., Morris J. G.. . 1987. Myocardial failure in cats associated with low plasma taurine: a reversible cardiomyopathy. Science. 237:764–768. doi: 10.1126/science.3616607 [DOI] [PubMed] [Google Scholar]
- Pion, P. D., Lewis J., Greene K., Rogers Q. R., Morris J. G., and Kittleson M. D.. . 1991. Effect of meal-feeding and food deprivation on plasma and whole blood taurine concentrations in cats. J. Nutr. 121(11 Suppl):S177–S178. doi: 10.1093/jn/121.suppl_11.S177 [DOI] [PubMed] [Google Scholar]
- Plantz, B. 2017. Grain-free dry dog, cat food no longer a niche market. Available from https://www.petfoodindustry.com/articles/6576-grain-free-dry-dog-cat-food-no-longer-a-niche-market [August 4, 2017, accessed March 17, 2021].
- Rakhshandeh, A., Htoo J. K., Karrow N., Miller S. P., and De Lange C. F. M.. . 2014. Impact of immune system stimulation on the ileal nutrient digestibility and utilisation of methionine plus cysteine intake for whole-body protein deposition in growing pigs. Br. J. Nutr. 111:101–110. doi: 10.1017/S0007114513001955 [DOI] [PubMed] [Google Scholar]
- Rasmusson, R. L., Davis D. G., and Lieberman M.. . 1993. Amino acid loss during volume regulatory decrease in cultured chick heart cells. Am. J. Physiol. 264(1 Pt 1):C136–C145. doi: 10.1152/ajpcell.1993.264.1.C136 [DOI] [PubMed] [Google Scholar]
- Rebouche, C. J., and Engel A. G.. . 1983. Kinetic compartmental analysis of carnitine metabolism in the dog. Arch. Biochem. Biophys. 220:60–70. doi: 10.1016/0003-9861(83)90387-9 [DOI] [PubMed] [Google Scholar]
- Robinson, J. L., McBreairty L. E., Randell E. W., Brunton J. A., and Bertolo R. F.. . 2016. Restriction of dietary methyl donors limits methionine availability and affects the partitioning of dietary methionine for creatine and phosphatidylcholine synthesis in the neonatal piglet. J. Nutr. Biochem. 35:81–86. doi: 10.1016/j.jnutbio.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Robinson, J. L., McBreairty L. E., Randell E. W., Harding S. V., Bartlett R. K., Brunton J. A., and Bertolo R. F.. . 2018. Betaine or folate can equally furnish remethylation to methionine and increase transmethylation in methionine-restricted neonates. J. Nutr. Biochem. 59:129–135. doi: 10.1016/j.jnutbio.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Rodrigues, L. A., Wellington M. O., González-Vega J. C., Htoo J. K., Van Kessel A. G., and Columbus D. A.. . 2021. Functional amino acid supplementation, regardless of dietary protein content, improves growth performance and immune status of weaned pigs challenged with Salmonella typhimurium. J. Anim. Sci. 99:skaa365. doi: 10.1093/jas/skaa365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, S., Rossi G., Giordano A., and Paltrinieri S.. . 2008. Homocysteine measurement by an enzymatic method and potential role of homocysteine as a biomarker in dogs. J. Vet. Diagn. Invest. 20:644–649. doi: 10.1177/104063870802000520 [DOI] [PubMed] [Google Scholar]
- Sachan, D. S., Hongu N., and Johnsen M.. . 2005. Decreasing oxidative stress with choline and carnitine in women. J. Am. Coll. Nutr. 24:172–176. doi: 10.1080/07315724.2005.10719462 [DOI] [PubMed] [Google Scholar]
- Shinohara, Y., Hasegawa H., Ogawa K., Tagoku K., and Hashimoto T.. . 2006. Distinct effects of folate and choline deficiency on plasma kinetics of methionine and homocysteine in rats. Metabolism. 55:899–906. doi: 10.1016/j.metabol.2006.02.017 [DOI] [PubMed] [Google Scholar]
- Söder, J., Höglund K., Dicksved J., Hagman R., Eriksson Röhnisch H., Moazzami A. A., and Wernersson S.. . 2019. Plasma metabolomics reveals lower carnitine concentrations in overweight Labrador Retriever dogs. Acta Vet. Scand. 61:1–12. doi: 10.1186/s13028-019-0446-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead, L. M., Brosnan J. T., Brosnan M. E., Vance D. E., and Jacobs R. L.. . 2006. Is it time to reevaluate methyl balance in humans? Am. J. Clin. Nutr. 83:5–10. doi: 10.1093/ajcn/83.1.5 [DOI] [PubMed] [Google Scholar]
- Sunvold, G., Tetrick M., and Davenport G.. . 2000. Process and product for promoting weight loss in overweight dogs. U. S. Patent No. US 6,204,291 B1. [Google Scholar]
- Thurston, J. H., Hauhart R. E., and Naccarato E. F.. . 1981. Taurine: possible role in osmotic regulation of mammalian heart. Science 214:1373–1374. doi: 10.1126/science.7313699 [DOI] [PubMed] [Google Scholar]
- Vance, D. E., and Ridgway N. D.. . 1988. The methylation of phosphatidylethanolamine. Prog. Lipid Res. 27:61–79. doi: 10.1016/0163-7827(88)90005-7 [DOI] [PubMed] [Google Scholar]
- Vester, B., and Rasmussen K.. . 1991. High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur. J. Clin. Chem. Clin. Biochem. 29:549–554. doi: 10.1515/cclm.1991.29.9.549 [DOI] [PubMed] [Google Scholar]
- Zello, G. A., Pencharz P. B., and Ball R. O.. . 1993. Dietary lysine requirement of young adult males determined by oxidation of l-[1-13C] phenylalanine. Am. J. Physiol. Endocrinol. Metab. 264:E677–E685. doi: 10.1152/ajpendo.1993.264.4.E677 [DOI] [PubMed] [Google Scholar]