Abstract
Autoimmune pancreatitis (AIP) is a rare form of chronic pancreatitis that is often overlooked and is usually characterised clinically by frequent presentations with obstructive jaundice. Serum IgG4 testing as a means to ‘rule out’ IgG4-related disease may not be as helpful as initially thought and may lead to a missed diagnosis if suspicion is low. We present a patient with a years long history of recurrent pancreatitis ultimately found to have AIP after undergoing evaluation with a relatively new technology, SpyGlass, which allows for direct cholangioscopy and enabled us to make the correct diagnosis.
Keywords: autoimmune biliary disease, pancreatic disorders, endoscopic retrograde pancreatography, chronic pancreatitis, biliary endoscopy
Autoimmune pancreatitis (AIP) is a rare form of chronic pancreatitis that is often overlooked in clinical practice and can be challenging to diagnose. We discuss a patient who initially presented to our hospital in 2015 with a 2-year history of abdominal pain secondary to recurrent pancreatitis. He was seen and evaluated by the gastroenterology service and underwent a traditional workup including a serologic evaluation for AIP by IgG4 testing, with serum IgG4 level 7.3 (normal 2–120). He had a history of heavy alcohol but was reportedly sober by the time he was seen in the gastroenterology clinic. Over the course of the following 5 years, the patient had numerous hospital admissions for recurrent pancreatitis and complications including pseudocyst formation and chronic abdominal pain that led to long-term narcotic use. During that timeframe, he underwent a total of four endoscopic ultrasounds (EUS) and three endoscopic retrograde cholangiopancreatography (ERCP) procedures to diagnose and treat complications of recurrent pancreatitis including evaluation by biopsy and drainage of pseudocysts, stenting of pancreatic duct leak and of a stricture in the common bile duct. EUS biopsies with FNA in this patient were obtained and were negative for malignancy but did demonstrate groups of reactive ductal cells in a background of lymphocytes compatible with chronic pancreatitis. In 2020, we acquired SpyGlass technology (Boston Scientific) for direct cholangioscopy and used this advanced diagnostic technique when the patient was next readmitted for complications of chronic pancreatitis. During this particular admission, the patient presented with his typical picture of nausea, vomiting and abdominal pain. Physical examinaton was significant for sinus tachycardia and epigastric tenderness with guarding. Labs were remarkable for lipase 1321, alkaline phosphatase 472, total bilirubin 5.16 (direct 4.67), alanine transaminase 46, aspartate transaminase 61 and white blood cells 13. CT of the abdomen was obtained, which demonstrated a known previously placed biliary stent extending from the common bile duct to the duodenum and peripancreatic fat stranding along with scattered calcifications and pancreatic atrophy consistent with changes of acute on chronic pancreatitis. With concern for biliary obstruction in the setting of existing stent, coupled with leukocytosis, we elected to proceed with ERCP, this time using the SpyGlass technology for direct observation and guided tissue sampling. Cholangioscopy revealed strictures in the lower and middle third of the main duct and exophytic tissue in the lower bile duct (figure 1), all of which was biopsied with SpyBite forceps (Boston Scientific). The strictures were then dilated and restented. Pathology of the strictured areas was negative for malignancy, but after a review of our findings and the clinical history of the patient, we requested additional staining for IgG4 cells, and resultant immunohistochemical stains demonstrated strong and diffusively positive IgG4 plasma cells along with obliterative phlebitis and storiform fibrosis (figure 2). Based on these results, the patient was diagnosed with autoimmune cholangiopathy secondary to AIP. He was started on prednisone and within days demonstrated gradual but clear clinical improvement.
Figure 1.
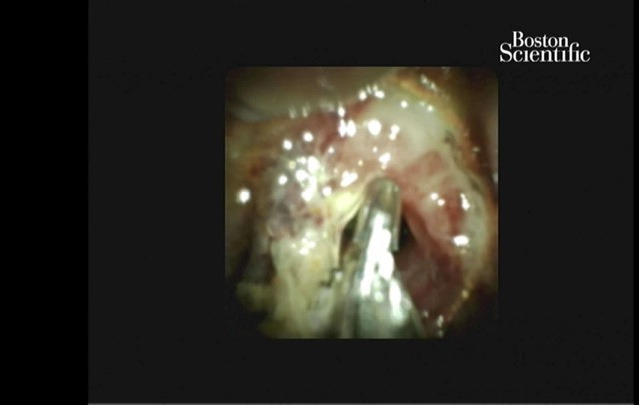
Exophytic tissue in the common bile duct visualised with SpyGlass cholangioscopy and biopsied with SpyBite forceps.
Figure 2.
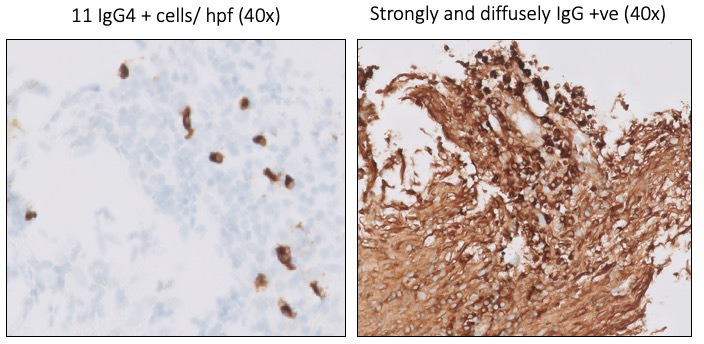
Tissue obtained with the SpyBite forceps from the common bile duct with positive IgG4 staining on pathology examination.
AIP is diagnosed using the Mayo Clinic HISORt criteria, which uses a combination of histological findings, characteristic imaging, serologic testing, other organ involvement and/or clinical response to steroids to arrive at a diagnosis.1 The patient met established criteria for AIP with classic histological findings on pathology and having a clinical response to steroids.2 In addition, the presence of biliary strictures on ERCP is consistent with concomitant autoimmune (IgG4) cholangiopathy, which, in turn, is strongly associated with cases of AIP.3 Because of his normal serum IgG4 level and non-classical CT imaging, a diagnosis of AIP had not been previously pursued, and his episodes of recurrent pancreatitis had been attributed to surreptitious alcohol use versus other idiopathic causes. This assumption had unknowingly led to a markedly delayed diagnosis, long-term narcotic use and significant morbidity leading to multiple hospitalizations.
With the advent and availability of the SpyGlass cholangioscope and consequent incorporation of cholangioscopy into clinical practice, we propose that all patients with recurrent pancreatitis without a clear causation be considered for cholangioscopy and directed tissue sampling, especially when other modalities (eg, endoscopic ultrasound) have been non-diagnostic or unable to obtain adequate tissue samples. Furthermore, we advocate for considering AIP in all patients with recurrent pancreatitis, as earlier diagnosis could significantly improve outcomes by decreasing long-term complications from recurrent pancreatitis, decreasing hospitalizations and reducing dependence on narcotics.
Footnotes
Contributors: AMP, DS, and AR researched and wrote up the case report. AMP and DS made the revisions to the case report. SCG participated in care of the patient and was an integral contributor to making the diagnosis. HS and DG provided pathological interpretation and pathology slides.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study. Not applicable.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo clinic's HISORt criteria. J Gastroenterol 2007;42 Suppl 18:39–41. 10.1007/s00535-007-2046-8 [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Ohara H, Kim MH, et al. Role of endoscopy in the diagnosis of autoimmune pancreatitis and immunoglobulin G4-related sclerosing cholangitis. Dig Endosc 2014;26:627–35. 10.1111/den.12289 [DOI] [PubMed] [Google Scholar]
- 3.Okazaki K. Autoimmune Pancreatitis and IgG4-Related Disease: The Storiform Discovery to Treatment. Dig Dis Sci 2019;64:2385–94. 10.1007/s10620-019-05746-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study. Not applicable.


