Abstract
Primary pneumomediastinum is the presence of air in the interstitium of the mediastinum. The exact aetiology is unclear; nevertheless, it has been reported more frequently in patients with asthma and in individuals who use recreational drugs. It is commonly preceded by a sharp rise in intrathoracic pressure as in a Valsalva-like manoeuvre. We describe a rare case of severe pneumomediastinum with a small pneumothorax related to cannabis smoking and aggravated by vigorous sexual intercourse. The patient was successfully treated conservatively due to clinical and radiological stability and the absence of secondary cause.
Keywords: pneumomediastinum, air leaks, pneumothorax, respiratory medicine, smoking and tobacco
Background
Pneumomediastinum refers to the presence of air in the interstitium of the mediastinum. It is called primary pneumomediastinum when there is no clear aetiology; however, tobacco and marijuana smokers, as well as patients with asthma, have a higher risk of developing the disease. In several cases precipitating factors may be identified. Sexual intercourse has been cited as an aggravating factor in a few case reports.1 2 We present a case of primary pneumomediastinum in an adult man who regularly smoked cannabis and developed pneumomediastinum after cannabis inhalation and intense sexual intercourse.
Case presentation
A 24-year-old Caucasian man presented to the emergency department with sudden pleuritic central chest pain and chest and neck swelling. Both the pain and the swelling developed while he was engaged in intense sexual intercourse with a woman (his female partner) on top. The pain was sharp, 8 out of 10 in severity, and worsened with deep inspiration and movements. The severity of pain limited his activities. The swelling started under his collar bone but rapidly spread from his ear to the middle of the chest. He described the swelling as air bubbles under the skin. There was no history of coryzal symptoms, cough, breathlessness and palpitations. History of nausea, vomiting and haematemesis was absent as well. There was also no history of trauma or corrosives intake. The patient did not engage in any straining or exertional activity before the sexual intercourse.
The patient was otherwise fit and well without any medical history. He also denied any family history of relevant medical disorders. He has been a regular cannabis smoker for 10 years; on average, he smokes five joints (rolled cannabis cigarette) a day, each weighing 0.3 mg. On this occasion, the patient smoked one joint before intimacy.
The patient drinks one to two bottles of 500 mL lager every week; however, he denied having any alcohol before the current incident. He never smoked nicotine. He worked as a vehicle assister (assisting the movement of vehicles). There was no history of occupational exposure to fumes or smoke. He denied having any pets or birds.
The patient was 178 cm in height and weighed 74 kg. There were no marfanoid features. His initial vital signs showed a heart rate of 88 beats per minute, blood pressure of 123/65 mm Hg, temperature of 37.6℃, respiratory rate of 23 and oxygen saturation of 96% on room air. There was mild but noticeable surgical emphysema palpable on the upper part of the chest to the angle of the mandible. Neck veins were not distended. The trachea was central in position; the percussion note was resonant and the chest was clear to auscultation. There were no added heart sounds. The abdomen was soft and non-tender and the bowel sounds were normal. There was no clinical evidence of deep venous thrombosis.
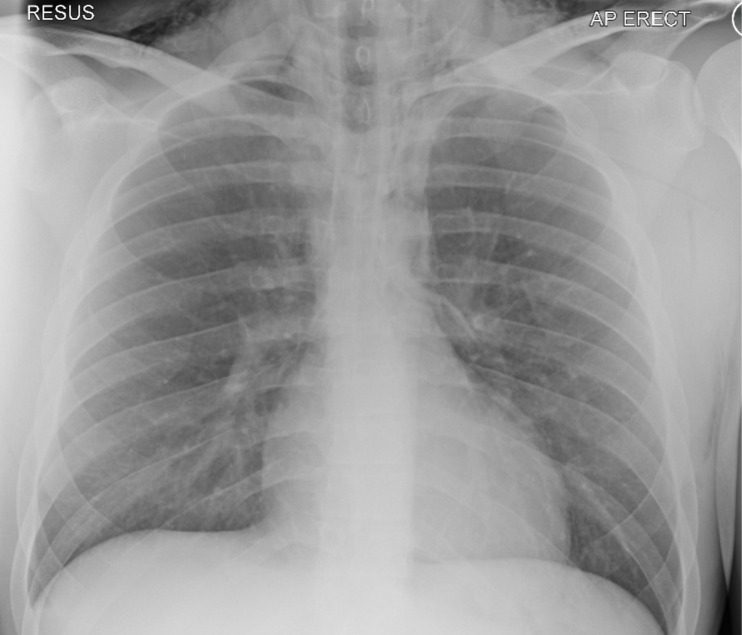
His chest X-ray showed pneumomediastinum with surgical emphysema involving the upper chest and neck, as shown in figure 1.
Figure 1.
Chest X-ray on admission showing surgical emphysema and pneumomediastinum. AP, anteroposterior.
He had raised white cell count of 15.9×109/L (normal range 4–13×109/L); however, there was no clinical or radiological evidence of infection. The rest of his blood counts and biochemical laboratories were normal.
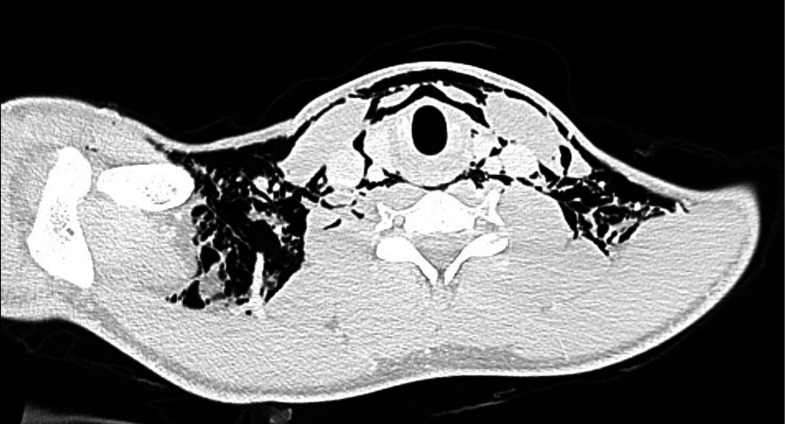
We started the patient on 15 L/min high-flow oxygen (using non-rebreathing mask) to help with the resolution of pneumomediastinum. Non-contrast CT of the chest revealed an extensive pneumomediastinum/pneumopericardium (figure 2). The central airways were patent. The exact site of perforation was not apparent.
Figure 2.
Non-contrast CT of the chest on admission showing pneumomediastinum/pneumopericardium and air under the pectoralis major muscle bilaterally.
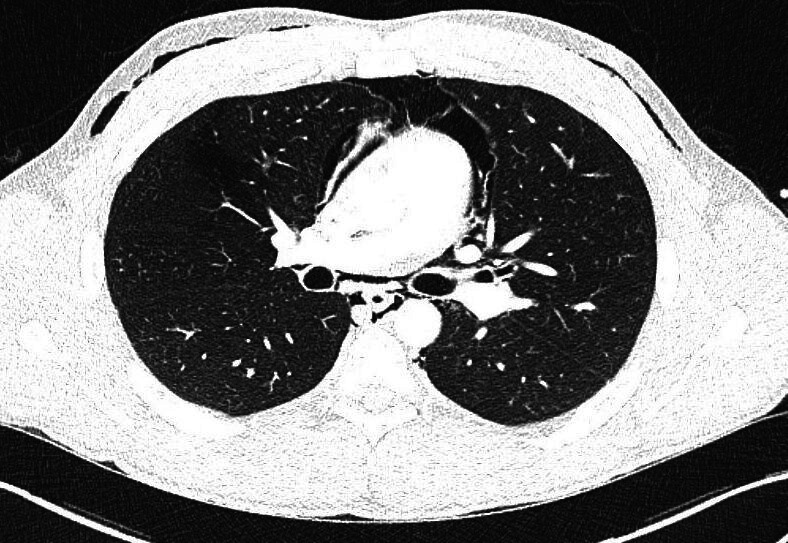
Subsequent CT of the chest with intravenous contrast done to delineate the aetiology of pneumomediastinum showed resolution of the pneumothorax. However, pneumomediastinum was still present (as shown in figure 3). Furthermore, there was no apparent cause of pneumomediastinum since the outline of the oesophagus and the abdominal viscera were normal. Additionally, lung parenchyma was normal and there was no lymphadenopathy or fractures.
Figure 3.
CT scan with intravenous contrast showing extensive pneumomediastinum and surgical emphysema.
After consulting with the thoracic surgery team, we decided to treat the patient conservatively because he remained clinically stable with no radiological deterioration, and more importantly there was no apparent source of pneumomediastinum.
Differential diagnosis
The differential diagnosis of pneumomediastinum includes pneumothorax, oesophageal rupture due to ingested material, Boerhaave’s syndrome, blunt chest trauma with injury to the airways, tracheal perforation, infection from gas-producing bacteria, intestinal or gastric rupture, and sinus fracture. Acute pulmonary embolism, acute coronary syndrome and pericarditis are other differentials. Differentiation is aided by history, clinical examination, ECG, blood tests, X-rays, CT scan and oesophagography.
Outcome and follow-up
The patient was discharged home with instructions to avoid air travel and strenuous exercise, for example, swimming and scuba diving. On subsequent clinic visit, there was complete resolution of his symptoms and chest X-ray findings (figure 4). The patient was referred to drug and alcohol liaison services after his agreement.
Figure 4.
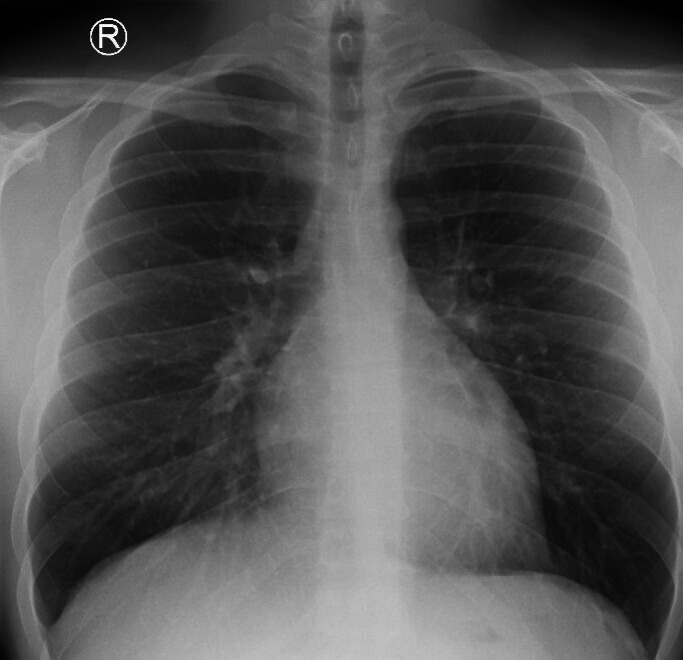
Follow-up chest X-ray in 2 weeks showing complete resolution of pneumomediastinum and surgical emphysema.
Discussion
Pneumomediastinum, also called mediastinal emphysema, is the air in the interstitium of the mediastinal cavity. Laënnec3 was the first to describe it in 1819. It is classified into primary and secondary pneumomediastinum. Secondary pneumomediastinum is frequently related to trauma. Other common causes of secondary pneumomediastinum include therapeutic procedures (iatrogenic) and exacerbations of respiratory diseases.
The incidence of pneumomediastinum is variable in different studies and has been reported to be 1 in 800 to 1 in 42 000 in patients admitted to hospitals.4 While the cause of primary spontaneous pneumomediastinum is unknown, an increased incidence has been proclaimed in patients with asthma, smokers and consumers of inhaled illicit drugs such as cannabis. In most cases, it is precipitated by Valsalva-like behaviours such as coughing, sneezing, defecating, vomiting and childbirth. Inflating party balloons, playing the trombone, Xiao-Lin temple boxing, vocal exercise and karaoke-related events are among other incidental associations of primary pneumomediastinum.
In a sample of 21 patients with primary spontaneous pneumomediastinum, 14 were found to have used cannabis.1 It was attributed to coughing in 43% of cases and concurrent vomiting in 57% of cases. None of the patients in this study had pneumomediastinum in association with sexual activity.
There have only been two cases of pneumomediastinum linked to sexual activity to the best of our knowledge. One of them occurred during lesbian partner intimacy when the patient held her breath for an extended time.2 This patient had previously used cannabis as a recreational drug. The second case of pneumomediastinum appeared in a man during sexual intercourse with his male partner,5 the very act of which was not described. So far, no case of pneumomediastinum has been attributed to sexual activity in straight couples.
Interestingly a case of sex-related pneumoperitoneum has been reported in a woman during orogenital sex; however, there was no pneumomediastinum or pneumothorax in this patient and there was no history of cannabis smoking.6
The exact pathogenesis of spontaneous pneumomediastinum is not clear. The Macklin effect explains the possible mechanism: increased pressure in the alveolar spaces causes them to rupture, allowing air to migrate to the mediastinum through the perivascular and bronchovascular sheaths.7 A substantial increase in mediastinal or a significant decrease in mediastinal intravascular pressure may provide another explanation.8 The air can then pass through the skin, causing surgical emphysema,9 or into the pleural or peritoneal cavity, causing pneumothorax and pneumoperitoneum, respectively.10
Pneumomediastinum can present in several ways. A retrospective comparative study of 74 patients found that 54% had chest pain, 32% had shortness of breath and 39% had subcutaneous emphysema. Vomiting was cited as a precipitating factor in 36% of cases, while asthma exacerbation was cited as a precipitating factor in 36%. There was no triggering factor in 21% of the patients.11 Symptoms such as light-headedness, fatigue, neck swelling, torticollis, dysphonia, stomach pain and back pain are uncommon.
Clinical examination may be normal. Patients generally appear well; however, some may look anxious with tachypnoea and tachycardia. Surgical emphysema is present in 30%–90% of cases2 and can be classified in five grades based on severity/extent of spread. A crunchy sound with a heart beat, called Hamman’s sign, is specific and is present in about one-fifth of the patients.12 In secondary causes like oesophageal perforation or if there is tension mediastinum, the patient may become unwell.
In malignant pneumomediastinum, also referred to as tension pneumomediastinum, the accumulation of a significant amount of air exerts pressure on the mediastinal structures, resulting in tamponade signs, including distended veins, decreased venous return and hypotension.
Chest X-ray may show air bubbles or lucent lines alongside the mediastinal structures. A lucent line separating the heart from the underlying diaphragm is known as the continuous diaphragm sign. The V sign is caused by air lining the confluence of the brachiocephalic veins. A ring around the artery symbol on lateral chest X-ray is the air around the right pulmonary artery. The surgical emphysema may appear as a ginkgo leaf sign in which the air outlines the fibres of the pectoralis major muscle, giving the appearance of a ginkgo leaf on chest X-ray. Pleural effusion may indicate an oesophageal rupture. Additionally, surgical emphysema and pneumothorax are also seen in spontaneous pneumomediastinum.13
On chest ultrasound, pneumomediastinum may appear as an ‘Air Gap Sign’, where the air in the pericardial cavity prevents the visualisation of the underlying heart during cardiac systole.
CT scan shows the extent of disease and sometimes the aetiology. When an oesophageal rupture is suspected, a water-soluble contrast oesophagography or a CT scan with oral contrast can reveal the leak and confirm the diagnosis. An ECG is performed to rule out pericarditis, which is an important differential diagnosis.
Conclusion
Patients with asthma and individuals who smoke cannabis or tobacco have more propensity to have primary pneumomediastinum, which is frequently preceded by triggering factors. Sexual intercourse is a rare triggering factor for primary pneumomediastinum. Most patients can be managed conservatively with close monitoring for development of tension pneumothorax.
Learning points.
There is a possible association between primary pneumomediastinum and cannabis smoking.
Sexual intercourse could be one of the triggering factors for pneumomediastinum.
Pneumomediastinum can be managed conservatively irrespective of radiological severity unless there are signs of tension like hypotension and distended neck veins or a secondary cause.
Footnotes
Contributors: HE: directly looked after the patient. MI: indirectly looked after the patient, did the literature review and helped in writing the article. AH, ZM: literature review and helped in writing the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Weiss ZF, Gore S, Foderaro A. Pneumomediastinum in marijuana users: a retrospective review of 14 cases. BMJ Open Respir Res 2019;6:e000391. 10.1136/bmjresp-2018-000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flatman S, Morrison E, Elahi M. Spontaneous pneumomediastinum associated with sex. J Radiol Case Rep 2010;4:25–9. 10.3941/jrcr.v4i4.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laënnec RT. De L’auscultation Médiate ou Traité du Diagnostic des Maladies des Poumon et du Coeur. 1st ed. Paris: Brosson & Chaudé, 1819. [Google Scholar]
- 4.McMahon DJ. Spontaneous pneumomediastinum. Am J Surg 1976;131:550–1. 10.1016/0002-9610(76)90008-8 [DOI] [PubMed] [Google Scholar]
- 5.Packham CJ, Stevenson GC, Hadley S. Pneumomediastinum during sexual intercourse. Br Med J 1984;288:1196–7. 10.1136/bmj.288.6425.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawich SO, Johnson PB, Williams E, et al. Non-Surgical pneumoperitoneum after oro-genital intercourse. Int J Surg Case Rep 2013;4:1048–51. 10.1016/j.ijscr.2013.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MACKLIN MT, MACKLIN CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions. Medicine 1944;23:281–358. 10.1097/00005792-194412000-00001 [DOI] [Google Scholar]
- 8.Agut A, Talavera J, Buendia A, et al. Imaging DIAGNOSIS-SPONTANEOUS pneumomediastinum secondary to primary pulmonary pathology in a Dalmatian dog. Vet Radiol Ultrasound 2015;56:E54–7. 10.1111/vru.12223 [DOI] [PubMed] [Google Scholar]
- 9.Russo A, Del Vecchio C, Zaottini A, et al. Role of emergency thoracic ultrasonography in spontaneous pneumomediastinum. two case report. G Chir 2012;33:285–96. [PubMed] [Google Scholar]
- 10.Pooyan P, Puruckherr M, Summers JA, et al. Pneumomediastinum, pneumopericardium, and epidural pneumatosis in DKA. J Diabetes Complications 2004;18:242–7. 10.1016/S1056-8727(03)00059-X [DOI] [PubMed] [Google Scholar]
- 11.Caceres M, Ali SZ, Braud R, et al. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann Thorac Surg 2008;86:962–6. 10.1016/j.athoracsur.2008.04.067 [DOI] [PubMed] [Google Scholar]
- 12.Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007;31:1110–4. 10.1016/j.ejcts.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 13.Çelik B, Pirzirenli MG, Büyükkarabacak YB, et al. Clinical and radiological characteristics of patients treated with the diagnosis of spontaneous pneumomediastinum. Curr Thorac Surg 2021;6:1–6. 10.26663/cts.2021.0001 [DOI] [Google Scholar]