Abstract
Objective:
Gestation weight (GW), body mass index (BMI), and blood 25-hydroxyvitamin D [25(OH)D] level during pregnancy are important determinants of the gestational outcomes. This study aimed to study how these parameters vary between antenatal vitamin D recipients and non-recipients in gestational diabetes mellitus (GDM) patients.
Material and Methods:
The randomized controlled trials comparing these outcomes between vitamin D recipient and non-recipient GDM patients were searched in electronic databases (PubMed, Embase, and Scopus). The reviewed studies’ data were abstracted and critically appraised using the Cochrane tool. The estimation of the weighted mean difference for GW and BMI and standardized mean difference (SMD) for 25(OH)D levels occurred by juxtaposing the interventions meta-analytically (random-effect model). The statistical inconsistency was determined by Chi2 and I2 method. The statistical significance was estimated at p<0.05 and 95% confidence interval (CI).
Results:
Eleven eligible trials (all Iran-based, except one), sourcing data from about 875 GDM patients, were reviewed. Overall, the risk of bias was low, except for selection and performance bias. On random-effect model meta-analysis, the 25(OH)D levels of the GDM patients favored the vitamin D recipients when compared to non-vitamin D (SMD 1.97, 95% CI: 1.06-2.88, p<0.001; I2 96.2%, p of Chi2 <0.001) and placebo (SMD 1.86, 95% CI: 0.95-2.77, p<0.001; I2 95.3%, p of Chi2 <0.001) recipients, respectively. On meta-regression, sample size was a predictor of the observed heterogeneity. For GW and BMI the interventions did not differ statistically significantly.
Conclusion:
In GDM patients, antenatal use of vitamin D aids in the rise of blood 25(OH)D levels. However, vitamin D supplementation did not affect change in GW or BMI.
Keywords: Gestational diabetes, vitamin D, dietary supplement
Introduction
Gestational diabetes mellitus (GDM) refers to any degree of glucose intolerance that develops or is identified initially during gestation (1). Its global prevalence is about 7-10% (2,3,4,5). GDM diagnosis is made using glucose challenge tests between 24-28 weeks of gestation (1). Initial GDM management encompasses diet and exercise therapy, but if these fail to achieve glycemic control, physicians start insulin therapy (1).
GDM is a crucial health burden since it can affect both the GDM patient and her neonate. Gestational weight (GW) and body mass index (BMI) in pregnancy are two important anthropometric determinants of GDM-related outcomes. Studies in GDM patients suggest that an excessive GW accumulation increases the risk of maternal complications, such as increased likelihood of cesarean delivery, large for gestational age, and gestational hypertension and fetal problems, such as macrosomia, large for gestational age, hypoglycemia in newborns, and poor APGAR score (6,7,8,9,10,11). It remains unclear if the Institute of Medicine’s guideline (2009) for recommended GW gain for respective BMI categories can be applied to the GDM subpopulation or not (8,12). However, studies on overweight and obese GDM patients found that gaining GW less than that recommended for their respective BMI categories resulted in favorable obstetric and neonatal outcomes (8,13,14). Maintaining an optimum weight before and during pregnancy, therefore, can decrease the complications of pregnancy (9). Nevertheless, it remains unclear if any antenatal intervention in GDM patients may be beneficial in achieving an acceptable GW and BMI.
In this respect, vitamin D has emerged as a potentially useful agent that has attracted attention. In GDM patients, various clinical trials (15,16,17,18) have tested the maternal health effect of antenatal vitamin D supplementation, and due to the different relationships between GDM and vitamin D status in the body, such testing appears pertinent. For instance, inadequate vitamin D levels in the body are associated with an increased risk of developing GDM (19,20,21,22,23). Vitamin D deficiency (<20 ng/mL) has a nearly fourfold increased risk of GDM development than women with sufficient vitamin D level (>30 ng/mL) after adjusting for the age of the mother, race, ethnicity, and family history of type 2 diabetes among first-degree family members (24). Moreover, studies showed a decreased GDM prevalence in prenatal vitamin D recipients (25,26). Given this evidence it is important to understand how vitamin D supplementation in GDM mothers can affect GW and their BMI. Additionally, as the fetus entirely depends on maternal 25-hydroxyvitamin D [25(OH)D] levels, maternal levels in GDM mothers after vitamin D supplementation also requires evaluation (27).
Intervention description
The inactive forms of the fat-soluble vitamin D are D2 (ergocalciferol) and D3 (cholecalciferol) (28,29). Both forms are available from diet and supplements and vitamin D3 is also produced in the skin on exposure to sunlight (28,29). On hydroxylation of pre-vitamin D in the liver, the main circulating form, 25(OH)D, of vitamin D is produced (27). In blood, 25(OH)D is either present in the bound form (to albumin) or free form (27). For its physiologic role, it is converted to the active form, calcitriol 1,25-dihydroxyvitamin D (28,30). The physiological effect of Vitamin D in pregnancy is mediated via calcitriol’s action on the vitamin D receptors in uteroplacental tissue (28,30). Compared to calcitriol, which has a half-life of 4-6 hours (27), the relatively longer half-life of 25(OH)D of between two and three weeks (31) makes the latter an ideal marker for vitamin D status (32).
In GDM patients, contemporary trials have supplemented vitamin D at various dosages. For oral preparations, while some trials used it at a dose of 50,000 IU, two to three weeks apart for three to eight weeks (33,34,35,36), other trials used it twice daily at 200-500 IU for six to sixteen weeks (17,37). One trial used a single intramuscular injection of vitamin D at a dose of 300,000 IU (38). Furthermore, while few trials used the vitamin as a single supplement (33,37,38), others co-supplemented it with various micronutrients, including zinc, magnesium, and calcium (17,34).
What this review adds?
In contemporary medicine, several clinical trials have tested the changes in GW, BMI, and plasma 25(OH)D level in GDM mothers, after antenatal vitamin D supplementation (15,34,35,36). Recent reviews have studied the effect of antenatal vitamin D supplementation on certain maternal complications such as cesarean section rate, pre-eclampsia, preterm delivery, macrosomia, and polyhydramnios and/or on neonatal complications including hyperbilirubinemia, hypoglycemia, and hospitalization (39,40,41). However, to the best of our knowledge, there is no systematic review and meta-analysis that studied how maternal GW, BMI, and 25(OH)D levels change in the blood on vitamin D supplementation in GDM patients. Therefore, this study explores this under-reviewed area of modern medicine by a systematic literature search, critical appraisal, and meta-analysis.
Aims
This study compared the GW, BMI, and 25(OH)D levels among vitamin D supplemented and not-supplemented GDM patients.
Material and Methods
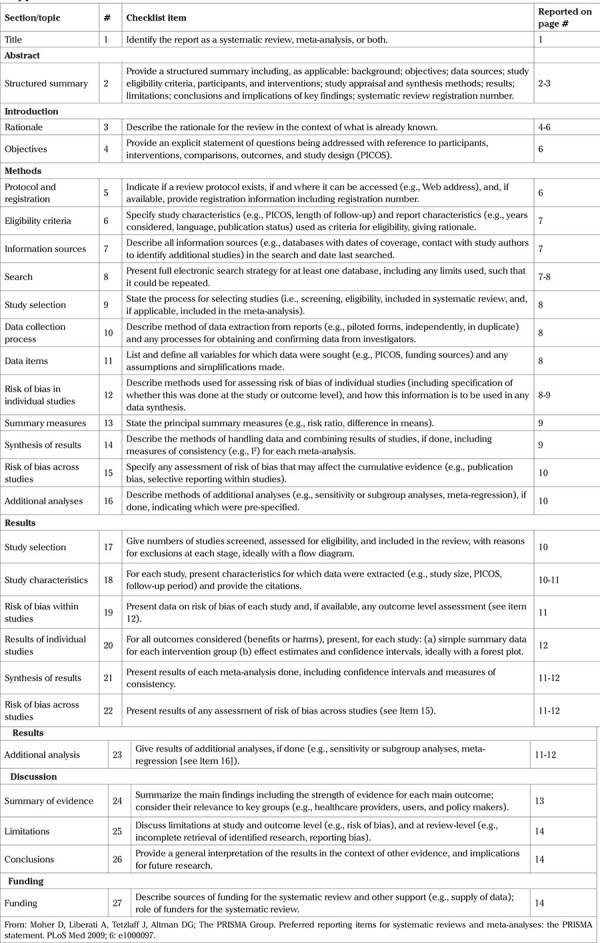
This systematic review is registered in the PROSPERO database (CRD42020149613) and has a pre-published protocol (42,43). This report adheres to the PRISMA 2009 reporting guideline (Supplemental Table 1) (44).
Supplemental Table 1. PRISMA checklist.
Inclusion criteria
1. Study design: Parallel arm randomized controlled trials of any number of intervention arms.
2. Population: Pregnant females of any age were eligible, irrespective of their pre-pregnancy BMI and 25(OH)D levels. They must be diagnosed with GDM during their concurrent pregnancy.
3. Intervention arm: The treatment arm/s should have received vitamin D as a sole or co-supplement.
4. Comparator arm: The comparator arm/s may have received a placebo or any other supplement except vitamin D. Comparator arm/s not receiving any intervention were also eligible.
5. Outcomes: The trials must report the GW (kg), BMI (kg/m2), and 25(OH)D (in ng/mL or mmol/L) in the above GDM patients before and after receiving these interventions and before childbirth.
We accepted the diagnosis and management of GDM and the dosage and regimen of interventions received by the participants in the respective treatment arms as per the trialists.
Exclusion criteria
1. Study design other than those described above, e.g., observational studies and crossover studies.
2. Participants with diabetes types besides GDM, like type 1 or type 2 diabetes.
3. Studies conducted on animals.
4. Editorials, abstracts from conference presentations (where a full published manuscript is not available), letters, or any other brief communications.
Database search
We searched the title and abstract of prospective trials matching the above eligibility criteria in PubMed, Embase, and Scopus databases, irrespective of the date and language of publication and geographical boundary. The following search terms were used “vitamin D” OR “calciferol” OR “vitamin D2” OR “ergocalciferol” OR “vitamin D3” OR “cholecalciferol” AND “GDM” OR “gestational diabetes” along with these MeSH terms- “Cholecalciferol”, “ergocalciferols”, and “diabetes, gestational”. To identify the clinical trials in PubMed [(Clinical Trial) and (Randomized Controlled Trial)] and Embase [(controlled clinical trial) and (randomized controlled trial)], we used filters. In Scopus, instead of filters, the following search terms were used: “trial,” “randomised,” “randomized,” and “controlled.” The last date of the search was 17 September, 2020. Additionally, we reviewed the references of the papers included in this review.
We uploaded the retrieved citations (from database search) in the Rayyan systematic review software (45) and eliminated the duplicate articles. Successively, skimming of the remaining citations’ titles and abstracts against the eligibility criteria commenced. Articles were read in full-text when it seemed to meet the inclusion criteria, or if the suitability for incorporation in this review was doubtful.
Data abstraction and risk of bias assessment
We extracted data about the study design, consent, ethics, registration number of the trial, participant features, interventions contrasted, and the outcomes of interest in a pre-piloted form. With the Cochrane collaboration tool, individual trial's risk of selection bias, performance bias, detection bias, attrition bias, reporting bias, and any other bias was determined, and each of these risk of bias (RoB) components was categorized as low, high, or unclear (46). To assess selection bias, the random allocation sequence generation method, and its concealment method from participants, were judged. The blinding mechanism of study participants and personnel and that of outcome assessors were used to evaluate the performance and detection bias, respectively. By evaluating missing outcome data, and its reason among the intervention arms, the risk of attrition bias, was evaluated. Any additional bias, besides the above, comprised the other bias type. For a visual presentation of the RoB, we prepared an RoB graph and an RoB summary using the Review Manager (RevMan) software (46,47).
The review authors independently performed study selection, data abstraction, and RoB assessment, and resolved any disagreement in an opinion by discourse.
Meta-analysis
The juxtaposed interventions’ effect on each of the outcomes was contrasted by random-effects meta-analysis (using DerSimonian and Laird method) since we assumed clinical heterogeneity among the trials attributable to the different types of vitamin D co-supplements used in these. The use of endpoint means of the respective outcomes and their SDs ensued to conduct the meta-analysis. We estimated the meta-analytic effect sizes of GW and BMI in weighted mean differences (WMD) and that of 25(OH)D levels in standardized mean differences (SMD) due to the identical and non-identical types of measuring units used in the trials, respectively. A decrease in the summary effect of GW and BMI, and its increase in 25(OH)D levels, denoted a favorable meta-analytic finding. For any outcome, when multiple treatment arms tested an intervention, the post-intervention means and their SDs of those intervention groups were combined for meta-analysis (46). Outcome reported in the median were not considered for meta-analysis.
Heterogeneity and meta-regression
The statistical heterogeneity was determined by Chi2 (statistically significant at p<0.1) and I2 (categorized as low, moderate, and high at I2 values of 25, 50, and 75%, respectively) statistics (48). To account for any substantial heterogeneity, we performed univariate meta-regression by presence or absence of missing outcome data and sample size (categorized as <100 and ≥100). Using the predictor identified by meta-regression, we did a subgroup analysis to see how heterogeneity changed across the different categories of the predictor.
Publication bias and sensitivity analysis
The publication bias assessment incorporated visual inspection of funnel plots and Egger’s test. For each outcome, a sensitivity analysis included iteration of the meta-analysis using a fixed-effect model and by dropping a trial each time.
Statistical analysis
Using random-effect and fixed-effect models, all outcomes were compared meta-analytically between vitamin D and placebo-receiving GDM patients.
We estimated the statistical significance of meta-analysis derived effect sizes at p<0.05 and 95% confidence interval (CI). Stata statistical software v16 (StataCorp, College Station, TX) was used for analysis.
Results
Scope of the review
The database search retrieved 271 citations. After eliminating the duplicates, 188 citations underwent skimming against the eligibility criteria. Out of the 22 articles needing full-text reading, 11 trials sourcing data from about 875 participants published between 2014-19, were included in this review (Figure 1) (15,16,17,18,33,34,35,37,49,50,51). All trials except the Chinese one (37) were Iran-based, and the average age of participants in the respective intervention arms was approximately 28-32 years. The intervention period of Iranian (16,17,18,33,49,50,51) and Chinese (37) trials were 6-8 and 16 weeks, respectively. In most trials (15,16,17,18,34,35,37,49,50,51), GDM was diagnosed primarily using the American Diabetes Association criteria (52,53). Insulin was not used during the intervention period, except in the trial by Yazdchi et al. (33). Eight trials (15,16,18,33,34,37,49,50) used the D3 form of the vitamin while this was not clear among the remaining trials (17,35,51). In most of the trials (81.8%), a co-supplement (e.g., calcium, magnesium, zinc, omega-3 fatty acid, evening primrose oil, probiotic) accompanied the vitamin D supplementation (15,16,17,18,34,35,37,50,51). The intervention was given between 24-28 weeks of gestation in nine trials (15,16,17,18,33,34,35,49,50), at 16 weeks of gestation in one trial (37), and in the remaining one, this was unclear (51). Table 1 depicts the salient features of the trials.
Figure 1.
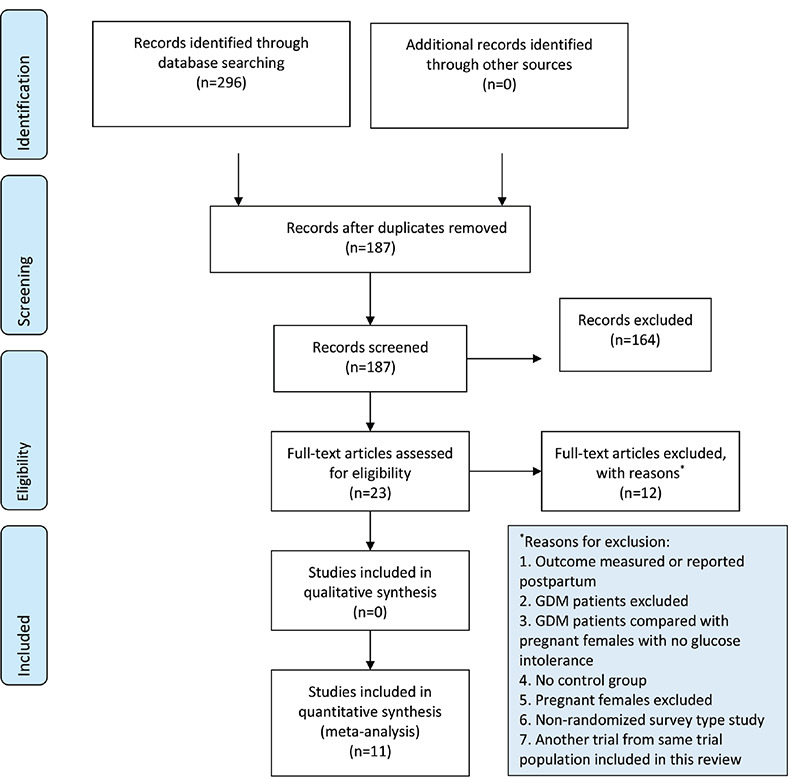
Study selection process [PRISMA flow chart (58)]
Table 1. Summary table.
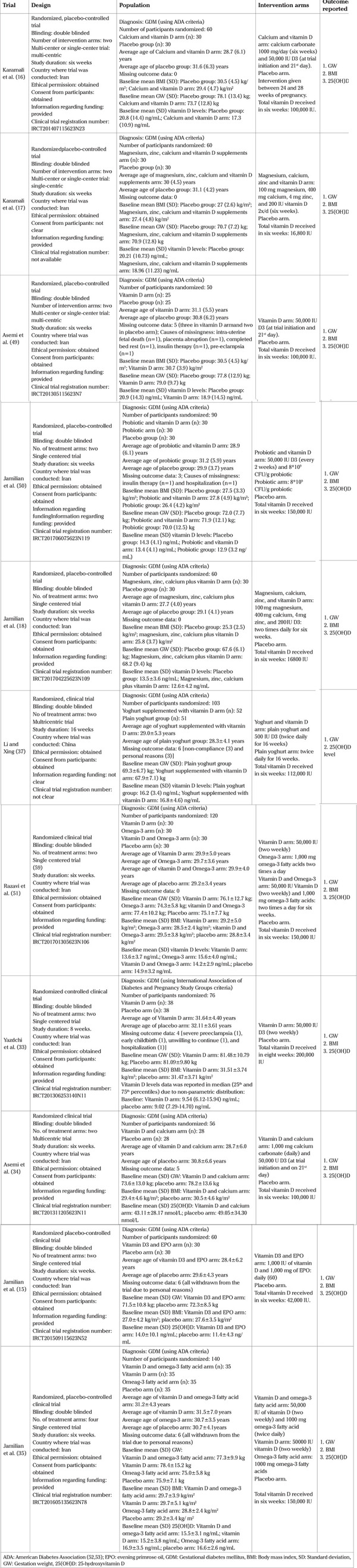
RoB assessment
In most studies, the allocation concealment component of the selection bias and performance bias was unclear (Table 2 and Figure 2). Otherwise, the RoB was low.
Table 2. Risk of bias assessment.
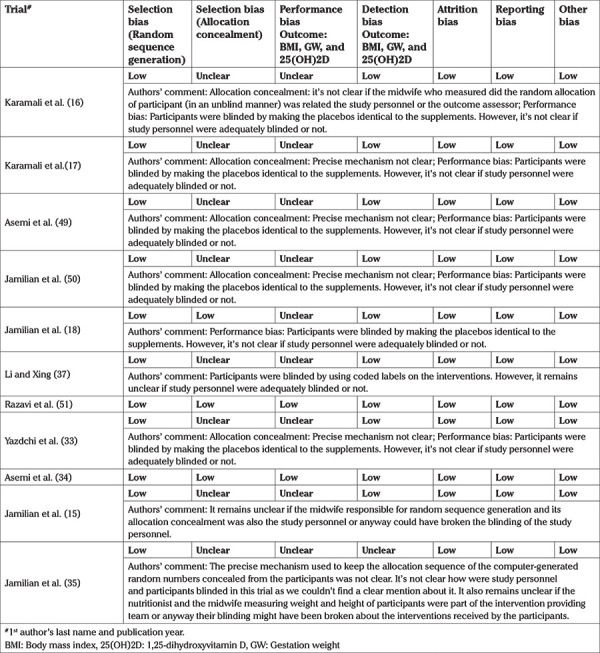
Figure 2.
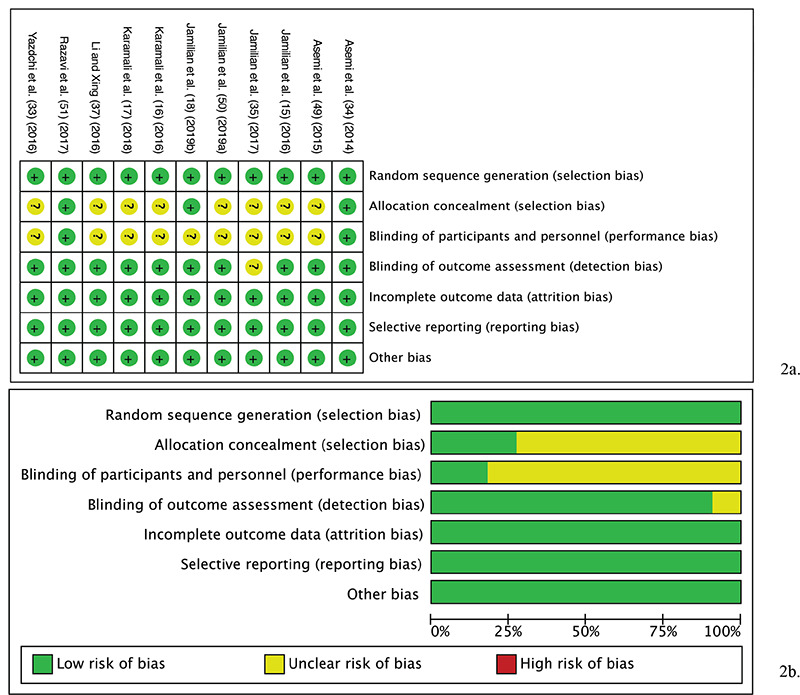
a) Risk of bias graph: review authors' evaluation of respective risk of bias items presented across all studies included in the review. b) Risk of bias summary: review authors’ evaluation of respective risk of bias item for each included study
Meta-analysis findings
Eleven trials comparing GW (15,16,51,17,18,33,34,35,37,49,50), and 10 trials contrasting BMI (15,16,17,18,33,34,35,49,50,51) with one study (37) excluded as it did not report the follow up BMI, and 25(OH)D with one trial (33) excluded for reporting follow up value in median, were included in the meta-analytic juxtaposition between vitamin D recipients and its non-recipients.
The antenatal vitamin D use in GDM patients favored plasma 25(OH)D level attainment compared to its non-supplementation (random-effect model: SMD 1.97, 95% CI: 1.06-2.88, p<0.001; I2 96.2%, p of Chi2 <0.001).
The post-intervention GW (random-effect model: WMD 0.18, 95% CI, -1.10-1.47, p=0.773; I2 0%, p of Chi2 0.559) and BMI (random-effect model: WMD 0.27, 95% CI, -0.28-0.82, p=0.331; I2 0%, p of Chi2 0.838) were not statistically significantly different between the juxtaposed interventions (Figure 3).
Figure 3.
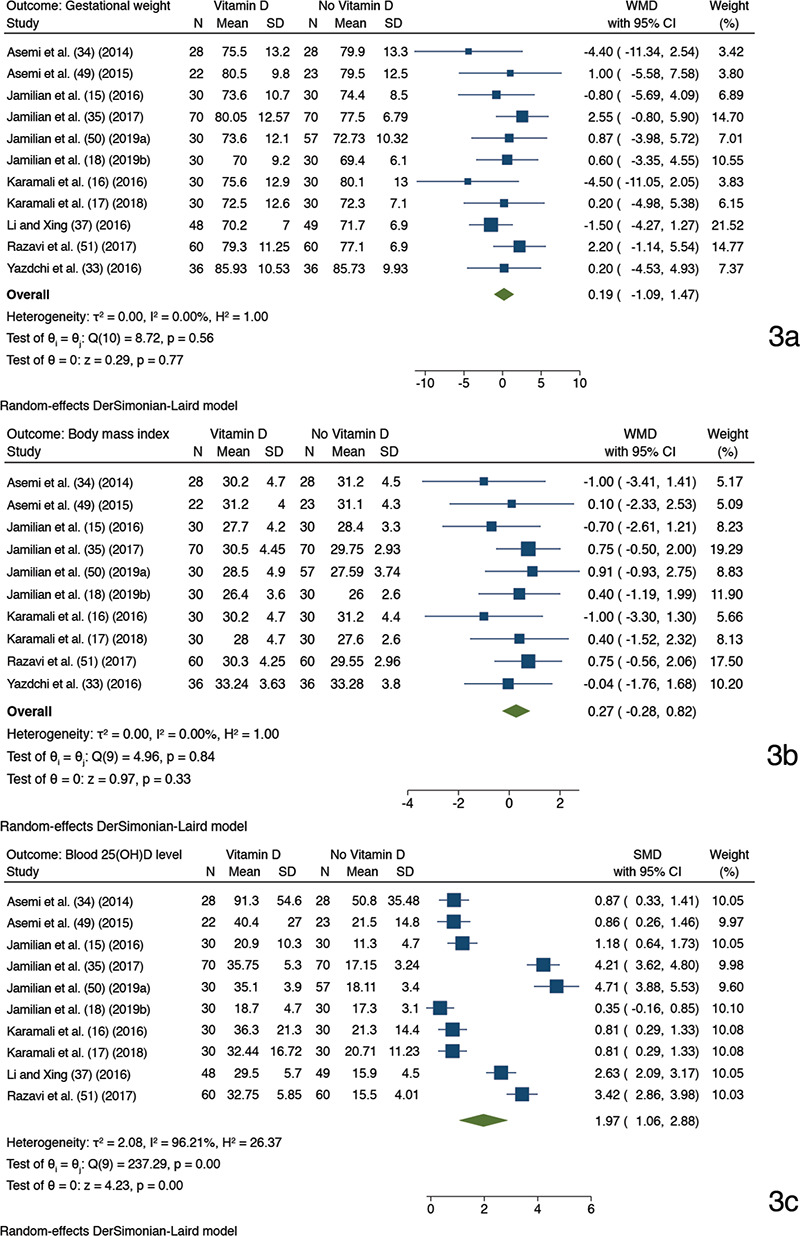
Forest plots depicting meta-analysis findings (random-effect model). Outcome: gestational weight a), body mass index b), and 25(OH)D level in blood c). A comparison between antenatal vitamin D supplementation (as the only or cosupplement with other supplements) and non-vitamin D based supplementation; Two trials had with identical trial author name and year have been suffixed with alphabet “a” (50) and “b” (18) after the study name and year
SD: Standard deviation, CI: Confidence interval, SMD: Standardized mean difference, 25(OH)D: 25-hydroxyvitamin D
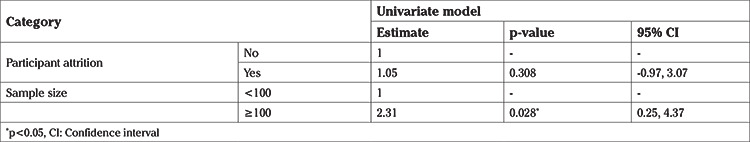
Meta-regression and subgroup analysis
The univariate meta-regression suggested that sample size was a statistically significant predictor of the observed heterogeneity in the effect size of 25(OH)D level (Supplemental Table 2). Upon subgroup analysis by the sample size, heterogeneity was moderate when sample size was ≥100, and the effect size increased (random-effect model: SMD 3.81, p<0.001; 95% CI, 3.03-4.59; I2 72.5%) (Supplemental Figure 1).
Supplemental Table 2. Univariate meta-regression analysis.
Supplemental Figure 1.
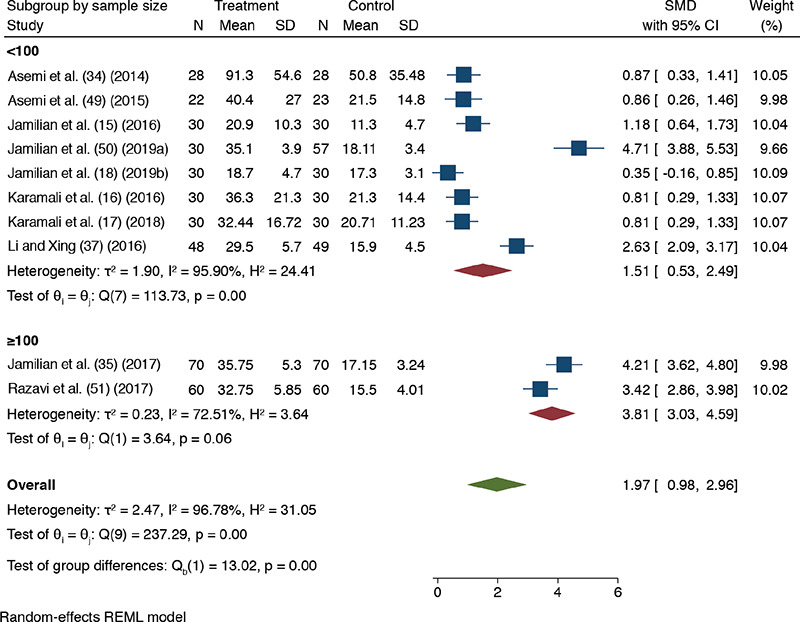
Forest plot showing meta-analysis comparing between antenatal vitamin D supplementation (as a sole or co-supplement with other supplements) and non-vitamin D supplementation results on 25(OH)D level in blood using random-effect model. Subgroup by sample size (<100 and ≥100 category); Two trials have identical trial author name and year that have been suffixed with alphabet “a” (61) and “b” (27) after the study year
SD: Standard deviation, CI: Confidence interval, SMD: Standardized mean difference, 25(OH)D: 25-hydroxyvitamin D
Publications bias
For 25(OH)D, a small study effect was suggested by the asymmetric funnel plots (Supplemental Figure 2) and Egger’s test (p=0.005). On trim-and-fill analysis, no additional study was imputed. Funnel plots for the rest of the outcomes were approximately symmetric.
Supplemental Figure 2.
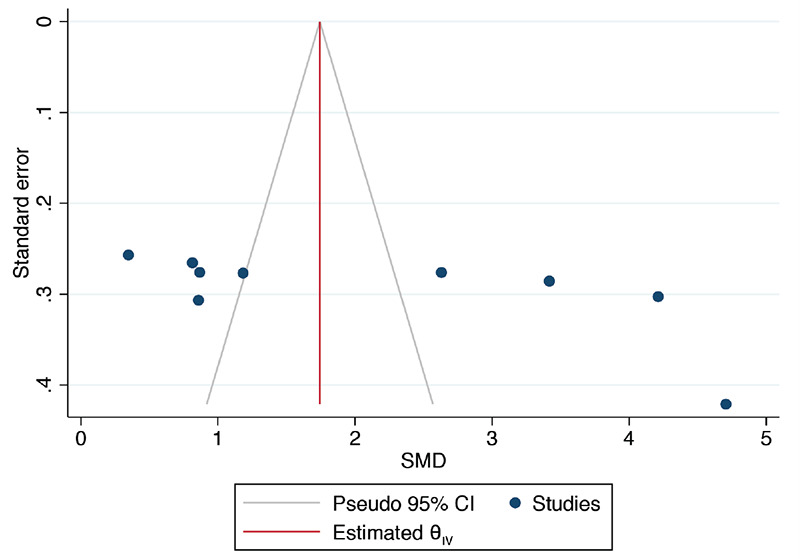
Funnel plot assessing publication bias between vitamin D supplemented and not supplemented GDM mothers for 25(OH)D levels in the blood
GDM: Gestational diabetes mellitus, SMD: Standardized mean difference, CI: Confidence interval, 25(OH)D: 25-hydroxyvitamin D
Sensitivity analysis
On using a fixed-effect model meta-analysis, the summary estimate of 25(OH)D level, reduced slightly (SMD 1.74, 95% CI, 1.57-1.92, p<0.001). The fixed-effect meta-analysis results for the rest of the outcomes were identical to the preliminary analysis. The meta-analysis findings for all outcomes remained unchanged on dropping a study each time and repeating the meta-analysis.
Supplementary meta-analysis
Between vitamin D and placebo, ten trials (15,16,17,18,33,34,35,49,50,51) compared GW and BMI, and nine trials (15,16,17,18,34,35,49,50,51) juxtaposed 25(OH)D levels, with one study (33) excluded because the study reported 25(OH)D values as medians. Vitamin D recipients achieved a favorable blood 25(OH)D level compared to the placebo recipients (random-effect model: SMD 1.86, 95% CI, 0.95-2.77, p<0.001; I2, 95.36%, p of Chi2 <0.001) (Figure 4). The effect size of 25(OH)D levels reduced slightly on using a fixed-effect meta-analysis model (SMD 1.45, 95% CI, 1.25-1.64). GW and BMI, when contrasted among the intervention arms, were not statistically significantly different. Since <10 studies were available for the 25(OH)D levels, we did not explore heterogeneity or assess the publication bias for it.
Figure 4.
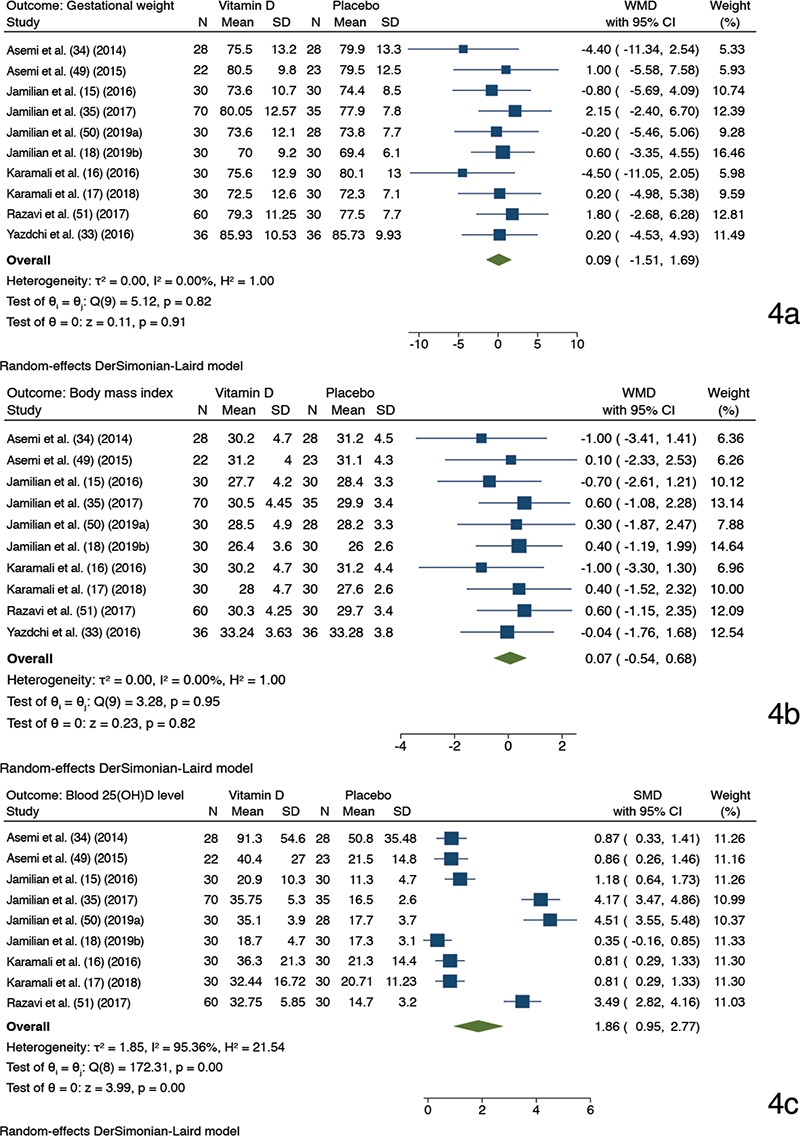
Forest plots depicting meta-analysis findings (random-effect model). Outcome: gestational weight a), body mass index b), and 25(OH)D level in blood c). A comparison between antenatal vitamin D supplementation (as a sole or cosupplement with other supplements) and placebo; Two trials had with identical trial author name and year have been suffixed with alphabet “a” (50) and “b” (18) after the study name and year
SD: Standard deviation, CI: Confidence interval, SMD: Standardized mean difference, 25(OH)D: 25-hydroxyvitamin D
Discussion
Overall, 11 trials, mostly Iranian, tested the effect of antenatal vitamin D complementation (as a co-supplement primarily) on GW, BMI, and 25(OH)D in 875 GDM patients, were retrieved. The intervention favored a rise in blood 25(OH)D levels, and the sample size was the plausible predictor of the observed heterogeneity.
Evidence quality
Utilizing the GRADE Working Group’s (2004) (54) approach of grading evidence quality we graded the evidence concerning the 25(OH)D level as of moderate-quality, due to the unclear RoB components and heterogeneity.
Comparison with what is known
As the context remains underexplored in contemporary literature, a direct juxtaposition of our findings to existing reviews is not possible. However, clinical trials studying the effect of vitamin D supplementation on 25(OH)D level in pregnant females with no glucose intolerance are available for a contrast. Two such trials found that vitamin D supplementation in the third trimester increased maternal plasma 25(OH)D levels compared to the control group (55,56). Another randomized trial found that vitamin D supplementation caused a statistically significantly greater increase in the 25(OH)D level than the placebo (57). Mirroring these trials’ findings (55,56,57), we observed that vitamin D3 supplementation in GDM patients increased the maternal 25(OH)D level.
Implications and strengths
The chief inference of this paper is that it informs about the rigor of the current evidence of the maternal benefits of prenatal vitamin D supplementation in GDM patients. From the perspective of maternal health, this study may help health authorities to determine if large scale supplementation for all GDM pregnancies will be an appropriate public health initiative or not, given the current evidence. Moreover, as the reviewed trials were primarily Iran-based, this paper might encourage future trialists to conduct identical trials globally to generate generalizable evidence. Concerning the strength, this systematic review is one of the preliminary efforts to investigate the maternal effects of antenatal vitamin D supplementation in GDM patients. A further strength was the unbound nature of our electronic database searche to include any date, language, or geographical boundary, thus adding comprehensiveness to our review. Finally, evidence generated from this systematic review and meta-analysis is likely to be rigorous, as it's grounded on the highest level of epidemiological evidence, randomized controlled trials.
Despite these strengths, this paper has a few weaknesses. As most trials were conducted in Iran, the external validity of this review is likely to be compromised. The heterogeneity observed for the 25(OH)D levels might have increased the risk of bias in our estimates, and this can be because one of the studies was not from Iran. Besides, the maternal health effects of vitamin D supplementation remains inseparable from other supplements that were simultaneously given to the participants in most trials.
Conclusion
Using vitamin D as the chief ingredient of antenatal supplements favors in blood 25(OH)D level rise in GDM patients. However, the effect of these supplements on GW and BMI was not distinguishable from those subjects who did not receive supplementation.
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest: No conflict of interest is declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Quintanilla Rodriguez BS, Mahdy H. Gestational Diabetes. [Updated: 2019 Dec 23]. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. Available from: [Internet] https://www.ncbi.nlm.nih.gov/books/NBK545196/ [PubMed]
- 2.Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet. 2001;75:221–8. doi: 10.1016/s0020-7292(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen CL, Pham NM, Binns CW, Duong D Van, Lee AH. Prevalence of Gestational Diabetes Mellitus in Eastern and Southeastern Asia: A Systematic Review and Meta-Analysis. J Diabetes Res. 2018;2018:6536974. doi: 10.1155/2018/6536974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–6. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 5.Adam S, Rheeder P. Screening for gestational diabetes mellitus in a South African population: Prevalence, comparison of diagnostic criteria and the role of risk factors. S Afr Med J. 2017;107:523–7. doi: 10.7196/SAMJ.2017.v107i6.12043. [DOI] [PubMed] [Google Scholar]
- 6.Gou BH, Guan HM, Bi YX, Ding BJ. Gestational diabetes: weight gain during pregnancy and its relationship to pregnancy outcomes. Chin Med J. 2019;132:154–60. doi: 10.1097/CM9.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macrì F, Pitocco D, di Pasquo E, Salvi S, Rizzi A, Di Leo M, et al. Gestational weight gain as an independent risk factor for adverse pregnancy outcomes in women with gestational diabetes. Eur Rev Med Pharmacol Sci. 2018;22:4403–10. doi: 10.26355/eurrev_201807_15490. [DOI] [PubMed] [Google Scholar]
- 8.Gante I, Amaral N, Dores J, Almeida MC. Impact of gestational weight gain on obstetric and neonatal outcomes in obese diabetic women. BMC Pregnancy Childbirth. 2015;15:249. doi: 10.1186/s12884-015-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Hong Z, Zhang L. Associations of prepregnancy body mass index and gestational weight gain with pregnancy outcomes in nulliparous women delivering single live babies. Sci Rep. 2015;5:12863. doi: 10.1038/srep12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan AM, Dennedy MC, Al-Ramli W, Heerey A, Avalos G, Dunne F. ATLANTIC-DIP: excessive gestational weight gain and pregnancy outcomes in women with gestational or pregestational diabetes mellitus. J Clin Endocrinol Metab. 2014;99:212–9. doi: 10.1210/jc.2013-2684. [DOI] [PubMed] [Google Scholar]
- 11.Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol. 2014;123:737–44. doi: 10.1097/AOG.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IOM (Institute of Medicine) and NRC (National Research Council) Weight Gain During Pregnancy. In: Rasmussen KM, Yaktine AL, editors. Washington DC, Washington (DC): National Academies Press. 2009. [Google Scholar]
- 13.Horosz E, Bomba-Opon DA, Szymanska M, Wielgos M. Maternal weight gain in women with gestational diabetes mellitus. J Perinat Med. 2013;41:523–8. doi: 10.1515/jpm-2012-0254. [DOI] [PubMed] [Google Scholar]
- 14.Park JE, Park S, Daily JW, Kim SH. Low gestational weight gain improves infant and maternal pregnancy outcomes in overweight and obese Korean women with gestational diabetes mellitus. Gynecol Endocrinol. 2011;27:775–81. doi: 10.3109/09513590.2010.540597. [DOI] [PubMed] [Google Scholar]
- 15.Jamilian M, Karamali M, Taghizadeh M, Sharifi N, Jafari Z, Memarzadeh MR, et al. Vitamin D and Evening Primrose Oil Administration Improve Glycemia and Lipid Profiles in Women with Gestational Diabetes. Lipids. 2016;51:349–56. doi: 10.1007/s11745-016-4123-3. [DOI] [PubMed] [Google Scholar]
- 16.Karamali M, Asemi Z, Ahmadi-Dastjerdi M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects pregnancy outcomes in gestational diabetes: randomized, double-blind, placebo-controlled trial. Public Health Nutr. 2016;19:156–63. doi: 10.1017/S1368980015000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karamali M, Bahramimoghadam S, Sharifzadeh F, Asemi Z. Magnesium-zinc-calcium-vitamin D co-supplementation improves glycemic control and markers of cardiometabolic risk in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. 2018;43:565–70. doi: 10.1139/apnm-2017-0521. [DOI] [PubMed] [Google Scholar]
- 18.Jamilian M, Mirhosseini N, Eslahi M, Bahmani F, Shokrpour M, Chamani M, et al. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth. 2019;19:107. doi: 10.1186/s12884-019-2258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 20.Lu M, Xu Y, Lv L, Zhang M. Association between vitamin D status and the risk of gestational diabetes mellitus: a meta-analysis. Arch Gynecol Obstet. 2016;293:959–66. doi: 10.1007/s00404-016-4010-4. [DOI] [PubMed] [Google Scholar]
- 21.Poel YH, Hummel P, Lips P, Stam F, van der Ploeg T, Simsek S. Vitamin D and gestational diabetes: a systematic review and meta-analysis. Eur J Intern Med. 2012;23:465–9. doi: 10.1016/j.ejim.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26:889–99. doi: 10.3109/14767058.2013.765849. [DOI] [PubMed] [Google Scholar]
- 23.Zhang MX, Pan GT, Guo JF, Li BY, Qin LQ, Zhang ZL. Vitamin D Deficiency Increases the Risk of Gestational Diabetes Mellitus: A Meta-Analysis of Observational Studies. Nutrients. 2015;07:8366–75. doi: 10.3390/nu7105398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spaight C, Gross J, Horsch A, Puder JJ. Gestational Diabetes Mellitus. Endocr Dev. 2016;31:163–78. doi: 10.1159/000439413. [DOI] [PubMed] [Google Scholar]
- 25.Sablok A, Batra A, Thariani K, Batra A, Bharti R, Aggarwal AR, et al. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol (Oxf) 2015;83:536–41. doi: 10.1111/cen.12751. [DOI] [PubMed] [Google Scholar]
- 26.Rostami M, Tehrani FR, Simbar M, Bidhendi Yarandi R, Minooee S, Hollis BW, et al. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J Clin Endocrinol Metab. 2018;103:2936–48. doi: 10.1210/jc.2018-00109. [DOI] [PubMed] [Google Scholar]
- 27.Curtis EM, Moon RJ, Harvey NC, Cooper C. Maternal vitamin D supplementation during pregnancy. Br Med Bull. 2018;126:57–77. doi: 10.1093/bmb/ldy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medicine I, Board FN, Calcium CRDRIVD, Valle HBD, Yaktine AL, Taylor CL, et al. Institute of Medicine, Food and Nutrition Board, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In: Del Valle HB, Yaktine AL, Taylor CL, Catharine Ross A, editors. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US) 2011. [Google Scholar]
- 29.Chauhan K, Shahrokhi M, Huecker MR. Vitamin D. 2021 Jul 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: [Internet] http://www.ncbi.nlm.nih.gov/pubmed/28722941 .
- 30.Knabl J, Vattai A, Ye Y, Jueckstock J, Hutter S, Kainer F, et al. Role of Placental VDR Expression and Function in Common Late Pregnancy Disorders. Int J Mol Sci. 2017;18:2340. doi: 10.3390/ijms18112340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99:3373–81. doi: 10.1210/jc.2014-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollis BW, Wagner CL. Clinical review: Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98:4619–28. doi: 10.1210/jc.2013-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yazdchi R, Gargari BP, Asghari-Jafarabadi M, Sahhaf F. Effects of vitamin D supplementation on metabolic indices and hs-CRP levels in gestational diabetes mellitus patients: a randomized, double-blinded, placebo-controlled clinical trial. Nutr Res Pract. 2016;10:328–35. doi: 10.4162/nrp.2016.10.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asemi Z, Karamali M, Esmaillzadeh A. Effects of calcium-vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: a randomised placebo-controlled trial. Diabetologia. 2014;57:1798–806. doi: 10.1007/s00125-014-3293-x. [DOI] [PubMed] [Google Scholar]
- 35.Jamilian M, Samimi M, Ebrahimi FA, Hashemi T, Taghizadeh M, Razavi M, et al. The effects of vitamin D and omega-3 fatty acid co-supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. J Clin Lipidol. 2017;11:459–68. doi: 10.1016/j.jacl.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am J Clin Nutr. 2013;98:1425–32. doi: 10.3945/ajcn.113.072785. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Xing B. Vitamin D3-Supplemented Yogurt Drink Improves Insulin Resistance and Lipid Profiles in Women with Gestational Diabetes Mellitus: A Randomized Double Blinded Clinical Trial. Ann Nutr Metab. 2016;68:285–90. doi: 10.1159/000447433. [DOI] [PubMed] [Google Scholar]
- 38.Hosseinzadeh-Shamsi-Anar M, Mozaffari-Khosravi H, Salami MA, Hadinedoushan H, Mozayan MR. The efficacy and safety of a high dose of vitamin d in mothers with gestational diabetes mellitus: a randomized controlled clinical trial. Iran J Med Sci. 2012;37:159–65. [PMC free article] [PubMed] [Google Scholar]
- 39.Saha S, Saha S. The risk of morbidities in newborns of antenatal vitamin D supplemented gestational diabetes mellitus patient. Int J Health Sci. 2020;14:3–17. [PMC free article] [PubMed] [Google Scholar]
- 40.Saha S, Saha S. A comparison of the risk of cesarean section in gestational diabetes mellitus patients supplemented antenatally with vitamin D containing supplements versus placebo: A systematic review and meta-analysis of double-blinded randomized controlled trials. J Turk Ger Gynecol Assoc. 2020;21:201–12. doi: 10.4274/jtgga.galenos.2020.2019.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saha S. Obstetric and neonatal outcomes in vitamin D supplemented gestational diabetes mellitus patients: an abridgment of systematic reviews. AIMS Med Sci. 2020;7:298–300. [Google Scholar]
- 42.Saha S, Saha S. A Comparison of the Changes in Gestational Weight, Body Mass Index, and Serum Vitamin D Level in Gestational Diabetes Mellitus Patients Complemented with Vitamin D in Contrast to Those Who Did Not Receive the Supplement: A Protocol for Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int J Diabetes Metab. 2019;25:74–9. [Google Scholar]
- 43.Saha S, Saha S. A comparison of changes in gestational weight, body mass index and serum vitamin D level in gestational diabetes mellitus patients complemented with vitamin D contrasted to those who did not receive the supplement: a protocol for systematic review and meta-analysis of randomised controlled trials. PROSPERO. 2020 [cited: 2020 Apr 11]. p. CRD42020149613. Available from: [Internet] https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020149613 .
- 44.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins JPT GS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. [Internet]. The Cochrane Collaboration. 2011 [cited: 2020 Aug 27]. Available from: [Internet] https://training.cochrane.org/handbook/archive/v5.1/
- 47.The Cochrane Collaboration. Review Manager (RevMan) [Computer program] [Internet]. The Cochrane Collaboration. Copenhagen: The Nordic Cochrane Centre; 2014. Available from: [Internet] https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download/download-and-installation .
- 48.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asemi Z, Karamali M, Esmaillzadeh A. Favorable effects of vitamin D supplementation on pregnancy outcomes in gestational diabetes: a double blind randomized controlled clinical trial. Horm Metab Res. 2015;47:565–70. doi: 10.1055/s-0034-1394414. [DOI] [PubMed] [Google Scholar]
- 50.Jamilian M, Amirani E, Asemi Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:2098–105. doi: 10.1016/j.clnu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Razavi M, Jamilian M, Samimi M, Afshar Ebrahimi F, Taghizadeh M, Bekhradi R, et al. he effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr Metab. 2017;14:80. doi: 10.1186/s12986-017-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossi G; American Diabetes Association. Diagnosis and classification of diabetes mellitus. Recenti Prog Med. 2010;101:274–6. [PubMed] [Google Scholar]
- 53.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37Suppl1:S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 54.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mallet E, Gügi B, Brunelle P, Hénocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol. 1986;68:300–4. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol. 2009;70:685–90. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 57.Roth DE, Al Mahmud A, Raqib R, Akhtar E, Perumal N, Pezzack B, et al. andomized placebo-controlled trial of high-dose prenatal third-trimester vitamin D3 supplementation in Bangladesh: the AViDD trial. Nutr J. 2013;12:47. doi: 10.1186/1475-2891-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asemi Z. Clinical trial of the effect of combined omega-3 fatty acids and vitamin D supplementation compared with the placebo on metabolic profiles and pregnancy outcomes in patients with gestational diabetes (Internet). Iranian Registry of Clinical Trials. 2017 (cited: 2019 Oct 18). p. Clinical Trial Protocol. Available from: [Internet] http://en.irct.ir/trial/6136 .
- 60.Clinical trial of the effect of combined vitamin D and evening primrose oil supplementation on metabolic profiles in gestational diabetes (Internet). IRCT (cited: 2020 Jun 9). Available from: [Internet] https://www.irct.ir/trial/6082 .


