A 33-year-old woman, P2L2, presented with complaints of intractable dry cough for three months with nausea, vomiting, loss of appetite, and occasional episodes of fever. She had no menstrual complaints. Her past and family histories were unremarkable. On clinical examination, her performance status score was “2” according to the Eastern Cooperative Oncology Group (ECOG). Vital signs were normal, except for low-grade fever and World Health Organization grade 3 anemia (hemoglobin: 7.6% gm). Abdominal examination revealed a firm to hard, abdominopelvic mass of 22-24 week’s size occupying hypogastrium, right and left iliac fossa with restricted mobility. Cervix and vagina were healthy on pelvic examination. Vaginal examination revealed a similar mass, uterus was not felt separately, pouch of Douglas and bilateral fornices were full. These findings were reconfirmed on rectal examination. Rectal mucosa, recto-vaginal septum, and parametrium were found to be healthy on P/V/R examination.
Complete fever profile including blood culture was normal. She tested negative for Coronavirus disease-2019 (COVID-19). Chest X-ray and contrast-enhanced computed tomography (CECT) of the chest were ordered to look for a cause of refractory cough, but the reports were absolutely normal. Any possibility of tuberculosis was also ruled out.
Ultrasonography (USG) of the abdomen and pelvis was suggestive of a large solid-cystic mass occupying the whole pelvic cavity. CECT showed an 18.5x16.5x15 cm lobulated heterogeneous mass lesion with multiple peripheral enhancing excrescences and nodules with a large, central, non-enhancing area suggestive of neoplastic etiology, uterus, and ovaries were not visualized separately (Figure 1). No enlarged lymph nodes were reported. Serum tumor markers including CA125 (408.2 U/mL) and CA19-9 (312.3 U/mL) were markedly raised while lactate dehydogenase, carcinoembryonic antigen, alpha-fetoprotein, and human chorionic gonadotropin were within normal limits. Pap smear was negative for malignant cells. USG of breast, and upper and lower gastrointestinal endoscopies were insignificant.
Figure 1.
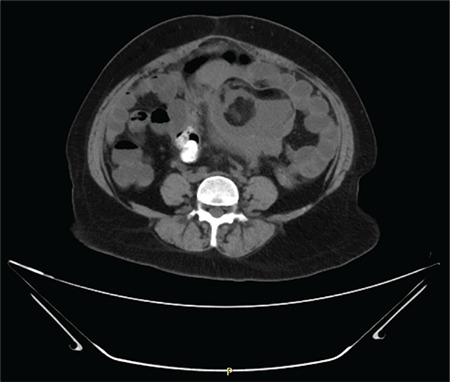
CECT abdomen & pelvis; axial section suggestive of neoplastic ovarian mass
CECT: Contrast-enhanced computed tomography
Her cough could not be relieved with broad-spectrum antibiotics, cough suppressants, and other conservative measures advised by a chest physician. She started having fever more frequently and her total leucocyte count was rising. Eventually, a plan of staging laparotomy was made because of high suspicion of malignancy and deterioration in the general condition of patient.
Answer
After obtaining informed written consent, she was taken up for surgery under combined spinal-epidural anesthesia. She was considered to be at very high risk of respiratory failure with general anesthesia due to persistent cough. The abdomen was entered with a midline vertical infra-umbilical incision. Intraoperatively, a huge, poorly defined, necrotic, friable tumor was found occupying the whole abdominopelvic cavity (Figure 2a,b). Fat planes were lost with anterior abdominal wall, omentum, and surrounding bowel loops. Adhesions were released and approximately 1000 mL sebaceous content was drained from the tumor. Macroscopically, the tumor measured 20x18x15 cm with an irregular surface, solid-cystic areas containing sebaceous fluid, and hair tuft (Figure 2c). There was no well-defined plane and the tumor mass was resected in pieces. The tumor was sent for frozen section to look for malignancy but yielded inconclusive results. After piecemeal removal of tumor mass, uterus could be seen deep down in the pelvis (Figure 2d). Bilateral tubes and ovaries were replaced by the tumor. Total abdominal hysterectomy with bilateral adnexectomy was performed. The omentum and the bowel loops were conglomerated with each other and the entire peritoneum was inflamed and friable. Therefore, pelvic and para-aortic lymph node dissection could not be carried out. However, lymph nodes were not enlarged on examination. Partial omentectomy was performed.
Figure 2.
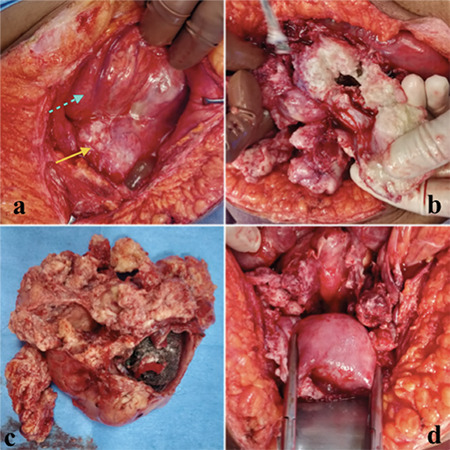
Intraoperative images; a,b) a huge, poorly defined, necrotic, friable tumor was found occupying the whole abdominopelvic cavity, mass (yellow arrow) adhered to bowel loops and omentum (shown by dotted arrow); c) Image shows tumor specimen with an irregular surface, solid-cystic areas containing sebaceous fluid and hair tuft; d) After removal of tumor, uterus was seen deep in the pelvis
Interestingly, the patient was completely relieved of her cough immediately after surgery and there were no fever episodes in the postoperative period. Her postoperative ECOG score was 1.
Histopathology suggested stage IIIC squamous cell carcinoma (SCC) arising from mature cystic teratoma (MCT) involving bilateral ovaries and posterior wall of the uterus up to an outer third of myometrium and atypical cells in omental tissue (Figure 3). Cytology for malignant cells was negative. The peritoneal and cystic fluids were sterile and negative for mycobacterium tuberculosis. Tumor markers were repeated on postoperative day 2 and showed a significant fall in CA125 (87.9 U/mL) and CA19-9 (12.46 U/mL) values. The case was discussed in the institutional tumor board and a decision was taken to give adjuvant chemotherapy with paclitaxel and carboplatin. She was discharged on the 5th postoperative day in stable condition.
Figure 3.
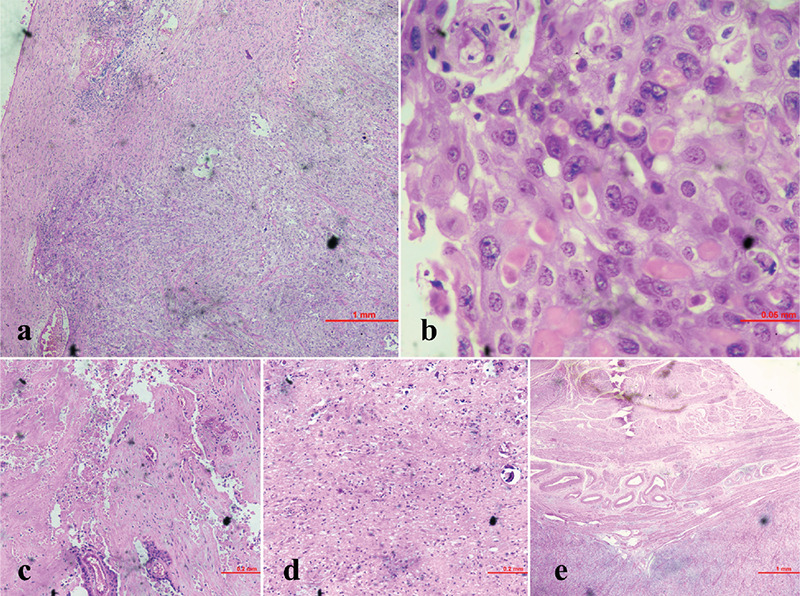
Histopathological images; a) 40x haemotoxylin & eosin (H&E) stain, showing squamous cell differentiation; b) 40x H&E stain, showing squamous cells with individual cell keratinization; c) H&E stain, showing necrotic areas; d) H&E stain, showing glial tissue (neuroectoderm) with calcification; e) H&E stain, showing myometrial involvement
Discussion
MCT is the most common ovarian germ cell tumor. It can occur in 10-20% of women in their lifetime (1). The biological behavior of most MCTs is benign and the clinical course remains uneventful. However, malignant transformation of ovarian MCT is extremely rare (0.17-2%) (2). Most MCTs are diagnosed during reproductive age, while malignant transformation usually occurs in postmenopausal women. Patients with malignant transformation usually present with abdominal pain and mass (2). Whereas, in our case, MCT malignant transformation occurred in a young, reproductive age woman who presented with a complaint of intractable cough.
MCT can have a malignant transformation to various types such as SCC, adenocarcinoma, sarcoma, small cell carcinoma, or malignant melanoma (3). SCC is the most common among all histological types, accounts for >80% of all the malignant transformations (2).
The detection of SCC transformation in MCT is often incidental during examination, imaging or postoperative pathological examination as the presenting complaints are non-specific (4). Patients usually present with abdominal pain, palpable mass, and bloating (2,5). Sometimes, a patient may present with an acute abdomen due to torsion or rupture of tumor (6,7). Our case is unique as the patient’s chief complaint was intractable cough. In the literature review, we could not find any patient with malignant transformation with such presenting complaints. The exact reason behind her cough is unclear. Still, it might be because of diaphragmatic irritation due to tumor cells or the other possibility is prior rupture of MCT, but less likely as there was no feature of peritonitis, such as high-grade fever or acute abdominal pain.
Like any other ovarian cancer, SCC transformation in MCT should be treated with staging surgery including total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and lymph node dissection. Li et al. (2) described that hysterectomy and omentectomy may improve survival while lymph node dissection doesn’t affect long-term survival. Nevertheless, they recommend lymphadenectomy as complete cytoreduction should improve the outcome. Fertility sparing surgery may be offered in women <45 years of age with stage IA/IC malignancy. In our case, hysterectomy and partial omentectomy were performed due to high suspicion of advanced malignancy.
The role of adjuvant chemotherapy and the ideal regimen is not well recognized in SCC transformation of MCT. In a systemic review by Li et al. (2), overall survival was not improved with adjuvant chemotherapy. On the contrary, Hackethal et al. (8) reported that adjuvant chemotherapy (paclitaxel and carboplatin) may have a better prognosis in the advanced stage.
Prognostic factors are not yet defined for this entity. Staging of the tumor appears to have an impact on the outcome. The reported 5-year survival rate in stage I, II, III, IV is 85.8%, 39.1%, 26.2%, 0% respectively (2). Therefore, diagnosis in early stages is crucial for long-term survival. Early diagnosis is a challenge as there is no specific imaging or laboratory test that can detect malignancy in MCT. Hence, MCT in women >30 years old with solid areas or firm, friable, myxomatous, or variegated areas or unusual adherence to surrounding structures should be treated with suspicion.
To the best of our knowledge, this is the first case report to describe intractable cough as a presenting complaint in SCC transformation from ovarian MCT. This disease always poses a diagnostic challenge due to its rarity and aggressive clinical course. Diagnostic criteria and treatment protocols are yet to be determined. Available data is limited. Hence, the reporting of each case is important to elucidate its biology and clinical course. Preoperative suspicion of malignant transformation in MCT may result in an optimum outcome. Health care providers should be aware of this disease entity and intractable cough as a presenting complaint.
References
- 1.Kim MJ, Kim NY, Lee DY, Yoon BK, Choi D. Clinical characteristics of ovarian teratoma: age-focused retrospective analysis of 580 cases. Am J Obstet Gynecol. 2011;205:32.e1–4. doi: 10.1016/j.ajog.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Zhang Q, Zhang S, Dong R, Sun C, Qiu C, et al. Squamous cell carcinoma transfor-mation in mature cystic teratoma of the ovary: a systematic review. BMC Cancer. 2019;19:217. doi: 10.1186/s12885-019-5393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rim SY, Kim SM, Choi HS. Malignant transformation of ovarian mature cystic teratoma. Int J Gynecol Cancer. 2006;16:140–4. doi: 10.1111/j.1525-1438.2006.00285.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi EJ, Koo YJ, Jeon JH, Kim TJ, Lee KH, Lim KT. Clinical experience in ovarian squa-mous cell carcinoma arising from mature cystic teratoma: a rare entity. Obstet Gynecol Sci. 2014;57:274–80. doi: 10.5468/ogs.2014.57.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarmohammadi H, Mansoori B, Wong V, Tacher V, Wilkins LR, Pavlidakey PG, et al. Squamous cell carcinoma arising from ovarian mature cystic teratoma and causing small bowel obstruction. J Cancer Res Ther. 2014;10:770–2. doi: 10.4103/0973-1482.136051. [DOI] [PubMed] [Google Scholar]
- 6.Gooneratne AT, James AO, Gupta J, Abdulaal Y. Squamous cell carcinoma arising in a ma-ture cystic teratoma invading the sigmoid colon: a rare presentation. BMJ Case Rep. 2015;2015:bcr2014208472. doi: 10.1136/bcr-2014-208472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Malignant transformation of mature cystic teratoma of the ovary: Experience at a single institution. Eur J Obstet Gynecol Reprod Biol. 2008;141:173–8. doi: 10.1016/j.ejogrb.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg HR, Münstedt K. Squa-mous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol. 2008;9:1173–80. doi: 10.1016/S1470-2045(08)70306-1. [DOI] [PubMed] [Google Scholar]


