Abstract
Introduction
The neutrophil-to-lymphocyte ratio (NLR) could be a predictive factor of severe COVID-19. However, most relevant studies are retrospective, and the optimal NLR cut-off point has not been determined. The objective of our research was identification and validation of the best NLR cut-off value on admission that could predict high in-hospital mortality in COVID-19 patients.
Methods
Medical files of all patients admitted for COVID-19 pneumonia in our dedicated COVID-units between March and April 2020 (derivation cohort) and between October and December 2020 (validation cohort) were reviewed.
Results
Two hundred ninety-nine patients were included in the study (198 in the derivation and 101 in the validation cohort, respectively). Youden’s J statistic in the derivation cohort determined the optimal cut-off value for the performance of NLR at admission to predict mortality in hospitalized patients with COVID-19. The NLR cut-off value of 5.94 had a sensitivity of 62% and specificity of 64%. In ROC curve analysis, the AUC was 0.665 [95% CI 0.530–0.801, p= 0.025]. In the validation cohort, the best predictive cut-off value of NLR was 6.4, which corresponded to a sensitivity of 63% and a specificity of 64% with AUC 0.766 [95% CI 0.651–0.881, p <0.001]. When the NLR cut-off value of 5.94 was applied in the validation cohort, there was no significant difference in death and survival in comparison with the derivation NLR cut-off. Net reclassification improvement (NRI) analysis showed no significant classification change in outcome between both NLR cut-off values (NRI:0.012, p=0.31).
Conclusion
In prospective analysis, an NLR value of 5.94 predicted high in-hospital mortality upon admission in patients hospitalized for COVID-19 pneumonia.
Keywords: neutrophil-to-lymphocyte ratio, coronavirus disease, SARS-CoV-2 infection, COVID-19, risk factors, laboratory markers
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, secondary to SARS-CoV-2 infection, is a serious disease worldwide.1 Risk factors for severe COVID-19 are age, male sex, genetic variants (in Eurasians),2 inborn errors, or auto-antibodies interfering with interferon or immunity3 and chronic diseases such as high blood pressure, cardiovascular disease, and diabetes.4 While research for effective treatment of COVD-19 and large-scale vaccination campaigns are ongoing, identifying biomarkers on admission that could predict in-hospital mortality remain important. Abnormal laboratory markers on admission that have been associated with mortality1,5,6 are elevated serum levels of creatinine, D-dimer, troponin I, lactate dehydrogenase (LDH) and IL-6 as well as thrombocytopenia. Many prognostic scores have been developed7–9 and differ in their predicted outcome measure and clinical parameters.7
Recently, Knight et al8 developed the 4 C Mortality Score. The score ranges from 0 to 21 points and includes the usual clinical and biological variables, such as age, sex, number of comorbidities, breathing rate, peripheral oxygen saturation, Glasgow coma scale, urea, and CRP levels. A score of ≥15 had a 62% mortality risk compared with 1% mortality risk for those with a score of ≤ 3, which is better than previously developed scores (ROC analysis with AUC range 0.61–0.76). Many studies have reported that the neutrophil-to-lymphocyte ratio (NLR) can predict severe disease.9–14 However, most of the studies were retrospective, and the optimal NLR cut-off point is lacking.
The objective of our research was to identify and validate the best NLR cut-off value on admission which could predict high in-hospital mortality in COVID-19 patients.
Methods
Setting and Patients
The study was conducted in one of the largest teaching hospitals in Belgium. Medical files of patients with COVID-19 pneumonia admitted to our dedicated COVID-19 units were reviewed. Patients admitted between March 2020 and April 2020 (derivation cohort) were retrospectively analyzed to identify the NLR cut-off point on admission that enabled mortality prediction. We then prospectively included 101 patients between 01 October 2020 and 25 December 2020 (validation cohort) to validate the NLR cut-off point. Only patients with a positive RT-PCR nasopharyngeal swab were included. The definition of severe COVID-19 was (≥1 positive criteria): (1) respiration rate ≥30 breaths per minute; (2) mean oxygen saturation <94% while breathing room air; (3) arterial blood oxygen partial pressure/oxygen concentration ≤ 300 mm Hg (1 mm Hg = 0.133kPa). Patients were excluded if <18 years old, undergoing palliative care, pregnant, or under chemotherapy for solid cancer or hematological disease (lymphoma, leukemia, myeloma).
Ethical Issues
The institutional ethical board approved the study (CEHF 2020/06AVR/201, Comité d’Ethique Hospitalo-Facultaire, Cliniques Universitaires Saint-Luc). Since the study was not an interventional study and that we analysed routine laboratory tests, which were already performed in all patients, informed consent was not necessary according to Belgian and local ethics law. The study was performed in accordance with the principles stated in the Declaration of Helsinki, and confidentiality of patients was guaranteed.
Data Analysis
We used our institutional database to collect the following data of all hospitalized COVID-19 patients: Demographic characteristics (age, sex, ethnicity); tobacco use; symptoms; clinical parameters; laboratory data (neutrophil count, lymphocyte count, eosinophil count, NLR, LDH, renal function, uric acid, troponin, C-reactive protein, ferritin, and D-dimer); treatment against SARS-CoV-2 and results of chest CT scan. Blood processing machines (Cobas® 8100 [Roche] and XN9000 [Sysmex]) were used to enumerate neutrophils and lymphocytes in the blood. Neutrophil-to-lymphocyte index was obtained by machine-derived cell differentials (neutrophil count divided by lymphocyte count). Chest CT images were classified as 1) compatible or not compatible with COVID-19 pneumonia; 2) the percent of lung involved (<10%, 10–25%, 25–50%, or >50%), based on visual assessment of radiological lung lesions. Risk factors associated with severe COVID-19, such as age, malignancy (without chemotherapy), cardiac disease, high blood pressure, diabetes, COPD, liver disease, the need for oxygen supplementation or ventilation support, admission to the intensive care unit (ICU), death, and length of stay in the dedicated COVID-19 unit were collected.
Statistical Analysis
Continuous variables were expressed as mean and standard deviations and categorical variables as counts and percentages. Categorical and continuous variables were compared with the Chi-squared test and the unpaired Student’s t-test, respectively. Youden’s J statistics was used in both cohorts to identify the best predictive cut-off values of NLR on admission associated with high in-hospital mortality. Net reclassification improvement analysis was calculated to assess whether the NLR cut-off evaluated in both cohorts led to classification changes. Receiver-operating characteristic (ROC) curves were computed to measure the discrimination performance of cut-off values. The odds ratio (OR) of NLR for predicting mortality was calculated with univariate binary logistic regression.
All analyses were conducted with SPSS 27 software (IBM SPSS Statistics for Windows, Version 27.0, Armonk, NY: IBM Corp). All tests were 2-sided with 0.05 as the significance threshold.
Results
Clinical Characteristics and Outcome
Demographics and clinical characteristics of patients included in the validation and derivation cohorts are summarized in Table 1. Age, comorbidity, and severity of the diseases were similar between the two cohorts.
Table 1.
Clinical Characteristics of Patients with COVID-19
| Characteristics | Derivation Cohort (N=198) | Validation Cohort (N=101) | P value |
|---|---|---|---|
| Sexe (Male) | 110 (55%) | 65 (64%) | 0.14 |
| Mean Age | 64.4 [14] | 62.3 [17.2] | 0.269 |
| BMI (kg/m2) | 28 [5] | 27 [5] | 0.444 |
| Smoking | 8 (4%) | 2 (2%) | 0.35 |
| Mean SpO2* | 90% [4.6] | 89% [4.5] | 0.004 |
| Nbr of patients with OT | 179 (91%) | 99 (98%) | 0.02 |
| Mechanical and non-mechanical respiratory support | |||
| HFNC | 29 (15%) | 16 (15.8%) | 0.78 |
| Oxygen mask | 87 (44%) | 55 (54.5%) | 0.09 |
| CPAP | 56 (28%) | 43 (42%) | 0.13 |
| Invasive mechanical ventilation | 18 (9%) | 8 (7.9%) | 0.73 |
| Co-morbidities | |||
| Cardiovascular disease | 107 (54%) | 45 (44.6%) | 0.12 |
| Hypertension | 101 (51%) | 52 (51.5%) | 0.94 |
| Chronic pulmonary disease | 33 (17%) | 10 (9.9%) | 0.11 |
| Diabetes | 49 (25%) | 22 (21.8%) | 0.6 |
| Immunosuppression | 24 (12%) | 18 (17.8%) | 0.18 |
| Chronic liver disease | 10 (5%) | 2 (2%) | 0.2 |
| Chronic kidney disease | 36 (18%) | 16 (15.8%) | 0.54 |
| Malignancy | 10 (5%) | 6 (5.9%) | 0.75 |
| Biological data | |||
| CRP on admission (mg/dl) | 103.4 [81.7] | 100 [74.8] | 0.735 |
| WBC count on admission | 6.7 [3.3] | 7 [3.7] | 0.431 |
| Absolute Neutrophil Count on admission (x10 3/mm3) | 5.13 [2.99] | 5.5 [3.3] | 0.398 |
| NLR on admission | 7 [7.4] | 7.3 [6.1] | 0.767 |
| Eosinophil count on admission (x10 3/mm3) | 0.02 (0.04) | 0.02 (0.05) | 0.930 |
| LDH (UI/L) | 385 [189] | 369 [136] | 0.445 |
| AST (UI/L) | 54 [81] | 43 [26] | 0.195 |
| ALT (UI/L) | 39 [74] | 36 [31] | 0.696 |
| D-Dimer (ng/mL) | 2634 [4077] | 1926 [3349] | 0.177 |
| Creatinine (mg/dl) | 1.4 [1.6] | 1.4 [3] | 0.867 |
| Troponin T (ng/L) | 31.2 [69.1] | 15.2 [19.2] | |
| Lung CT scan | 188 (95%) | 80 (79.2%) | 0.001 |
| Stratification of lung lesions on CT scan | |||
| <10% | 15 (7.6%) | 7 (6.9%) | 0.2 |
| 10–25% | 90 (45.5%) | 28 (27.7%) | |
| 25–50% | 53 (26.8%) | 25 (24.8%) | |
| >50% | 30 (15.2%) | 20 (19.8%) | |
| Outcome | |||
| Overall death | 29 (15%) | 19 (18.8%) | 0.35 |
| ICU admission | 37 (19%) | 16 (15.8%) | 0.54 |
| Death in ICU | 11 (6%) | 6 (5.9%) | 0.9 |
| Treatment | |||
| HCQ | 144 (72.7%) | / | |
| HCQ with AZT | 18 (9.1%) | / | |
| HCQ combined with CS | 19 (9.6%) | / | |
| HCQ with AZT and CS | 17 (8.6%) | / | |
| Dexamethasone | / | 101 (100%) |
Notes: *When breathing ambient air. Data are mean (SD), Interquartile range [IQR] or percentage (%).
Abbreviations: OT, oxygen therapy; HCQ, hydroxychloroquine; AZT, azithromycin; CS, corticosteroids; HFNC, high Flow Nasal Cannula; NLR, neutrophil to lymphocyte ratio; CPAP, continuous positive airway pressure; CRP, C-reactive protein, ICU: intensive care unit.
ROC Curve and Youden Index Analysis in the Derivation (n=198) and Validation (n=101) Cohorts
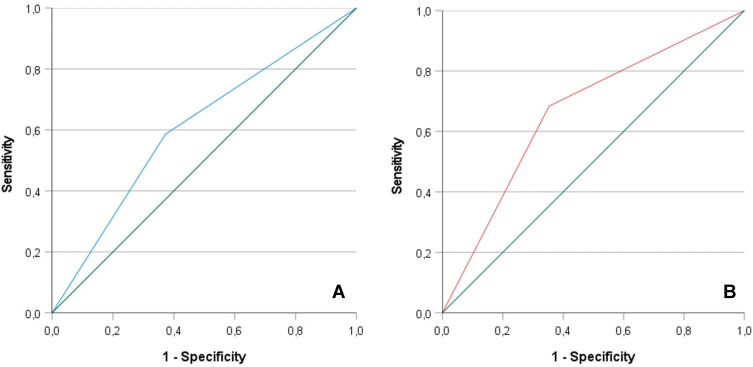
In the derivation cohort, the best predictive cut-off value of NLR on admission was 5.94, which was associated with 62% sensitivity and 64% specificity. Discrimination performances by ROC analysis (Figure 1A) for predicting mortality in hospitalized patients with COVID-19 had an AUC of 0.665 [95% CI 0.530–0.801, p = 0.025]. In the validation cohort, the optimal cut-off value of NLR was slightly different (6.4), with corresponding sensitivity of 63% and specificity of 64%. ROC analysis (Figure 1B) showed an AUC of 0.766 [95% CI 0.651–0.881, p <0.001]. When the NLR cut-off value of 5.94 was applied in the validation cohort, no significant differences in death and survival between the 2 cut-off values were found (Table 2). Net reclassification improvement (NRI) analysis confirmed that there were no statistically significant classification changes in terms of outcome, by using both NLR cut off values (NRI: 0.012, p= 0.31). Univariate analysis showed that the NLR cut-off value of 5.94 was associated with an odds ratio of 3.9 for death (CI 95% 1.13–11.50, p=0.012).
Figure 1.
Receiver-operating characteristic (ROC) curves showing the discrimination performance of NLR cut off values in the derivation (1A) and validation (1B) cohort.
Table 2.
Overall Death According to Different NLR Cut-off Value in the Validation Cohort and Evaluation by Net Reclassification Improvement
| NLR | Death | Alive | p value | NRI |
|---|---|---|---|---|
| NLR <5.94 | 6 (32%) | 53 (65%) | 0.008 | 0.012 (p value =0.31) |
| NLR >5.94 | 13 (68%) | 29 (35%) | ||
| NLR <6.4 | 6 (32%) | 54 (67%) | 0.006 | |
| NLR >6.4 | 13 (68%) | 28 (34%) |
Abbreviations: NRI, net reclassification improvement; NLR, neutrophil to lymphocyte ratio.
Discussion
The main outcome of this study was the identification of the neutrophil-to-lymphocyte ratio (NLR) cut-off point on admission that predicted high in-hospital mortality from COVID-19 pneumonia; the cut-off value of 5.94 was associated with an odds ratio of 3.9 for death. Interest in NLR is keen because it is a simple and cheap biomarker. While many prognostic tools have been developed for COVID-19, the simplicity of the NLR likely will make it useful in a broad range of health-care systems, especially in limited-resource settings.
Increased NLR is a risk factor for mortality in various diseases, such as hip fractures, infection, malignant diseases, acute myocardial ischemia, and polymyositis.15–18 Several studies have found that that NLR is associated with progression and mortality of COVID-19.19–24 However, most of these studies were retrospective, and prospective studies have been needed. Li et al10 included 19 studies in their meta-analysis, and only one was prospective. Li et al25 found, in a meta-analysis including 34 studies (25,074 COVID-19 patients) that high NLR was an independent risk factor for high mortality. Thirteen studies (1579 patients) found that NLR was predictive of disease severity with an AUC 0.85 (95% CI 0.81–0.88). Ten studies (2967 patients) reported that NLR was predictive of mortality, with 83% sensitivity and specificity. In their subgroup analysis, 10 studies showed that an NLR cut-off value ≥ 6.5 and < 6.5 were predictive of mortality with AUC 0.92 (95% CI 0.89–0.94) and 0.84 (95% CI 0.80–0.87), respectively. This cut-off value is in line with our NLR 5.94 and 6.4 in the derivation and validation cohort, respectively. Compared with the results of the meta-analysis of Hariyanto et al,26 NLR is as efficient as C-reactive protein, D-dimer, LDH, and procalcitonin in predicting severe outcome on admission in patients with COVID-19.
The mechanism by which NLR is associated with poor outcomes was first proposed by Zahorec et al27 They showed that in stress, values of inflammatory cytokines and neutrophils are increased, which may induce a decrease in lymphocyte counts and apoptosis. Since lymphocytes are involved in the regulation of the inflammatory response, the decrease in their numbers may be harmful and give rise to a high inflammatory state.28 COVID-19 infection is characterized by lymphopenia and high cytokine production, such as in haemophagocytic lymphohistiocytosis, with increased levels of IL-1, IL-2, IL-6, IL-7, and tumour necrosis factor-α.29 Biomarkers such as IL-6 and IL-1 are associated with poor outcome, but since these biomarkers are not widely available, others are needed. The NLR is an easily calculated blood test that can help to quickly identify patients at high risk of death and thereby improve their management.
Several other biomarkers (COVID-GRAM, NEWS 2, and 4C mortality score) have been proposed to help identify patients who may have life-threatening COVID disease.8,12,30 The COVID-GRAM, constructed by Liang et al,12 is based on 10 variables, with NLR being one of its components. However, many parameters such as creatinine, D-dimer, ferritin, and sex that are associated with high mortality are not included in the COVID-GRAM. In a previous study,9 we retrospectively validated COVID-GRAM and found that NLR on admission and day 3 may predict patients at risk of critical disease as effectively as does COVID-GRAM. NEWS2 score seems to be significantly associated with intubation, whereas 4C mortality score was predictive of mortality.8,30,31 Recently, Yildiz et al31 prospectively validated these scores in a cohort of 114 patients; 4C mortality score had the highest discrimination for mortality prediction. NEWS2 on admission seems to be a better predictor of ICU admission than are CURB-65, COVID-GRAM, and 4C mortality score.31 Compared with the four scores cited above, NLR on admission was also predictive of in–hospital mortality but not of ICU admission.31
Artificial intelligence systems have been studied to improve outcome of patients with COVID-19.32–36 These machine-learning systems can determine the relationships between clinical data and variables associated with outcome (mortality and ICU admission) without using linear or logistic regression. Studies using machine-learning systems showed that CRP, LDH, and procalcitonin were predictors of mortality and ICU admission, whereas D-dimer, age, and lymphocytes were better predictors of mortality than were ferritin, oxygen saturation, and temperature, which were better predictors of ICU admission.32–36 Most of the studies based on artificial systems need validation in prospective and multicentric study but seem promising. However, artificial systems should be used with caution in COVID-19 patients since they may exacerbate the health inequities already present in developing countries.37
Our study has limitations. 1) It is a monocentric study, and the sample size is small. Only Belgian patients are represented, so our findings need external validation with variable and larger populations. 2) Whether the NLR cut-off value can be used for more aggressive management and treatment of patients needs to be tested in multicenter, randomized, and prospective studies. 3) The effects of treatment of comorbidities associated with COVID-19 were not assessed. Drugs such as metformin, insulin, and DPP-4 inhibitors could affect survival. Several meta-analyses have studied the impact of diabetes drugs on outcome of patients infected with COVID-19.38–40 While insulin therapy seems to be associated with poor outcome, metformin use was associated with reduced mortality in COVID-19 patients,39 but two recent studies found that DPP-4 inhibitor use was not associated with poor outcome.40,41
Conclusions
In a prospective study, a neutrophil-to-lymphocyte ratio value of 5.94 was the best predictive value of in-hospital mortality for COVID-19. The neutrophil-to-lymphocyte ratio may be useful for clinicians in a broad range of health care systems, especially in limited-resource settings where other inflammatory markers (interleukins, ferritin, and D-dimer) and CT scan are not available.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Halil Yildiz and Diego Castanares-Zapatero are co-first authors for this study. The authors report no conflicts of interest in this work.
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeberg H, Pääbo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020;587(7835):610–612. doi: 10.1038/s41586-020-2818-3 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570. doi: 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett S, Tafuro J, Mayer J, et al. Clinical features and outcomes of adults with COVID-19: a systematic review and pooled analysis of the literature. Int J Clin Pract. 2020;75:e13725. doi: 10.1111/ijcp.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynants L, Calster BV, Bonten MMJ, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. bmj. 2020;369:m1328. doi: 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight SR, Ho A, Pius R. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yildiz H, Yombi JC, Castanares-Zapatero D. Validation of a risk score to predict patients at risk of critical illness with COVID-19. Infect Dis (Lond). 2021;53(1):78–80. doi: 10.1080/23744235.2020.1823469 [DOI] [PubMed] [Google Scholar]
- 10.Li X, Liu C, Mao Z, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care. 2020;24(1):647. doi: 10.1186/s13054-020-03374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Zheng Y, Cai L, et al. Neutrophil-to-lymphocyte ratio, a critical predictor for assessment of disease severity in patients with COVID-19. Int J Lab Hematol. 2021;43(2):329–335. doi: 10.1111/ijlh.13374 [DOI] [PubMed] [Google Scholar]
- 12.Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081. doi: 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte count predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18:206. doi: 10.1186/s12967-020-02374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niessen R, Bihin B, Gourdin M, Yombi JC, Cornu O, Forget P. Prediction of postoperative mortality in elderly patient with hip fractures: a single-centre, retrospective cohort study. BMC Anesthesiol. 2018;18:183. doi: 10.1186/s12871-018-0646-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faria SS, Fernandes PJ, Silva MJ. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azab B, Zaher M, Weiserbs KF. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST elevation myocardial infarction. Am J Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062 [DOI] [PubMed] [Google Scholar]
- 18.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 19.Lian J, Jin C, Hao S, et al. High neutrophil-to-lymphocyte ratio associated with progression to critical illness in older patients with COVID-19: a multicenter retrospective study. Aging (Albany NY). 2020;30(12):13849–13859. doi: 10.18632/aging.103582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JJ, Cao YY, Tan G, et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2021;76(2):533–550. doi: 10.1111/all.14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu J, Kong J, Wang W, et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb Res. 2020;192:3–8. doi: 10.1016/j.thromres.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7(9):e671–e678. doi: 10.1016/S2352-3026(20)30217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nalbant A, Kaya T, Varim C, Yaylaci S, Tamer A, Cinemre H. Can the neutrophil/lymphocyte ratio (NLR) have a role in the diagnosis of coronavirus 2019 disease (COVID-19)? Rev Assoc Med Bras (1992). 2020;66(6):746–751. doi: 10.1590/1806-9282.66.6.746 [DOI] [PubMed] [Google Scholar]
- 24.Ma A, Cheng J, Yang J, Dong M, Liao X, Kang Y. Neutrophil-to-lymphocyte ratio as a predictive biomarker for moderate-severe ARDS in severe COVID-19 patients. Crit Care. 2020;24(1):288. doi: 10.1186/s13054-020-03007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Hou H, Diao J, Wang Y, Yang H. Neutrophil-to-lymphocyte ratio is independently associated with COVID-19 severity: an updated meta-analysis based on adjusted effect estimates. Int J Lab Hematol. Epub 2021 Jan 27. doi: 10.1111/ijlh.13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hariyanto TI, Japar KV, Kwenandar F, et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: a systematic review and meta-analysis. Am J Emerg Med. 2021;41:110–119. doi: 10.1016/j.ajem.2020.12.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zahorec R. Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 28.Heffernan DS, Monaghan SF, Thakkar RK, Machan JT, Cioffi WG, Ayala A. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit Care. 2012;16:R12. doi: 10.1186/cc11157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gidari A, Vittorio De Socia G, Sabbatini S, et al. Predictive value of National Early Warning Score 2 (NEWS2) for intensive care unit admission in patients with SARS-CoV-2 infection. Infect Dis (Lond). 2020;52(10):698–704. doi: 10.1080/23744235.2020.1784457 [DOI] [PubMed] [Google Scholar]
- 31.Yildiz H, Castanares-Zapatero D, Hannesse C, Vandermeersch D, Pothen L, Yombi JC. Prospective validation and comparison of COVID-GRAM, NEWS2, 4C mortality score, CURB-65 for the prediction of critical illness in COVID-19 patients. Infect Dis (Lond). Epub 2021 Mar 10. doi: 10.1080/23744235.2021.1896777 [DOI] [PubMed] [Google Scholar]
- 32.Hou W, Zhao Z, Chen A, Li H, Duong TQ. Machining learning predicts the need for escalated care and mortality in COVID-19 patients from clinical variables. Int J Med Sci. 2021;18(8):1739–1745. doi: 10.7150/ijms.51235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu JS, Ge P, Jiang C, et al. Deep-learning artificial intelligence analysis of clinical variables predicts mortality in COVID-19 patients. J Am Coll Emerg Physicians Open. 2020;1(6):1364–1373. doi: 10.1002/emp2.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu JQ, Musheyev B, Peng Q, Duong TQ. Neural network analysis of clinical variables predicts escalated care in COVID-19 patients: a retrospective study. PeerJ. 2021;9:e11205. doi: 10.7717/peerj.11205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang X, Coffee M, Bari A, et al. Towards an artificial intelligence framework for data-driven prediction of coronavirus clinical severity. Computers, Materials and Continua. 2020;63(1). doi: 10.32604/cmc.2020.010691 [DOI] [Google Scholar]
- 36.Li X, Ge P, Zhu J, et al. Deep learning prediction of likelihood of ICU admission and mortality in COVID-19 patients using clinical variables. PeerJ. 2020;8:e10337. doi: 10.7717/peerj.10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abrams EM, Szefler SJ. COVID-19 and the impact of social determinants of health. Lancet Respir Med. 2020;8(7):659–661. doi: 10.1016/S2213-2600(20)30234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hariyanto TI, Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020;19:100290. doi: 10.1016/j.obmed.2020.100290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hariyanto TI, Lugito NPH, Yanto TA, Siregar JI, Kurniawan A. Insulin therapy and outcome from coronavirus disease 2019 (COVID-19): a Systematic Review, Meta-Analysis, and Meta-Regression. Endocr Metab Immune Disord Drug Targets. Epub 2021 Jul 09. doi: 10.2174/1871530321666210709164925 [DOI] [PubMed] [Google Scholar]
- 40.Hariyanto TI, Kurniawan A. Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021;20(1):1–8. doi: 10.1007/s40200-021-00777-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Diabetes Endocrinol. 2021;9:586–594. doi: 10.1016/S2213-8587(21)00180-7 [DOI] [PMC free article] [PubMed] [Google Scholar]