Abstract
Background
Knowledge regarding factors predicting the SARS-COV-2 reinfection risk is scarce and it has major implications in public health policies. We aimed to identify factors associated with the risk of symptomatic SARS-COV-2 reinfection.
Methods
We conducted a nationwide retrospective cohort study and 99,993 confirmed cases of COVID-19 were analyzed.
Results
The overall risk of reinfection (28 or more elapsed days between both episodes onset) was 0.21% (incidence density, 2.5 reinfections per 100,000 person-days) and older subjects and those with the mild primary disease were at reduced risk of the event. Healthcare workers and immunosuppressed or renal patients had at greater risk of SARS-COV-2 reinfection.
Conclusions
If replicated in other populations, these results may be useful to prioritize efforts focusing on the reduction of SARS-COV-2 spread and the related burden.
Keywords: COVID-19, Severe Acute Respiratory Syndrome Coronavirus 2, Health Personnel, Risk Reduction Behavior
Background
The COVID-19 (coronavirus disease 2019) by SARS-COV-2 (severe acute respiratory coronavirus 2) pandemic is a complex phenomenon and reinfection is one of the many ongoing related debates [1]. Current knowledge regarding factors predicting the SARS-COV-2 reinfection risk is scarce and it has major implications in public health policies, including vaccination strategies and relaxation of social distancing measures [2].
The social and economic burden of the COVID-19 pandemic in Mexico has been high and by mid-February 2021, nearly 2 million laboratory-positive cases and nearly 170 thousand deaths had been registered [3]. Current vaccination efforts in Mexico started in late December 2020 and are slowly progressing; they first targeted health-care personnel directly involved in the attention of COVID-19 patients. Our study aimed to identify factors associated with the risk of SARS-COV-2 symptomatic reinfection in a large and nationwide cohort of laboratory-confirmed COVID-19 survivors.
Methods
A nationwide and retrospective cohort study was conducted in Mexico including adults (aged 20 years or above) with laboratory-confirmed (quantitative reverse transcription polymerase chain reaction, RT-qPCR) COVID-19 by SARS-COV-2. This analysis took place in September 2020 and a broader description of the methods has already been published [4]. Adults whose symptoms appeared from March to June 2020 and who recovered to primary infection were analyzed.
The main binary outcome was symptomatic reinfection of SARS-COV-2 and was defined by the reappearance of symptoms of COVID-19 at 28 days or more after initial laboratory-confirmed illness [1] and a positive RT-qPCR result during second-time illness. Risk ratios (RR) and 95% confidence intervals (CI), calculated using generalized linear regression models, were used to identify factors associated with the risk of reinfection. All methods were performed following the relevant guidelines and regulations.
Results
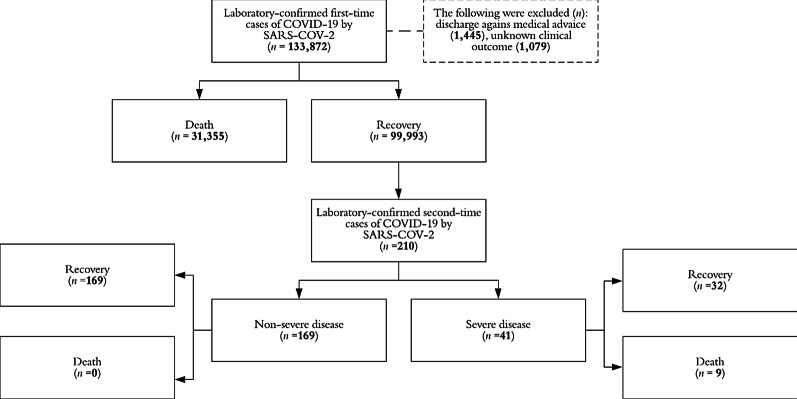
Data from 99,993 participants were analyzed for a total follow-up of 8,268,237 person-days. The overall risk of SARS-COV-2 symptomatic reinfection was 0.21% (n = 210) and the incidence density was 2.5 reinfections per 100,000 person-days. The mean elapsed days (± standard deviation) between both COVID-19 episodes was 61.0 ± 31.0 and ranged from 28 to 116 days. Mild subsequent illness was documented in 169 patients (80.5%) of reinfected subjects and the observed fatality rate was 4.3% (n = 9). Figure 1 shows the study profile.
Fig. 1.
Study profile, Mexico 2020. COVID-19 Coronavirus disease 2019, SARS-COV-2 Severe acute respiratory syndrome coronavirus 2
Table 1 shows the characteristics of the study sample according to the reinfection status for selected variables. Patients with SARS-COV-2 reinfection were younger and were more likely to be healthcare professionals or other related employments. They were also more likely to have had milder symptoms at primary disease and had a significantly higher prevalence of chronic kidney disease or immunosuppression (any cause except for type 2 diabetes mellitus or kidney disease).
Table 1.
Characteristics of the study sample according to symptomatic SARS-COV-2 reinfection status, Mexico 2020
| Overall | SARS-COV-2 reinfection | p | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| n = 99,993 | n = 99,783 | n = 210 | |||||
| Gender | |||||||
| Female | 50,916 | (50.9) | 50,805 | (50.9) | 111 | (52.9) | 0.574 |
| Male | 49,077 | (49.1) | 48,978 | (49.1) | 99 | (47.1) | |
| Age (mean ± SD, years) | 42.2 ± 13.1 | 42.2 ± 13.1 | 39.2 ± 10.4 | < 0.001 | |||
| Age group (years) | |||||||
| 20–49 | 73,069 | (73.1) | 72,888 | (73.1) | 181 | (86.2) | < 0.001 |
| 50–59 | 16,755 | (16.8) | 16,735 | (16.7) | 20 | (9.5) | |
| 60–69 | 6644 | (6.6) | 6638 | (6.7) | 6 | (2.9) | |
| 70 + | 3525 | (3.5) | 3522 | (3.5) | 3 | (1.4) | |
| Occupation | |||||||
| Housewife | 10,685 | (10.7) | 10,679 | (10.7) | 6 | (2.9) | < 0.001 |
| Healthcare worker | 10,183 | (10.2) | 10,151 | (10.2) | 32 | (15.2) | |
| Other healthcare-related | 22,303 | (22.3) | 22,195 | (22.2) | 108 | (51.4) | |
| Student | 919 | (0.9) | 919 | (0.9) | 0 | (0) | |
| Other | 55,903 | (55.9) | 55,839 | (56.0) | 64 | (30.5) | |
| Disease severity (at primary infection) a | |||||||
| Mild-moderate | 81,018 | (81.0) | 80,827 | (81.0) | 191 | (91.0) | < 0.001 |
| Severe | 18,975 | (19.0) | 18,956 | (19.0) | 19 | (9.0) | |
| Personal history of: | |||||||
| Obesity (BMI 30 or higher) | |||||||
| No | 81,531 | (81.5) | 81,353 | (81.5) | 178 | (84.8) | 0.228 |
| Yes | 18,462 | (18.5) | 18,430 | (18.5) | 32 | (15.2) | |
| Type 2 diabetes mellitus | |||||||
| No | 86,909 | (86.9) | 86,718 | (86.9) | 191 | (91.0) | 0.082 |
| Yes | 13,084 | (13.1) | 13,065 | (13.1) | 19 | (9.0) | |
| Arterial hypertension | |||||||
| No | 82,167 | (82.2) | 81,991 | (82.2) | 176 | (83.8) | 0.535 |
| Yes | 17,826 | (17.8) | 17,792 | (17.8) | 34 | (16.2) | |
| Immunosuppressionb | |||||||
| No | 98,830 | (98.8) | 98,627 | (98.8) | 203 | (96.7) | 0.003 |
| Yes | 1163 | (1.2) | 1156 | (1.2) | 7 | (3.3) | |
| Chronic kidney disease | |||||||
| No | 98,440 | (98.5) | 98,239 | (98.5) | 201 | (95.7) | 0.001 |
| Yes | 1553 | (1.5) | 1544 | (1.5) | 9 | (4.3) | |
| Chronic obstructive pulmonary disease | |||||||
| No | 98,872 | (98.9) | 98,667 | (98.9) | 205 | (97.6) | 0.083 |
| Yes | 1121 | (1.1) | 1116 | (1.1) | 5 | (2.4) | |
| Asthma | |||||||
| No | 96,906 | (96.9) | 96,705 | (96.9) | 201 | (95.7) | 0.315 |
| Yes | 3087 | (3.1) | 3078 | (3.1) | 9 | (4.3) | |
| Cancer (any site) | |||||||
| No | 99,744 | (99.7) | 99,535 | (99.7) | 209 | (99.5) | 0.508 |
| Yes | 249 | (0.3) | 248 | (0.3) | 1 | (0.5) | |
SARS-COV-2 Severe acute respiratory coronavirus 2, SD Standard deviation, BMI Body mass index
1) The absolute and relative (%) frequencies are presented, except if the mean is specified; 2) p-value from chi-square or t-tests are presented as corresponding.
aSevere illness included the register of dyspnea requiring hospital admission.
bImmunosuppression referred to any cause of the related deficiency except for type 2 diabetes mellitus or renal impairment.
In multiple analyses (Table 2), increasing age was associated with a reduced risk of reinfection (RRper year = 0.99997, 95% CI 0.99814–0.99958), as well as those with severe primary illness (RR = 0.9989, 95% CI 0.9981–0.9997). When compared with housewives, healthcare workers (RR = 1.0042, 95% CI 1.0030–1.0055) and other healthcare-related employees (RR = 1.0025, 95% 1.0012–1.0039) showed an increased reinfection risk. Other high-risk conditions included the personal history of immunosuppression (RR = 1.0038, 95% 1.0011–1.0065) or chronic kidney disease (RR = 1.0039, 95% CI 1.0016–1.0063).
Table 2.
Predictors of symptomatic laboratory-confirmed SARS-COV-2 reinfection, Mexico 2020
| RR (95% CI), p | ||||||
|---|---|---|---|---|---|---|
| Bivariate analysis | Multiple analysis | |||||
| Gender | ||||||
| Female | 1.0000 | 1.0000 | ||||
| Male | 0.9998 | (0.9993–1.0004) | 0.574 | 1.0004 | (0.9997–1.0009) | 0.256 |
| Age group (years) | ||||||
| 20–49 | 1.0000 | 1.0000 | ||||
| 50–59 | 0.9987 | (0.9980–0.9995) | 0.001 | 0.9989 | (0.9981–0.9997) | 0.006 |
| 60–69 | 0.9984 | (0.9973–0.9996) | 0.007 | 0.9984 | (0.9972–0.9996) | 0.009 |
| 70 + | 0.9983 | (0.9968–0.9999) | 0.039 | 0.9982 | (0.9966–0.9999) | 0.032 |
| Occupation | ||||||
| Housewife | 1.0000 | 1.0000 | ||||
| Healthcare worker | 1.0043 | (1.0032–1.0054) | < 0.001 | 1.0042 | (1.0030–1.0055) | < 0.001 |
| Other healthcare-related | 1.0026 | (1.0013–1.0038) | < 0.001 | 1.0025 | (1.0012–1.0039) | < 0.001 |
| Student | 0.9994 | (0.9964–1.0025) | 0.721 | 0.9993 | (0.9961–1.0024) | 0.646 |
| Other | 1.0006 | (0.9996–1.0015) | 0.227 | 1.0005 | (0.9994–1.0016) | 0.337 |
| Disease severity (at primary infection)a | ||||||
| Mild-moderate | 1.0000 | 1.0000 | ||||
| Severe | 0.9987 | (0.9979–0.9994) | < 0.001 | 0.9989 | (0.9981–0.9997) | 0.007 |
| Personal history of: | ||||||
| Obesity (BMI 30 or higher) | ||||||
| No | 1.0000 | 1.0000 | ||||
| Yes | 0.9996 | (0.9988–1.0003) | 0.228 | 0.9997 | (0.9989–1.0004) | 0.360 |
| Type 2 diabetes mellitus | ||||||
| No | 1.0000 | 1.0000 | ||||
| Yes | 0.9993 | (0.9984–1.0001) | 0.082 | 0.9996 | (0.9987–1.0005) | 0.333 |
| Immunosuppressionb | ||||||
| No | 1.0000 | 1.0000 | ||||
| Yes | 1.0040 | (1.0013–1.0066) | 0.003 | 1.0038 | (1.0011–1.0065) | 0.005 |
| Chronic kidney disease | ||||||
| No | 1.0000 | 1.0000 | ||||
| Yes | 1.0038 | (1.0015–1.0061) | 0.001 | 1.0039 | (1.0016–1.0063) | 0.001 |
RR Risk ratio, CI Confidence interval, BMI Body mass index
1) Generalized linear regression models were used to obtain RR and 95% CI; 2) Multiple regression coefficients were adjusted by variables listed in the table
aSevere illness included the register of dyspnea requiring hospital admission
bImmunosuppression referred to any cause of the related deficiency except for type 2 diabetes mellitus or renal impairment
Discussion
Our results suggest that symptomatic SARS-COV-2 reinfection is a rare phenomenon and factors associated with its risk were characterized. However, these results must be carefully considered since currently there is not a well-defined criterion for SARS-COV-2 reinfection [1].
All enrolled subjects reported disappearance of symptoms from primary infection and the used cutout point to identify potential cases of reinfection (at least 28 days between both laboratory-positive episodes) seemed to be epidemiologically useful since is according to the observed IgG antibodies titers decay in recovered COVID-19 patients [5]. In our study, no PCR testing was performed to avoiding the inclusion of potential cases of persistent viral shedding.
However, and despite this later, the computed incidence density in our analysis was considerable lower (2.5 vs. 7.6 reinfections per 100,000 person-days) than that estimated in a large cohort study where PCR and antibodies testing were available [6]. Therefore, the criteria proposed by Tomassini et al. [1] and which was used in our study may be particularly relevant to identify reinfection cases in sources limited healthcare settings, where no genetic sequencing of viral strains are systematically performed.
According to our findings, healthcare workers and other related employees (i.e. medical assistants, dentists, etc.) are at increased risk of SARS-COV-2 symptomatic reinfections, which sounds plausible given the increased risk of exposure among these subjects [7]. Similar findings were recently observed in a cohort that took place in two cities in the USA [8].
Mild COVID-19 patients at primary episodes also may be at greater risk of reinfection, which may be secondary to lower antibodies titers when compared with pneumonia patients [9]. The association between immunosuppression and [2] renal impairment with COVID-19 risk has been widely discussed [4, 10].
If later replicated, further research is needed to identify factors determining a decreased reinfection risk among older participants and after adjusting by multiple exposures. We hypothesize that a reduced COVID-19 awareness among younger subjects may be implied, at least partially. Besides, longer isolation after the first episode among older subjects and those with more severe disease may be determining the observed scenario.
Conclusions
To the best of our knowledge, this is the first study evaluating predictors of symptomatic SARS-COV-2 reinfection in a large subset of individuals and populations at high-risk were identified. Clinical and epidemiological research regarding SARS-COV-2 reinfection has immediate implications for public health policies focusing on the reduction of viral spread.
Acknowledgements
None to declare.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-COV-2
Severe acute respiratory coronavirus 2
- RT-qPCR
Quantitative reverse transcription polymerase chain reaction
- RR
Risk ratio
- CI
Confidence interval
Authors' contributions
EM-Z and OM-C conceived and designed the study, collected and analyzed data and wrote the manuscript. XT and MH designed the study, analyzed data, and interpreted results and critically reviewed the manuscript. MR-S designed the study, interpreted results and critically reviewed the manuscript. FA-S collected and analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
Funding
None to declare.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Health Research Committee 601 of the Mexican Institute of Social Security provided approval (R-2020-601-022). Prior to the study, all the participants provided informed consent for the analysis of their de-identified data in this study. The methods performed were made following the relevant guidelines and regulations, at the national and local level, for the ethical conduct of research in human subjects (Norm IMSS 2000-001-009, updated on September 29, 2017, that establishes the provisions for health research in the Mexican Institute of Social Security).
Consent for publication
Not applicable.
Competing interests
None to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Efrén Murillo-Zamora, Email: efren.murilloza@imss.gob.mx.
Felipe Aguilar-Sollano, Email: qfaguilar1@gmail.com.
Oliver Mendoza-Cano, Email: oliver@ucol.mx.
References
- 1.Tomassini S, Kotecha D, Bird PW, Folwell A, Biju S, Tang JW. Setting the criteria for SARS-CoV-2 reinfection - six possible cases. J Infect. 2021;82(2):282–327. doi: 10.1016/j.jinf.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol. 2020;101(8):791–797. doi: 10.1099/jgv.0.001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Government of Mexico. COVID-19 in Mexico: General data. Available at: https://coronavirus.gob.mx/datos/. Accessed 10 Apr 2021. [Webpage in Spanish]
- 4.Murillo-Zamora E, Trujillo X, Huerta M, Ríos-Silva M, Mendoza-Cano O. Male gender and kidney illness are associated with an increased risk of severe laboratory-confirmed coronavirus disease. BMC Infect Dis. 2020;20(1):674. doi: 10.1186/s12879-020-05408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand SP, Prévost J, Nayrac M, Beaudoin-Bussières G, Benlarbi M, Gasser R, et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep Med. 2021;2(6):100290. doi: 10.1016/j.xcrm.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielicki JA, Duval X, Gobat N, Goossens H, Koopmans M, Tacconelli E, et al. Monitoring approaches for health-care workers during the COVID-19 pandemic. Lancet Infect Dis. 2020;20(10):e261–e267. doi: 10.1016/S1473-3099(20)30458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veronica F, Anne R, Christopher B, Kenneth C, Jon R. Incidence of COVID-19 recurrence among large cohort of healthcare employees. Ann Epidemiol. 2021;60:8–14. doi: 10.1016/j.annepidem.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko JH, Joo EJ, Park SJ, Baek JY, Kim WD, Jee J, et al. Neutralizing antibody production in asymptomatic and mild COVID-19 patients, in comparison with pneumonic COVID-19 patients. J Clin Med. 2020;9(7):2268. doi: 10.3390/jcm9072268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thng ZX, De Smet MD, Lee CS, Gupta V, Smith JR, McCluskey PJ, et al. COVID-19 and immunosuppression: a review of current clinical experiences and implications for ophthalmology patients taking immunosuppressive drugs. Br J Ophthalmol. 2021;105(3):306–310. doi: 10.1136/bjophthalmol-2020-316586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.